Abstract
Cyclin-dependent kinase-6 (CDK6) is required for early thymocyte development and tumorigenesis. To mechanistically dissect the role of CDK6 in thymocyte development, we generated and analyzed mutant knock-in mice and found that mice expressing a kinase-dead Cdk6 allele (Cdk6K43M) had a pronounced reduction in thymocytes and hematopoietic stem cells and progenitor cells (Lin−Sca-1+c-Kit+ [LSK]). In contrast, mice expressing the INK4-insensitive, hyperactive Cdk6R31C allele displayed excess proliferation in LSK and thymocytes. However, this is countered at least in part by increased apoptosis, which may limit progenitor and thymocyte expansion in the absence of other genetic events. Our mechanistic studies demonstrate that CDK6 kinase activity contributes to Notch signaling because inactive CDK6 kinase disrupts Notch-dependent survival, proliferation, and differentiation of LSK, with concomitant alteration of Notch target gene expression, such as massive up-regulation of CD25. Further, knockout of CD25 in Cdk6K43M mice rescued most defects observed in young mice. These results illustrate an important role for CDK6 kinase activity in thymocyte development that operates partially through modulating Notch target gene expression. This role of CDK6 as a downstream mediator of Notch identifies CDK6 kinase activity as a potential therapeutic target in human lymphoid malignancies.
Introduction
Cyclin-dependent kinase-6 (CDK6) acts as an important cell cycle regulator of the G1-S phase transition1 by negatively regulating the retinoblastoma protein and titrating Cip/Kip CDK inhibitors.2 It also modulates differentiation of certain cells.3 Because CDK6 plays an essential role linking growth-regulatory signals to cell division, its kinase activity is very tightly controlled by association with 2 families of cyclin-dependent kinase inhibitors, including CIP/KIP family and INK4 family proteins.4
Predominant expression of CDK6 in hematopoietic cells5,6 and overexpression in human T-cell lymphoblastic lymphoma/leukemia6 suggest a potential role in T-cell development and malignancies. Moreover, recent analysis of CDK6 knockout (KO) animals reported significantly decreased thymic cellularity and tumorigenesis, underscoring the specific importance of CDK6 in the thymus.7,8 However, the mode of action is unclear because wholesale loss of CDK6 not only impairs its kinase function but also alters the function of other CDKs because of loss of titration common CDK inhibitors. Further, effects of CDK6 loss may also be underestimated because the liberated D cyclins may activate other CDKs.2,8 Therefore, it is necessary to study Cdk6 point mutants in vivo. To address this, we generated mice with targeted mutations of the Cdk6 gene. In the present study, we tested the role of different functional domains of CDK6 in murine thymocyte and hematopoietic stem cell development and homeostasis. We report that CDK6 kinase activity is required for T-cell development at multiple stages, for Lin−Sca-1+c-Kit+ (LSK) production and maintenance, and for their Notch-dependent lineage commitment, survival, proliferation, and differentiation. These studies identify a key role for CDK6 activity in stem and progenitor cells and strongly support CDK6 as a specific therapeutic target in human lymphoid malignancies.
Methods
Construction of Cdk6 KO and knockin mice, Cd25−/− mice, shRNA (GATA3), and γ-secretase inhibitor
To make Cdk6 mutant mice, the LoxP-flanked transcriptional STOP cassette (LSL) was inserted into intron 1 of the Cdk6 gene as described.7 Site-directed mutagenesis was used to introduce point mutations into exon 1 to make conditional mutant alleles Cdk6R31C, K43M, DM. Each strain was mated to nestin-cre mice to excise the LSL cassette. Resulting heterozygotes Cdk6R31C, K43M, DM, WT−Δ were mated to homozygosity.
Cd25−/− mice (BL6 background, stock no. 002952) were purchased from The Jackson Laboratory. All animal studies were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. PLKO.1 target gene set was purchased from Open Biosystems (catalog no. RMM4534-NM_008091 [GATA3]). TRCN0000085482 clone was used in Figure 7D-E. γ-secretase inhibitor (DAPT, 565784 In Solution γ-Secretase Inhibitor IX) was purchased from Calbiochem.
Western blotting, immunoprecipitation, in vitro kinase assays
Thymi were dissected and lysed. Western blotting, immunoprecipitation Westerns, and in vitro kinase assays were performed as described.7
Flow cytometry
Purification of bone marrow cells and OP-9DL1 cocultures
Purification of BM cells and subsequent coculture with OP9-DL1 supporting layer were done as described.7
RNA purification, RT-PCR array, and quantitative RT-PCR
Lin−c-Kit+ cells were sorted by a MoFlo Cell Sorter and cultured on OP9-DL1 cells to initiate Notch signaling. Four days later, cells were sorted to exclude dead cells (PI+) and OP9-DL1 cells from the supporting layer (GFP+). Total RNA was prepared using RNeasy Mini Kit (QIAGEN), converted into double-stranded cDNA, and used for RT-PCR array (SA Biosciences) analysis or quantitative RT-PCR. All mRNA expression levels were normalized to the expression level of 26S RNA in all quantitative RT-PCR experiments. The specific primers used were as follows: CD25, AACCATAGTACCCAGTTGTCGG and TCCTAAGCAACGCATATAGACCA; GATA3, AGCCACATCTCTCCCTTCAG and AGGGCTCTGCCTCTCTAACC; Hes1, AATGCCGGGAGCTATCTTTCT and CCAGCCAGTGTCAACACGA; Beta-2 microglobulin (B2M), TTCTGGTGC-TTGTCTCACTGA and CAGTATGTTCGGCTTCCCATTC; and 26S, ATGATTATCCAAAATGCTTCATTG and AACAGCATATCCCGAATCTCA.
Statistics
Data from different pairs of mice were compared by a 2-tailed Student t test.
Results
Generation of Cdk6 KO or knockin mice
To prevent transcription from the Cdk6 locus and thus knock out CDK6 expression, we inserted an LSL into intron 1 of the Cdk6 gene.7 In knockin mice, point mutations were introduced into exon 1 adjacent to the LSL cassette, resulting in the conditional mutant alleles Cdk6R31C, K43M, DM (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The mutant mice are viable and fertile without gross abnormalities of body weight (within 3 months) or structure.
The Cdk6R31C knockin allele generates a hyperactive kinase, which has been shown to be insensitive to INK4 family inhibitors but does not cause gross structural alterations in the CDK6 protein.11,12 The Cdk6K43M knockin allele generates an inactive kinase because K43 is one of 3 residues forming a catalytic triad conserved in all eukaryotic kinases.13,14 Because this mutant lacks kinase activity, it is unable to inactivate retinoblastoma protein directly but should retain the ability to bind INK4 family proteins, D-type cyclins, and CIP/KIP proteins, thereby sequestering them from other CDKs.
A mutant allele with both mutations in cis (Cdk6DM) was also produced and used as a control for phenotypes arising both in the Cdk6R31C and Cdk6K43M mutant mice. Southern blot and PCR analysis confirmed the expected allele structure (supplemental Figure 1D), and sequence analysis of the entire coding region of Exon1 confirmed the presence of the expected mutations and no other changes (data not shown).
Biochemical properties of cell cycle regulators in KO, Cdk6R31C, Cdk6K43M, and Cdk6DM thymocytes
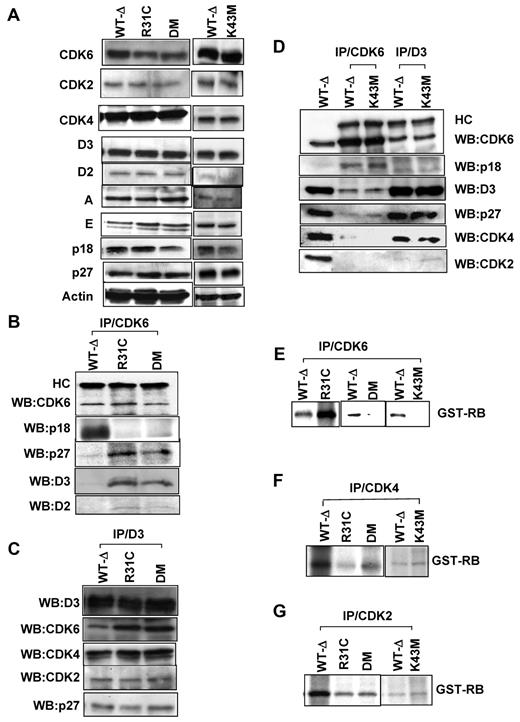
We next extracted proteins from thymocytes of the mutant mice and examined CDK6 protein expression and function. Proteins (designated as R31C, K43M, DM, and WT-Δ) derived from thymocytes of respective animals were readily detected (Figure 1A). The expression level of other cell cycle regulators in thymus extracts was comparable in all strains of mice (Figure 1A).
Analyses of cell cycle regulatory protein expression and function of kinases. (A) Immunoblots with different antibodies as indicated. Actin was used as loading control. (B-D). CDK6 or cyclin D3 was immunoprecipitated from thymocyte extracts and blotted with the indicated antibodies. HC indicates IgG heavy chain. Lane 1 (WT-Δ) represents input (50 μg). (E-G) In vitro kinase assay (CDK6, CDK4, or CDK2) was immunoprecipitated from thymocyte extracts, and an in vitro kinase assay was performed using the recombinant retinoblastoma protein (GST-RB) as the substrate.
Analyses of cell cycle regulatory protein expression and function of kinases. (A) Immunoblots with different antibodies as indicated. Actin was used as loading control. (B-D). CDK6 or cyclin D3 was immunoprecipitated from thymocyte extracts and blotted with the indicated antibodies. HC indicates IgG heavy chain. Lane 1 (WT-Δ) represents input (50 μg). (E-G) In vitro kinase assay (CDK6, CDK4, or CDK2) was immunoprecipitated from thymocyte extracts, and an in vitro kinase assay was performed using the recombinant retinoblastoma protein (GST-RB) as the substrate.
The function of mutant CDK6 proteins was examined by protein interaction assays. CDK6 protein was immunoprecipitated (Figure 1B-D) from the thymus lysates and the immunoprecipitates were probed with antibodies against CDK6, p18, p27, cyclin D3, and cyclin D2. Consistent with previous data,11,12 R31C and DM mutants lose interaction with INK4 family proteins, such as p18, and bind more p27 and D-cyclins (Figure 1B). The binding ability of K43M to p18, p27, and D cyclins was similar to that of WT-Δ (Figure 1D). Next, cyclin D3 was immunoprecipitated from thymus lysates and the immunoprecipitates were probed with antibodies against CDK6, p18, cyclin D3, p27, CDK4, and CDK2 (Figure 1C-D). In the absence of p18 binding (Figure 1B), the level of cyclin D3-bound R31C and DM was increased compared with that of WT-Δ (Figure 1C), whereas the level of cyclin D3-bound K43M was comparable with that of WT-Δ (Figure 1D). The levels of cyclin D3-bound CDK2, CDK4, and p27 were comparable in all thymus extracts (Figure 1C-D), suggesting that increased R31C and DM-bound p27 (Figure 1B,D) results predominantly from the increase in cyclin D2 association. These results suggest that K43M retains the ability to bind D cyclins and p18, to titrate p27, and that R31C and DM bind more p27 and D cyclins in the absence of INK4 family protein binding (supplemental Table 1).
CDK6 kinase activity in thymocyte lysates was examined by in vitro kinase assays (Figure 1E). K43M and DM had very little CDK6 kinase activity in anti-CDK6 immunoprecipitates, whereas R31C had higher kinase activity than that from WT-Δ. Thus, R31C had elevated kinase activity as predicted, whereas K43M and DM had no or low kinase activity. The K43M mutation (in DM) negated the activity of CDK6, and this clearly counteracted the elevated activity seen in R31C.
The liberation of p27 from other CDK complexes by R31C and DM (Figure 1B) may increase the kinase activity of CDK4 or CDK2. To test this possibility, we performed in vitro kinase assays to measure CDK2 and CDK4 kinase activity and showed that whole thymus lysates from mutant mice had similar (K43M) or slightly lower (R31C and DM) CDK2 and CDK4 kinase activity compared with that from WT-Δ (Figure 1F-G). Therefore, there is no obvious activation of CDK4 or CDK2 kinase activity in the thymocytes of mutant mice. These results clearly demonstrate that our Cdk6 mutant animals behave as expected at the biochemical level (supplemental Table 1), and thus are suitable to assess the relative contribution of CDK6 kinase activity and noncatalytic activity to the process of thymocyte and progenitor cell development.
Altered thymocyte numbers in CDK6 mutant mice
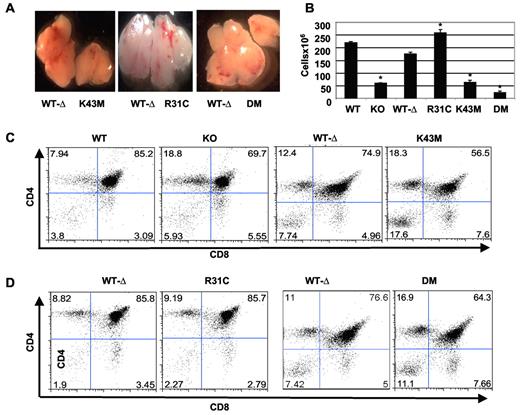
Similar to previous findings in KO mice,7 thymuses from Cdk6K43M and Cdk6DM mice contained approximately 3-fold and approximately 6-fold fewer thymocytes, respectively. In contrast, Cdk6R31C thymuses had approximately 1.5-fold more cells than those from Cdk6WT−Δ littermates (Figure 2B). Consistent with changes in cellularity, the Cdk6K43M and Cdk6DM thymuses appeared smaller, and Cdk6R31C thymus appeared bigger, than the Cdk6WT−Δ control (Figure 2A). In contrast, body weights were similar between the littermates at ages analyzed (supplemental Figure 2A), suggesting that the change in thymus size is tissue specific. These findings also suggest that loss of CDK6 kinase activity plays a major role in limiting thymic cellularity and that increasing CDK6 activity by preventing INK4 interaction significantly increases thymocyte numbers.
Defective thymocyte development in Cdk6 mutant mice. (A) Gross picture of thymi from the mutant mice and littermates. Images were acquired with Leica MZFL III dissection microscope at 2× magnification fitted with a SPOT RT KE/SE camera from Diagnostic Instruments Inc. SPOT software Version 4.1 for Macintosh was used for image production. (B) Total number of thymocytes from 1- to 3-month-old mice. The cell numbers are mean ± SE (n = 15 for WT and KO, n = 13 for R31C, n = 6 for K43M, and n = 17 for WT-Δ and DM). *P < .05, significantly different from the control levels. (C-D) Flow cytometric profiles of thymocytes from 2-month-old mice after staining with CD4 and CD8 antibodies. The percentage of cells in each quadrant is shown.
Defective thymocyte development in Cdk6 mutant mice. (A) Gross picture of thymi from the mutant mice and littermates. Images were acquired with Leica MZFL III dissection microscope at 2× magnification fitted with a SPOT RT KE/SE camera from Diagnostic Instruments Inc. SPOT software Version 4.1 for Macintosh was used for image production. (B) Total number of thymocytes from 1- to 3-month-old mice. The cell numbers are mean ± SE (n = 15 for WT and KO, n = 13 for R31C, n = 6 for K43M, and n = 17 for WT-Δ and DM). *P < .05, significantly different from the control levels. (C-D) Flow cytometric profiles of thymocytes from 2-month-old mice after staining with CD4 and CD8 antibodies. The percentage of cells in each quadrant is shown.
Altered thymocyte development in Cdk6 mutant mice
To determine how CDK6 activity impacts thymocyte development, we analyzed isolated cells from different developmental compartments using FACS. Double-positive (DP) cells were decreased in Cdk6K43M and Cdk6DM thymuses, whereas the percentage of CD4+ single-positive (SP) and CD8+ SP cells was increased (Figure 2C-D; supplemental Table 2). Notably, Cdk6K43M but not KO and Cdk6DM thymuses had a significant increase in the percentage of double-negative (DN) cells. Thus, animals expressing K43M strongly resembled KO mice with respect to changes in the more mature thymocyte subsets but more profoundly increased the DN population, indicating that specific inactivation of CDK6 differs from CDK6 loss in this measure of thymocyte development.
In Cdk6K43M and Cdk6DM mice, the actual number of cells in each thymocyte compartment was also reduced as observed in KO mice, with the exception of the DN compartment, in which Cdk6K43M mice had only slightly reduced numbers (supplemental Figure 2B) compared with the significant reductions seen in KO mice.7 Interestingly, the fraction of DP, CD8+, and DN populations was not altered in Cdk6R31C mice (Figure 2D; supplemental Figure 2; supplemental Table 2). Consistent with the increase in total thymic cellularity and the small changes in the fraction of each compartment, the absolute number of thymocytes in each compartment increased by approximately 50% (supplemental Figure 2C).
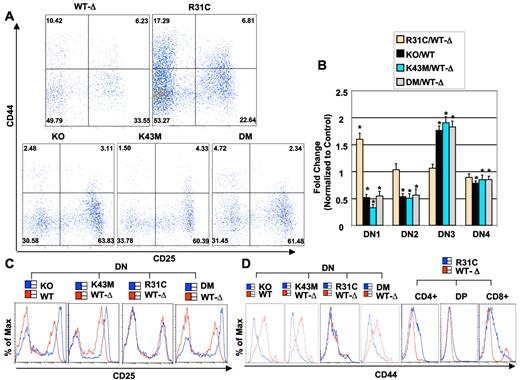
Cdk6K43M and Cdk6DM mice contained approximately 3 and 2 times fewer cells in the DN1 stage, respectively, than the Cdk6WT−Δ littermates, whereas Cdk6R31C mice had a significant increase (Figure 3A; supplemental Table 2). These data indicate that kinase activity affects stem and progenitor populations in the thymus because DN1 is a heterogeneous subset containing non–T-lineage cells and a c-kit+ CD4low subpopulation, accounting for most of the T-cell precursor activity in this compartment.
Alteration in DN subsets and in CD25 and CD44 expression in thymuses of Cdk6 mutant mice. (A) Flow cytometric analysis of CD44/CD25 expressing thymocytes from 2-month-old mice. The cells were stained with the “cocktail” of lineage-specific antibodies and CD44 and CD25 antibodies. Lineage-positive cells were electronically “gated out,” and CD44-versus-CD25 profiles of the lineage-negative compartments are presented. Numbers in quadrants indicate the percentage of cells in each subset. (B) Quantification of DN1–4 cells. The bar graph summarizes the percentage of DN1–4 populations from separate experiments. Data are mean ± SE. *P < .05, significantly different from the control levels, which were arbitrarily defined as 1 unit (100%). (See supplemental Table 2 for the animal numbers used.) (C) Flow cytometric analysis of CD25 expression in DN thymocytes. (D) Flow cytometric analysis of CD44 expression in DN thymocytes from WT and KO, and from WT-Δ and K43M, R31C, and DM. (Right) Flow cytometric analysis of CD44 expression in CD4+, DP, and CD8+ subsets from R31C thymocytes.
Alteration in DN subsets and in CD25 and CD44 expression in thymuses of Cdk6 mutant mice. (A) Flow cytometric analysis of CD44/CD25 expressing thymocytes from 2-month-old mice. The cells were stained with the “cocktail” of lineage-specific antibodies and CD44 and CD25 antibodies. Lineage-positive cells were electronically “gated out,” and CD44-versus-CD25 profiles of the lineage-negative compartments are presented. Numbers in quadrants indicate the percentage of cells in each subset. (B) Quantification of DN1–4 cells. The bar graph summarizes the percentage of DN1–4 populations from separate experiments. Data are mean ± SE. *P < .05, significantly different from the control levels, which were arbitrarily defined as 1 unit (100%). (See supplemental Table 2 for the animal numbers used.) (C) Flow cytometric analysis of CD25 expression in DN thymocytes. (D) Flow cytometric analysis of CD44 expression in DN thymocytes from WT and KO, and from WT-Δ and K43M, R31C, and DM. (Right) Flow cytometric analysis of CD44 expression in CD4+, DP, and CD8+ subsets from R31C thymocytes.
Cdk6K43M and Cdk6DM mice also had approximately 2 times fewer cells in the DN2 stage but had approximately 1.9-fold and approximately 1.8-fold more DN3 cells, respectively (Figure 3A-B). When assessed for DNA content, a smaller proportion of DN3 cells were in the S-G2-M phases of the cell cycle (supplemental Figure 3A,C), indicating a potential developmental delay at this stage. In contrast, only a marginal but still significant reduction in DN4 cells was detected in Cdk6K43M and Cdk6DM mice. Interestingly, we observed that the populations of DN2–4 cells from Cdk6R31C mice were comparable with that of WT-Δ (Figure 3B). Despite this, a greater proportion of DN2 and DN3 cells was in the S-G2-M phases of the cell cycle (supplemental Figure 3B), and a greater proportion of apoptotic cells was detected in the DN2 and DN3 compartments (supplemental Figure 3E). Thus, the increase in cycling cells in Cdk6R31C mice is accompanied by a higher apoptosis rate, probably compromising the effect of increased kinase activity on cell expansion, leading to a comparable fractional distribution of thymocyte subpopulations between Cdk6R31C and Cdk6WT−Δ. No change in the size of thymocytes in the DN stage was observed in any mutant animal (data not shown).
DN thymocytes were further analyzed for the expression of CD44 and CD25 (Figure 3C-D). There was a marked elevation in CD25 expression in Cdk6K43M and Cdk6DM thymocytes in the DN fraction as in KO, but not in Cdk6R31C (Figure 3C). Similarly, CD44 expression was lower in Cdk6K43M and Cdk6DM but unchanged in Cdk6R31C thymocytes in the DN fraction. However, CD44 was elevated in mature SP cells from Cdk6R31C mice (Figure 3D). The alterations of these markers may lead to partial developmental blocks in the differentiation of DN3 cells to DN4 and ultimately to the DP stage in KO, Cdk6K43M, and Cdk6DM animals. Taken together, these data suggest that loss of CDK6 kinase activity decreases thymocyte cell numbers in most developmental subsets, whereas hyperactive CDK6 kinase increases thymocyte cell numbers in most compartments, consistent with the expression profile of CDK6 in all thymocyte subsets.7 Further, loss of CDK6 kinase activity resulted in a reduced DN1 fraction, increased populations in the DN3 stage, increased CD25 expression, and reduced CD44 expression, whereas hyperactive kinase activity led to an increased DN1 fraction and increased expression of CD44 in more mature thymocyte populations, indicating that CDK6 kinase activity acts in part to regulate key T-cell markers and thus affects thymocyte development. Reduction in CDK6 kinase activity and alteration of CD25 and CD44 expression are sufficient to profoundly alter the DN3 compartment but cannot explain the hypoplasia in CDK6 mutant thymuses or the fact that this is more severe in Cdk6DM thymuses compared with those from Cdk6K43M thymuses (Figure 2B). Thus, additional effects of CDK6 loss or mutation may also influence T-cell development.
CDK6 and its kinase activity are required for stem cell/multipotent progenitor production and maintenance
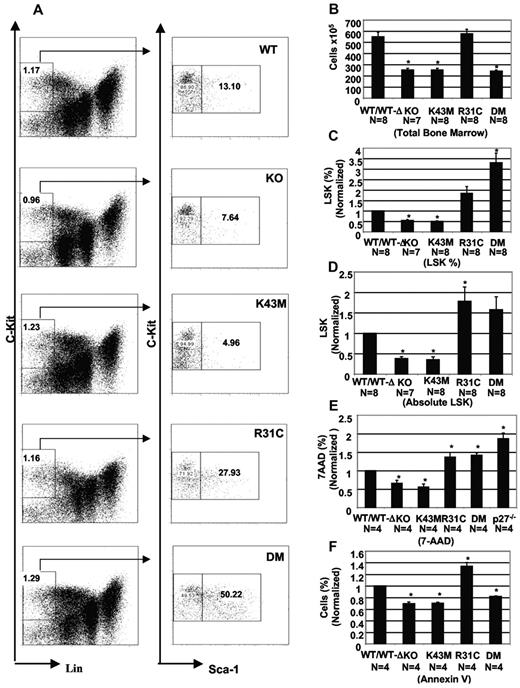
To investigate the function of CDK6 at early hematopoietic developmental stages, we assessed the impact of CDK6 loss or mutation on the profile of LSK cells in the BM using flow cytometry. We found that KO and Cdk6K43M mice had a 50% reduction in LSK cells compared with those from WT or Cdk6WT−Δmice (Figure 4C). The absolute number of LSK cells in KO and Cdk6K43M BM is approximately one-third of control. In contrast, Cdk6R31C BM had an approximately 2-fold increase in LSK fraction and in absolute number (Figure 4D). Cdk6DM BM had a similar absolute number of LSK cells compared with that of Cdk6WT−Δmice but had an approximately 4-fold increase in LSK fraction. Thus, both the kinase activity of CDK6 and its ability to titrate p27 probably govern LSK numbers in the BM.
CDK6 and its kinase activity are required for stem cell/multipotent progenitor development. (A) Staining for hematopoietic stem cells (LSK fraction). The relative percentage of selected cell populations in BM is shown. (B) Total BM cellularity from 2- to 3-month-old mice. The cell numbers are mean ± SE (N is indicated). *P < .05, significantly different from the control levels. (C) The percentage of LSK in the total cell population was calculated based on the percentage of Lin−c-Kit+ and c-Kit+ Sca-1+ populations and is the mean ± SE. *P < .05, significantly different from the WT/WT-Δ control, which was arbitrarily defined as 1 unit (100%). (D) The absolute cell numbers for the LSK were calculated based on the total number of cells (B) and the percentage of LSK (C) in the total cell population from BM, and are the mean ± SE (N is indicated). *P < .05, significantly different from the WT/WT-Δ controls, which was arbitrarily defined as 1 unit (100%). (E) Proliferative fraction of LSK. BM suspensions were surface-stained before permeabilization and then stained with 7-amino-actinomycin D (7-AAD). The percentage of (S-G2-M) from 4 separate experiments is the mean ± SE. *P < .05, significantly different from the WT/WT-Δ controls, which was arbitrarily defined as 1 unit (100%). (F) Apoptotic fraction from LSK cells using annexin V staining. Data are mean ± SE (N is indicated below each mutant mouse). *P < .05, significantly different from the WT/WT-Δ control, which was arbitrarily defined as 1 unit (100%).
CDK6 and its kinase activity are required for stem cell/multipotent progenitor development. (A) Staining for hematopoietic stem cells (LSK fraction). The relative percentage of selected cell populations in BM is shown. (B) Total BM cellularity from 2- to 3-month-old mice. The cell numbers are mean ± SE (N is indicated). *P < .05, significantly different from the control levels. (C) The percentage of LSK in the total cell population was calculated based on the percentage of Lin−c-Kit+ and c-Kit+ Sca-1+ populations and is the mean ± SE. *P < .05, significantly different from the WT/WT-Δ control, which was arbitrarily defined as 1 unit (100%). (D) The absolute cell numbers for the LSK were calculated based on the total number of cells (B) and the percentage of LSK (C) in the total cell population from BM, and are the mean ± SE (N is indicated). *P < .05, significantly different from the WT/WT-Δ controls, which was arbitrarily defined as 1 unit (100%). (E) Proliferative fraction of LSK. BM suspensions were surface-stained before permeabilization and then stained with 7-amino-actinomycin D (7-AAD). The percentage of (S-G2-M) from 4 separate experiments is the mean ± SE. *P < .05, significantly different from the WT/WT-Δ controls, which was arbitrarily defined as 1 unit (100%). (F) Apoptotic fraction from LSK cells using annexin V staining. Data are mean ± SE (N is indicated below each mutant mouse). *P < .05, significantly different from the WT/WT-Δ control, which was arbitrarily defined as 1 unit (100%).
Under homeostatic conditions, reduced cell proliferation, increased cell death, or both may cause the decreased LSK population in vivo in KO and Cdk6K43M mice. To assess these possibilities, we examined the proliferative profiles of LSK as determined by DNA content (supplemental Figure 5B; Figure 4E). We found a significantly decreased fraction of cycling cells in KO and Cdk6K43M mice (35% and 45% reduction, respectively). In contrast, Cdk6R31C and Cdk6DM mice had a significantly increased fraction of cycling cells (37% and 42% increase, respectively). This increase is similar to that observed in LSK cells derived from p27-deficient mice (86% increase).16 Thus, the increased proliferation of LSK cells in Cdk6R31C mice may partially result from both increased ability to bind to p27 and hyperactive kinase activity (Figure 1B; supplemental Figure 1; supplemental Table 1). Similarly, the liberation of p27 from other CDK complexes by DM may override the lack of CDK6 kinase activity in LSK cells but appears to be insufficient to support normal BM cellularity (Figure 4B), suggesting discrete and critical roles for CDK6 kinase activity in the LSK cells. Interestingly, as was observed in thymocyte populations, Cdk6R31C LSK cells show a profound increase in apoptosis (supplemental Figure 5A; Figure 4F), which probably largely counteracts the increased proliferation rate, thus limiting the overall expansion of these cells in the BM. Finally, it is noteworthy that this increase in apoptosis is not observed in Cdk6DM LSK cells, confirming that deregulated kinase activity is responsible for apoptosis.
CDK6 kinase activity is required for Notch-dependent proliferation, survival, and differentiation
Notch signaling has been shown to induce T-cell specification of multipotent hematopoietic progenitors.17 We previously showed that CDK6 loss interferes with the proliferation, differentiation, and survival signals instigated by Notch signaling in vitro.7 To dissect which functions of CDK6 mediate the effects of Notch, we took advantage of a cell culture system (OP9-DL1–T cell–promoting conditions) that has the capacity to induce differentiation of hematopoietic progenitors into T cells through expression of Delta-like–1 on BM stromal cells, thus mimicking the thymic stroma to support thymocyte development.18 c-Kit+Lin− (LK)19 BM cells were cocultured on OP9-DL1 cells and analyzed at day 12 in terms of expansion, differentiation, and survival. There was an approximately 9-fold increase in cellularity at day 12 in Cdk6WT−Δ and Cdk6R31C cultures. In contrast, LKs from KO, Cdk6K43M and Cdk6DM BM failed to expand throughout the coculture period (Figure 5A), suggesting that CDK6 kinase activity is required for Notch-mediated cell expansion. The loss of kinase activity in DM is clearly dominant over its ability to titrate p21/p27 in this assay.
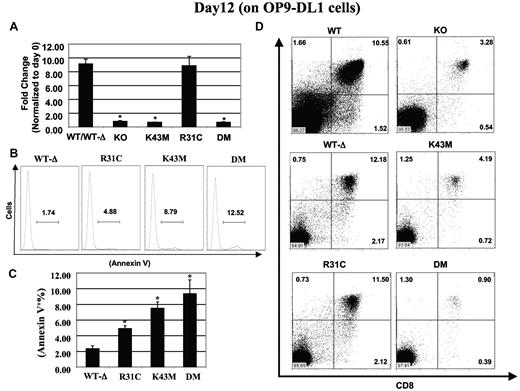
CDK6/kinase activity is required for Notch-dependent proliferation, survival, and differentiation. (A) Proliferation: the fold change in cell expansion was calculated based on the ratio of cocultured cells at day 12 to total c-Kit+Lin− cell number plated at day 0, which were arbitrarily defined as 1 unit. Data are mean ± SE (N = 5). *P < .05, significantly different from the control. (B) Apoptosis profiles after annexin V staining. (C) The percentage of cells stained with annexin V. Data are mean ± SE (N = 5). *P < .05, significantly different from the WT-Δ control. (D) Differentiation of stem cells into T cells. Flow cytometric profiles of thymocytes from day 12 cultures stained with CD4 and CD8 antibodies. The percentage of cells in each quadrant is shown.
CDK6/kinase activity is required for Notch-dependent proliferation, survival, and differentiation. (A) Proliferation: the fold change in cell expansion was calculated based on the ratio of cocultured cells at day 12 to total c-Kit+Lin− cell number plated at day 0, which were arbitrarily defined as 1 unit. Data are mean ± SE (N = 5). *P < .05, significantly different from the control. (B) Apoptosis profiles after annexin V staining. (C) The percentage of cells stained with annexin V. Data are mean ± SE (N = 5). *P < .05, significantly different from the WT-Δ control. (D) Differentiation of stem cells into T cells. Flow cytometric profiles of thymocytes from day 12 cultures stained with CD4 and CD8 antibodies. The percentage of cells in each quadrant is shown.
We next examined the levels of proliferation and apoptosis in cells from mutant mice cultured on OP9-DL1 cells. There was an approximately 1.8-fold increase in the proliferative fractions in Cdk6R31C cells at 12 days and a marginal but significant reduction in the proliferative fractions in K43M and DM cells (supplemental Figure 6B, 26% and 34% reduction, respectively). The apoptotic fraction of thymocyte subsets at day 12 was increased by 2-, 3-, and 4-fold in Cdk6R31C, Cdk6K43M, and Cdk6DM, respectively (Figure 5B-C), suggesting that aberrant CDK6 kinase activity increases apoptotic cell death in progenitor populations (Figure 4F) and lack of that activity (K43M and DM) greatly increases apoptosis in response to Notch signaling (Figure 5B-C). Thus, the reduced proliferation and increased apoptosis of Cdk6K43M and Cdk6DM progenitors cultured on OP9-DL1 cells support a role for CDK6 kinase activity in mediating proliferation and survival in response to Notch signals and may explain the significant reduction in thymic cellularity in vivo. In addition, the increased proliferative fractions (supplemental Figure 6) may counteract the increased apoptosis of Cdk6R31C progenitors cultured on OP9-DL1 cells and result in similar total cell numbers between Cdk6WT−Δ and Cdk6R31Cmice.
We also compared differentiation of different stem/progenitors plated on OP9-DL1 (Figure 5D). Notably, Cdk6K43M and Cdk6DM cells were defective in their ability to differentiate into DP cells. Cells accumulated in the DN3 stage (supplemental Figure 7A), and CD25 expression increased in Cdk6K43M and Cdk6DM cells (supplemental Figure 7B). However, the level of CD44 expression was unchanged (supplemental Figure 7C). In contrast, Cdk6R31C cells can differentiate into DP cells; the expression levels of CD25 and CD44 in Cdk6R31C cells were comparable with that of Cdk6WT−Δ mice, suggesting that CDK6 kinase activity might have a very important role downstream of Notch in suppressing CD25 gene expression. Thus, loss of kinase activity leads to a developmental delay in the differentiation of DN3 cells to DN4 and ultimately to the DP stage. Moreover, the unaltered CD44 expression in the OP-9DL1 coculture system using different CDK6 mutants suggests that the observed alteration in CD44 expression in thymocytes is Notch independent. Taken together, these data indicate that CDK6 kinase activity is required for Notch-mediated proliferation, survival, and differentiation.
CDK6/kinase activity modulates the expression of Notch target genes involved in cell survival and T-cell differentiation
To further characterize CDK6-mediated Notch signaling, we analyzed the expression of genes involved in Notch-mediated proliferation, survival, and differentiation using the Mouse Notch Signaling Pathway RT2 profiler PCR Array (SA Biosciences). LK cells were sorted from BM derived from KO, WT, and Cdk6K43M mice in an effort to focus solely on the effects of CDK6/kinase loss of function. The LK population was then used either to prepare RNA or to grow on OP9-DL1 to initiate Notch signaling for 4 days. At this time point, the first recognizable T-lineage cells are detected based on expression of Thy1 and CD25.19 RNA samples were prepared from day 4 sorted GFP−PI− cells to exclude supporting layer OP9-DL1 cells (which are GFP+) and dead cells (PI+). Of the 84 genes involved in the Notch signaling pathway in this array, we found that 94% and 83% were down-regulated in the stem cell population of KO and Cdk6K43M mice, respectively (data not shown). Expression of a large number of developmentally regulated genes, including several well-characterized Notch1 target genes, is dysregulated when there is loss of CDK6 function (supplemental Table 3). Among these were CD25, Pparg, Shh, DTX1, HES1, Hey1, Hr, Gli, Fzd2, DLL1, Wnt11, Zic2, CD44 Gsk3β, and CBL. Although there was no uniform degree or direction of misregulation in the response of individual genes, 4 of 4 (CD25, Dtx1, Hes1, and Hey1) well-characterized direct Notch1 target genes analyzed showed significant up-regulation in the context of CDK6 loss of function.
CDK6/kinase activity represses CD25 and GATA3 expression in Notch-stimulated cells
As described in supplemental Table 3, many Notch-responsive genes are misregulated in CDK6 mutant cells. Among these, the highly increased induction of CD25 was of particular interest, given the phenotypic alterations in thymocyte compartments of CDK6 mutant mice. The CD25 level was low in BM LK populations in KO and Cdk6K43M mice but highly up-regulated in response to Notch signaling (supplemental Table 3). Consistent with previous data,19 CD25 expression was also up-regulated in WT LK cells after Notch stimulation (35-fold). The increase in CD25 expression in KO (∼ 750 fold) and Cdk6K43M (∼ 250 fold) cells was dramatically greater than that in WT cells. Using quantitative RT-PCR with other CD25 primer pairs, we validated the expression pattern of CD25 (Figure 6A) seen in the RT-PCR array. Similarly, GATA3 expression was low in BM LK populations from KO and Cdk6K43M mice and was up-regulated in response to Notch signaling (Figure 6B). Activation of the Notch pathway consequent to OP9-DL1 coculture is required for the increased expression of CD25, GATA3, and Hes1 because inhibition of endogenous Notch activation by γ-secretase inhibitor significantly reduced the expression of CD25, GATA3, and Hes1 but not B2M, a non-Notch responsive gene (Figure 6C). In contrast, CD44 expression was little altered (supplemental Figure 7D), suggesting that changes in CD44 expression observed in thymocytes in vivo are not Notch dependent, consistent with the unaltered CD44 protein expression in the OP9-DL1 coculture system (supplemental Figure 7B). Together, these data suggest that CDK6 kinase activity suppresses GATA3 and CD25 gene expression.
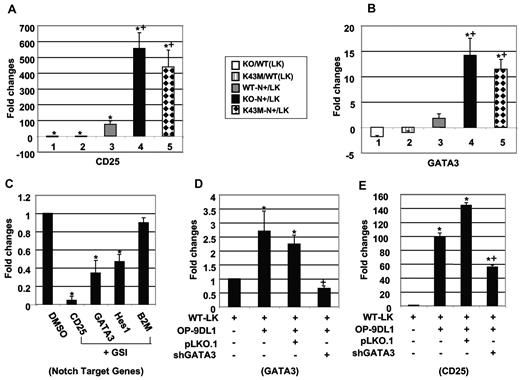
CDK6/kinase activity is required for repression of CD25 and GATA3. (A-B) The relative mRNA expression for CD25 (A) and GATA3 (B) between different mutants and their corresponding controls. LK indicates purified stem cell/progenitors; and N+, cells cocultured on OP-9DL1 for 4 days. RNA isolated from LK or N+ cells was used for quantitative RT-PCR according to the manufacturer's protocol. “KO/WT (LK)” indicates the ratio of LK cells derived from BM of KO compared with LK cells from WT, without OP9-DL1 stimulation. Similarly, “KO-N+/LK” indicates the ratio of LK cells from KO compared with the same cells after 4 days culture on OP9-DL1 stroma. Data are mean ± SE (N = 3). *P < .05, significantly different from the control levels (denominator), which were arbitrarily defined as 1 unit. +P < .05, significantly different from bar 3. All the mRNA expression levels were normalized to the expression level of 26S RNA. (C) Quantitative RT-PCR analysis of indicated gene expression after treatment of WT-LK cells with γ-secretase inhibitor (DAPT: 5 μm) for 4 days on OP9-DL1. Sorted GFP−PI− cells were used for quantitative RT-PCR. All the mRNA expression levels were normalized to the expression level of 26S RNA and calculated as values relative to DMSO-treated cells. Data are the mean ± SE (N = 3). *P < .05, significantly different from the control levels, which were arbitrarily defined as 1 unit. (D-E) Quantitative RT-PCR for GATA3 (D) and CD25 (E) gene expression. Sorted WT-LK cells were infected with control vector (pLKO.1) or shRNA GATA3 (shGATA3) and then cocultured with OP9-DL1 for 4 days. The optimal puromycin concentration used for selection is 0.5 μg/mL. Sorted GFP−PI− cells were used for quantitative RT-PCR. All the mRNA expression levels were normalized to the expression level of 26S RNA and calculated as values relative to WT-LK cells. Data are the mean ± SE (N = 3). *P < .05, significantly different from the WT-LK control level, which was arbitrarily defined as 1 unit. +P < .05, significantly different from the pLKO.1 level.
CDK6/kinase activity is required for repression of CD25 and GATA3. (A-B) The relative mRNA expression for CD25 (A) and GATA3 (B) between different mutants and their corresponding controls. LK indicates purified stem cell/progenitors; and N+, cells cocultured on OP-9DL1 for 4 days. RNA isolated from LK or N+ cells was used for quantitative RT-PCR according to the manufacturer's protocol. “KO/WT (LK)” indicates the ratio of LK cells derived from BM of KO compared with LK cells from WT, without OP9-DL1 stimulation. Similarly, “KO-N+/LK” indicates the ratio of LK cells from KO compared with the same cells after 4 days culture on OP9-DL1 stroma. Data are mean ± SE (N = 3). *P < .05, significantly different from the control levels (denominator), which were arbitrarily defined as 1 unit. +P < .05, significantly different from bar 3. All the mRNA expression levels were normalized to the expression level of 26S RNA. (C) Quantitative RT-PCR analysis of indicated gene expression after treatment of WT-LK cells with γ-secretase inhibitor (DAPT: 5 μm) for 4 days on OP9-DL1. Sorted GFP−PI− cells were used for quantitative RT-PCR. All the mRNA expression levels were normalized to the expression level of 26S RNA and calculated as values relative to DMSO-treated cells. Data are the mean ± SE (N = 3). *P < .05, significantly different from the control levels, which were arbitrarily defined as 1 unit. (D-E) Quantitative RT-PCR for GATA3 (D) and CD25 (E) gene expression. Sorted WT-LK cells were infected with control vector (pLKO.1) or shRNA GATA3 (shGATA3) and then cocultured with OP9-DL1 for 4 days. The optimal puromycin concentration used for selection is 0.5 μg/mL. Sorted GFP−PI− cells were used for quantitative RT-PCR. All the mRNA expression levels were normalized to the expression level of 26S RNA and calculated as values relative to WT-LK cells. Data are the mean ± SE (N = 3). *P < .05, significantly different from the WT-LK control level, which was arbitrarily defined as 1 unit. +P < .05, significantly different from the pLKO.1 level.
Knockdown of GATA3 reduces CD25 expression in Notch-stimulated cells
Like CD25, GATA3 is up-regulated in thymocytes between the DN3 and DN4 stages and tends to decrease by the DN4 stage.20 The CD25 promoter has binding sites for GATA factors.21 In view of the increased CD4+ SP and DN3 fractions in thymocytes lacking CDK6 kinase activity and the recent evidence that GATA3 functions in early T-cell commitment and CD4+ T-cell development,22 we hypothesized that GATA3 might be a downstream target of CDK6 in controlling T-cell proliferation and differentiation by modulating CD25 expression. To test this hypothesis, we infected sorted LK cells with lentivirus-encoded GATA3 shRNA or vector before coculture with OP-9DL1. Partial knockdown of GATA3 (Figure 6D) leads to equivalent down-regulation of CD25 (Figure 6E) but not B2M (data not shown) or 26S gene expression, suggesting that GATA3 regulates CD25 expression in CDK6 mutant cells.
Rescue of K43M phenotypes by KO of CD25
To determine the biologic relevance of increased CD25 expression in K43M thymocytes and progenitors, we used a Cd25 KO mouse.15 Cd25−/− mice were crossed with K43M mice, and thymocytes from the resulting K43M;CD25−/− animals were analyzed before 4 weeks of age because Cd25−/− mice have phenotypically normal development of T cells at this age.15 The deletion of CD25 in thymocytes was confirmed by genotyping (data not shown) and using CD25 antibody in FACS analysis (Figure 7A). In the absence of CD25, the DN3 (CD44−CD27−) and DN4 (CD44−CD27+) populations can be defined by differences in expression of CD27 (Figure 7C).23 As expected,15 at the age of 3 to 4 weeks, no differences were noted in developmental subpopulations defined by CD4 and CD8 between Cd25−/− mice and WT littermates (supplemental Table 5). Ablation of CD25 in the K43M background rescued some of the defects seen in K43M mice, such as total thymocyte cellularity (Figure 7D), LSK fraction (Figure 7B), absolute LSK numbers (Figure 7E), and accumulation in the DN3 stage (Figure 7C,F). The significantly increased number of LSK cells and the abrogation of the DN3 block in K43M;CD25−/− mice appear to restore total cellularity in the thymus to WT levels. However, there was no change in CD4+ and CD8+ populations between K43M and K43M;CD25−/− mice (supplemental Table 4), suggesting that the inhibitory role of CDK6 kinase activity in later thymocyte development is CD25 independent. Taken together, these data indicate that CDK6 kinase activity-mediated early thymocyte development is CD25 dependent.
Rescue of developmental defects in K43M mice by KO of CD25. (A) Flow cytometric analysis of CD25 expression in DN thymocytes. (B) Flow cytometric analysis of LSK fractions from BM. (C) Flow cytometric analysis of DN3 (CD44−CD27−) and DN4 (CD44−CD27+) populations in DN thymocytes. (D) Total number of thymocytes from 3- to 4-week-old mice. The cell numbers are mean ± SE (N = 3 for all the animals). *P < .05, significantly different from the WT control levels. +P < .05, significantly different from the K43M levels. (E) The absolute cell numbers for the LSK populations were calculated based on the percentage of LK (supplemental Table 5) and LSK (supplemental Table 5) in the total cell population from BM (supplemental Table 5). Absolute LSK = total BM cells × LK (%) × LSK (%), expressed as mean ± SE (N = 3). *P < .05, significantly different from the WT controls. +P < .05, significantly different from the K43M levels. (F) Quantification of DN3 cells. The bar graph summarizes the percentage of DN3 (CD44−CD27−) populations from 3 separate experiments. Data are mean ± SE. *P < .05, significantly different from the WT control levels, which were arbitrarily defined as 1 unit (100%). +P < .05, significantly different from the K43M levels.
Rescue of developmental defects in K43M mice by KO of CD25. (A) Flow cytometric analysis of CD25 expression in DN thymocytes. (B) Flow cytometric analysis of LSK fractions from BM. (C) Flow cytometric analysis of DN3 (CD44−CD27−) and DN4 (CD44−CD27+) populations in DN thymocytes. (D) Total number of thymocytes from 3- to 4-week-old mice. The cell numbers are mean ± SE (N = 3 for all the animals). *P < .05, significantly different from the WT control levels. +P < .05, significantly different from the K43M levels. (E) The absolute cell numbers for the LSK populations were calculated based on the percentage of LK (supplemental Table 5) and LSK (supplemental Table 5) in the total cell population from BM (supplemental Table 5). Absolute LSK = total BM cells × LK (%) × LSK (%), expressed as mean ± SE (N = 3). *P < .05, significantly different from the WT controls. +P < .05, significantly different from the K43M levels. (F) Quantification of DN3 cells. The bar graph summarizes the percentage of DN3 (CD44−CD27−) populations from 3 separate experiments. Data are mean ± SE. *P < .05, significantly different from the WT control levels, which were arbitrarily defined as 1 unit (100%). +P < .05, significantly different from the K43M levels.
Discussion
Previous observations support a crucial role for the CDK6 protein in T-cell development and tumorigenesis7,8 but were based solely on the complete loss of CDK6 in KO mice. Because CDK6 can act at multiple levels to impact cell proliferation and survival and because loss of individual cyclin/cdk subunits has been documented to allow reassortment of, and compensation by, the remaining components,2,8 we tested the specific role of CDK6 kinase and inhibitor titration functions. The data presented demonstrate that CDK kinase activity is predominantly responsible for proliferation control in developing thymocytes but acts together with CIP/KIP inhibitor titration functions to impact proliferation and survival of hematopoietic stem cells.
The CDK6 mutant protein K43M was found to be equivalent to wholesale loss of CDK6 in its impact on T-cell development. Thymocytes isolated from K43M and DM mice accumulate at the DN3 stage, consistent with a developmental delay in the thymi of KO, Cdk6K43M, and Cdk6DM mice. However, a considerable number of thymocytes were able to elude this inhibition in the DN3 stage and progressed with apparent increased efficiency to the CD4+ and CD8+ stage. Together, these results suggest that CDK6 kinase activity is required for the DN3 to DN4 transition but is then inhibitory for late developmental steps, and Cdk6K43M mice thus accumulate a higher than normal fraction of CD4+ and CD8+ cells.
The effects on the DN stages and the production of CD4+ and CD8+ cells seem insufficient to explain the approximately 75% reduction in the overall number of thymocytes in KO and Cdk6K43M mice. We show that this reduction is largely the result of a significantly reduced LSK cell fraction in the BM. Further, these LSK cells fail to proliferate, survive, and differentiate into DP cells when they are cultured under T-cell–promoting conditions in the presence of Notch ligand DL1. Thus, we conclude that, in addition to its role in thymocytes, CDK6 kinase activity plays a critical role in hematopoietic stem cells and in their commitment to the T-cell lineage on stimulation of Notch by DL1.
DM behaves in a manner quite similar to the K43M mutant in thymocytes and in LSK cells that have been stimulated by DL1. This implies that the ability of CDK6 to titrate these inhibitors, and thereby activate CDK2, is not sufficient to impact the effect of specific loss of CDK6 kinase activity. Thus, we predict that thymocyte production and T-cell development would be sensitive to CDK6 inhibitors.
A profound sensitivity of DM-expressing LSK cells to cell death after Notch stimulation was observed despite a more than 3-fold increase in the LSK fraction in Cdk6DM BM (Figure 4C). This increase corresponded to a significantly increased proliferative fraction in Cdk6DM LSK cells, and both effects were found to be quite similar to that observed on loss of p27KIP1. These data strongly imply that excessive titration of CIP/KIP proteins by CDK6 does play an important role in the stem/progenitor cell population. Indeed, the R31C mutant LSK cells also were found in somewhat higher fraction than in Cdk6WT−Δ mice and showed a significant increase in proliferative fraction as well. However, in contrast to LSK cells lacking CDK6 kinase activity, which show a reduced apoptotic fraction, those expressing the hyperactive R31C allele had a significantly increased fraction of apoptotic cells, suggesting that unrestrained CDK6 kinase activity is counterbalanced by cell death. Indeed, such increases in proliferative fraction, accompanied by increased cell death, were also observed in multiple thymocyte compartments in R31C mice, supporting the idea that R31C-driven abnormalities in the BM and T-cell compartments may be mitigated by apoptosis. Experiments designed to test this hypothesis are underway and may reveal an important role for CDK6 activity in hematopoietic malignancies.
Our data highlight the key role of CDK6 kinase activity in LSK proliferation and survival, and especially in progenitor cell responses to Notch receptor stimulation. We thus used the OP9-DL1 stroma-mimetic system to explore transcript changes in progenitor cells that lacked CDK6 or its kinase activity. These studies revealed that the developmental delay in vitro is accompanied by a failure to properly orchestrate expression of many Notch target genes (supplemental Table 3).
Despite our current lack of understanding of the mechanistic links between CDK6 activity and Notch-driven transcriptional changes, analysis of specific genes provides clues to the phenotypic responses observed in CDK6 mutant animals. For example, CD25 is consistently up-regulated both in vivo and in the Notch-dependent, in vitro coculture system, consistent with a previous study showing that CD25 expression is modulated by Notch signaling.24 In normal thymocytes, CD25 is expressed at relatively high levels in the DN2 and DN3 compartments but is rapidly down-regulated during the maturation of thymocytes from DN3 to DN4 stages.25 Persistently elevated CD25 levels in KO, Cdk6K43M, and Cdk6DM thymocytes indicate that CDK6 kinase activity is essential to repress CD25 in the DN compartment to ensure successful transition from the DN3 to the DN4 stage. Indeed, genetic deletion of CD25 rescued most of the phenotypes of kinase activity loss in early T-cell development and underscored the vital role CD25 plays during the early T-cell development as a downstream mediator of CDK6 loss. However, deletion of CD25 did not affect the increased populations of CD4+ and CD8+ in K43M mice, indicating that the inhibitory role of CDK6 kinase activity for late developmental steps is CD25 independent.
Consistent with this model of a key role for CD25 acting as a CDK6-sensitive mediator of thymocyte development, we also observed profound increases in GATA3 in Notch-stimulated thymocytes lacking CDK6 activity. A failure to repress GATA3 in cells lacking CDK6 activity may thus lead to the observed CD25-mediated inhibition of the DN3 to DN4 transition and may also facilitate differentiation of the CD4+ population in KO and Cdk6K43M mice because GATA3 also acts as a master transcriptional factor for differentiation of CD4+ cells. Indeed, we found that shRNA-mediated knockdown of GATA3 led to a strong decrease in expression of CD25 mRNA, suggesting that GATA3 acts upstream of CD25 on CDK6 activity loss. Alterations in GATA3 binding to the CD25 promoter region in CDK6 mutant cells are currently being assessed.
In conclusion, our data elucidate the role of CDK6 kinase activity in hematopoietic stem cell and thymocyte development at multiple stages. In the absence of CDK6 kinase activity, a reduction in proliferation and in stem and progenitor cell numbers, accompanied by a defect in response to Notch signaling, partially accounts for the reduction in total cellularity in the thymus. This is probably because cells lacking CDK6 kinase activity fail to properly regulate GATA3 and CD25 expression. Indeed, this is reminiscent of a similar role of cyclin D3 in thymocyte development.26 This aberrant gene regulation appears to be a component of a broader loss of proper regulation of a Notch-dependent transcriptome, but the mechanistic connection between CDK6 and this transcriptome remains to be determined.
In contrast, in the presence of hyperactive CDK6 kinase activity, an increase in proliferation and in the number of stem cells and progenitor cells with normal ability to respond to Notch signaling accounts for the observed increase in total cellularity in the thymus. However, these increases are countered, at least in part, by increased apoptosis in most T-cell subsets and in stem/progenitors, thus limiting cellular expansion and tumorigenesis in the absence of other genetic events, such as p53 loss. Therefore, our data strongly support further studies of CDK6 as a potential therapeutic target for both BM-derived cancers and immune diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Piotr Sicinski and Yan Geng for help in making K43M mice and Allen Parmelee (Flow Cytometry Core in Pathology, Tufts University School of Medicine) for his dedication and assistance.
This work was supported by the National Institutes of Health (grant CA127392, P.W.H.; and grant CA090576, R.A.V.E.), Tufts Medical Center Research Fund (M.G.H.), and a Tufts CTSI-Catalyst Award (M.G.H.).
National Institutes of Health
Authorship
Contribution: M.G.H. designed and performed experiments, analyzed data, interpreted results, and wrote the paper; A.D., N.S., and E.A.H. assisted in genotyping; C.M. and M.D. helped in FACS analysis; G.-f.H. and R.A.V.E. provided guidance for the group and assisted in manuscript preparation; F.G. provided guidance and reagents for the group; and P.W.H. designed experiments, interpreted results, provided guidance for the group, and assisted in manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of A.D. is Five Prime Therapeutics Inc, South San Francisco, CA.
Correspondence: Phil W. Hinds, Molecular Oncology Research Institute, Tufts Medical Center, 800 Washington St, #5609, Boston, MA 02111; e-mail: phinds@tuftsmedicalcenter.org; and Miaofen G. Hu, Molecular Oncology Research Institute, Tufts Medical Center, 800 Washington St, #5609, Boston, MA 02111; e-mail: mhu@tuftsmedicalcenter.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal