Abstract
In this study we investigated the role of CB1 receptor signaling in angiogenesis and the therapeutic exploitation of CB1 inactivation as an antiangiogenic strategy. We started from the observation that CB1 receptor expression is induced during angiogenesis and that the endocannabinoid anandamide stimulated bFGF-induced angiogenesis in the nanomolar physiologic range. To define the functional involvement of CB1 receptor signaling during angiogenesis, 2 different strategies have been carried out: siRNA-mediated knockdown and pharmacologic antagonism of CB1 receptors. CB1 receptors inactivation resulted in the inhibition of bFGF-induced endothelial proliferation, migration, and capillary-like tube formation, through prosurvival and migratory pathways involving ERK, Akt, FAK, JNK, Rho, and MMP-2. To corroborate the potential therapeutic exploitation of CB1 blockade as an antiangiogenic strategy, we performed in vivo assays founding that CB1 blockade was able to inhibit bFGF-induced neovascular growth in the rabbit cornea assay. A relevant finding was the ability to reduce ocular pathologic neo-vascularization in mouse oxygen-induced retinopathy. These results demonstrate that CB1 signaling participates to the proliferative response elicited by proangiogenic growth factors in angiogenesis and that for this reason CB1 receptor could represent a novel target for the treatment of diseases where excessive neoangiogenesis is the underlying pathology.
Introduction
The endocannabinoid system, composed of 2 cannabinoid receptors CB1 and CB2, their endogenous ligands (endocannabinoids, eg, anandamide), and the enzymes for their metabolism, has been pointed out to be implicated in several pathophysiologic conditions ranging from neurologic and psychiatric diseases to eating, cardiovascular, reproductive disorders, and cancer.1-3 The main effects of endocannabinoids rely on the activation of CB1, a 7-transmembrane-domain protein belonging to the Gαi protein-coupled receptor family (GPCR).4 CB1 receptors are highly expressed in the central nervous system, especially in the cortex, cerebellum, hippocampus, and basal ganglia, and their involvement in processes of memory and learning, in diseases affecting movement, mood, and anxiety and in conditions related to altered brain reward mechanisms, has been ascertained.5 Noteworthy, CB1 receptors are expressed also by a variety of peripheral tissues and cells and in the last years a role in several physiologic functions and pathologies is emerging. CB1 deficiency was reported to be associated to age-related osteoporosis6 and preterm birth in mice,7 highlighting a role for CB1 signaling in bone metabolism and in early pregnancy events. On the other hand, CB1 receptor antagonism and/or inactivation through genetic engineering as in CB1-deficient mouse strains,8 were shown to be useful in several pathologies in which the endocannabinoid system is unbalanced or CB1 more than expressed (eg, obesity, metabolic syndrome, diabetes, Alzheimer disease).9-11 Indeed, CB1 inactivation exerted an antifibrotic action in response to acute liver injuries, a systemic anti-inflammatory activity, antiatherosclerotic effects because of both improvement of serum cholesterol profile and decreased plaque inflammation,10,12-14 reversion of liver steatosis in obese mice,15 and improvement of albuminuria in diabetic nephropathy.16
Recent studies have proposed the endocannabinoid system as a new promising anticancer target, because endocannabinoids have a key role in the control of cell-signaling pathways involved in cancer cell growth, invasion, and metastasis processes.1,17,18 The antitumor mechanism of drugs targeting the endocannabinoid system depends on the tumor type and, according to the case, is CB1, CB2 or TRPV1 receptor-dependent or -independent. Blocking CB1 signaling has been reported to inhibit tumor growth in thyroid, mantle-cell lymphoma, and breast and colon tumors both in vitro and in vivo.19-22
Angiogenesis, the de novo development of blood vessels, occurs regularly in adult tissues in physiologic and pathologic conditions, including wound healing, inflammation, rheumatoid arthritis, endometriosis, diabetic retinopathy, macular degeneration, and tumor progression.23-25 Several complex steps are involved, the first one represented by localized enzymatic degradation of the basement membrane of the existing vessels, followed by the detachment of endothelial cells from adhesive proteins in the extracellular matrix and migration into the perivascular space, where cells proliferate. Then, the new endothelial cells morphologically differentiate into tube-like structures that eventually join to form new capillaries.26 The entire process is controlled by a finely tuned balance between positive and negative effectors.25 Both stimulating and inhibiting angiogenesis are fundamental strategies to human health. Recently, antiangiogenesis therapies have been introduced successfully in the clinic, heralding a new era for the treatment of several commonly occurring angiogenesis-related diseases.27,28
Interestingly, it has been reported that drugs acting at both CB receptors could inhibit tumor neoangiogenesis in vivo, thereby counteracting cancer growth, mainly through the inhibition of VEGF signaling and through the induction of endothelial cell apoptosis.19,29-32 We have to bear in mind that the pharmacologic effect of cannabinoid drugs, usually used at μM doses, does not necessarily reflect the action of endogenously produced endocannabinoids, that are at most in the nanomolar range.11 Indeed, endocannabinoids have been already reported to promote cell proliferation of neural progenitor cells through CB1 receptor.33,34
The role of the basic signaling of endocannabinoids lipid mediators in the angiogenic process has not yet been investigated. The present study arises from the observation that CB1 receptor expression is induced during angiogenesis in endothelial cells and that anandamide stimulated bFGF-induced proliferation in the nanomolar physiologic range. In light of these results, we were prompted to provide evidence for a specific role of CB1 receptor signaling in angiogenesis, investigating whether its genetic or pharmacologic inactivation was able to modulate the angiogenic process in vitro and in vivo. CB1 inactivation resulted in the inhibition of bFGF-induced proliferation, migration, and capillary-like tube formation of endothelial cells. Moreover, it markedly inhibited bFGF-induced neovascular growth in vivo in the rabbit cornea assay, being also effective in reducing pathologic neovascularization in the mouse model of oxygen-induced retinopathy (OIR). All together, our data strongly corroborate the new paradigm of endocannabinoids as positive modulators of cell proliferation, because CB1 receptor signaling participates to the proliferative response in angiogenesis and its blockade could therefore represent a new interesting antiangiogenic strategy.
Methods
Materials
The selective CB1 antagonist SR141716 was provided by Sanofi-Aventis. Anandamide was from Alexis Biochemicals. ERK (137F5), Akt (C67E7), pERK (D13.14.4E), pAKT (D9E), JNK (56G8), and pJNK (81E11) antibodies were from Cell Signaling Technology Inc; anti-FAK (C-20) and anti-FGFR1 (C-15) were from Santa Cruz Biotechnology Inc; anti-CB2 and anti-actin were from Abcam; anti-CB1 was from Pierce Antibodies (Thermo Scientific) and anti-tubulin (B512) was from Sigma-Aldrich.
Cell cultures
Human umbilical vein endothelial cells (HUVECs) isolated as described,31 were cultured in M199 with heparin, 10% inactivated FBS, 2mM l-glutamine and complete growth factors at 37°C in a humidified 5% CO2 atmosphere. HUVECs at 2 to 6 passages were used. Reagents were obtained from Gibco.
RT-PCR
Total RNA was extracted by guanidinium thiocyanate-isopropanol method. RT was performed using Moloney murine leukemia virus RT and random oligonucleotide primer. The sense primer CB1-F (5′-GATGTCTTTGGGAAGATGAACAAGC-3′) and the antisense primer CB1-R (5′-GACGTGTCTGTGGACACAGACATGG-3′) were used to amplify the CB1R; the primers for amplification of β2-microglobulin were β2M1 (5′-CCTGGATTGCTATGTGTCTGGGTTTCATCC-3′) and β2M2 (5′-GGAGCAACCTGCTCAGATACATCAAACATG-3′). PCRs were performed 30 seconds at 93°C, 1 minute at 59°C, and 1 minute at 69°C for 25 to 28 cycles. Amplified DNA was extracted with chloroform and electrophoresed in a 2% agarose gel.
siRNA transfection
Endothelial cells were transfected with control siRNA (100nM) or CB1 siRNA (100nM) with the Amaxa Nucleofection System, following the HUVEC Nucleofector kit instructions (Lonza). A 21-nucleotide small interfering RNA duplex (Dharmacon Research) was used for specific silencing of CB1 (siRNA-CB1), covering the sequence sense 5′-CCCAAGUGACGAAAACAUU-dTdT-3′. Specific silencing of CB1 gene was confirmed by RT-PCR and Western blot analysis.
Proliferation assay
Endothelial cell proliferation was evaluated by measuring BrdU incorporation into DNA (BrdU colorimetric assay kit; Roche). Cells (3 × 104)/mL transfected with siRNA or pretreated with CB1 antagonist (30 minutes) were seeded into 96-well plates with or without bFGF (10 ng/mL) or VEGF (10 ng/mL) for 24 hours at 37°C. Newly synthesized BrdU-DNA was determined using an ELISA reader at 450 nm (Biorad Laboratories).
Endothelial cell migration assay
Chemotactic motility of endothelial cells was assayed using Transwell (Corning Costar) with 6.5-mm diameter polycarbonate filters (8-μm pore size) coated with 55 μg of Matrigel. M199 medium (1% FBS) containing bFGF was placed in the lower wells as chemotactic stimulus. siRNA cells harvested after 24 hours, or cells pretreated for 30 minutes with CB1 antagonist SR141716 (0.3μM), were suspended in M199 1% FBS in the upper wells of the chamber and incubated at 37°C for 4 hours. Migrated cells were counted by a light microscope at 20× magnification: 10 randomly chosen microscopic fields were counted per well and the mean number was determined. Background levels of cells migrated in the absence of chemotactic bFGF (chemokinesis) were subtracted from all the experimental points.
Capillary-like tube formation assay
A 48-well plate was coated with 230 μL/well Matrigel for 30 minutes at 37°C. siRNA cells (1 × 104 cells/mL) or cells preincubated with CB1 antagonist (0.3μM, 30 minutes) were seeded in 250 μL of M199 medium, and then stimulated or not with bFGF (10 ng/mL). After 6 hours, capillary-like tube formation was examined under an inverted phase microscope, photographed and analyzed by Scion Image software 4.03.
Immunoprecipitation and Western blot analysis
Serum-starved cells were preincubated with CB1 antagonist for 30 minutes before stimulation with bFGF for various time points. siRNA cells were harvested at 24 hours. Protein extraction and Western blot were performed as described.31
Rho activation assay
Cells were pretreated with SR141716 (0.3μM, 30 minutes) and then stimulated with bFGF (10 ng/mL) for 24 hours. Rho activity was measured using the Rho activation kit (Upstate Biotechnology, Millipore). GTP-Rho in cell lysates was adsorbed to GST-Rhotekin Rho binding domain, which binds selectively to GTP-Rho. After precipitation, samples were processed for immunoblot analysis with anti-Rho antibody.
Gelatin zymography
Quiescent HUVECs were pretreated with SR141716 (0.3μM, 30 minutes) and then stimulated with bFGF (10 ng/mL, 24 hours). Conditioned media containing equal amounts of secreted proteins were mixed with non reducing Laemmli sample buffer (2×) and loaded on 7.5% SDS-polyacrylamide gels containing 1 mg/mL gelatin (Sigma-Aldrich), as described.31 Gelatinolytic activity of MMP-2 was detected as unstained bands on a blue background. HUVEC stimulated with PMA (phorbol myristate acetate; 0.1μM, 24 hours) without bFGF were used as positive control for MMP-2 activity.
Rabbit cornea assay
Experiments have been performed in accordance with the guidelines of the European Economic Community for animal care and welfare (EEC Law No. 86/609).
Substances were incorporated into sterile slow-release pellets of an ethynil-vinyl copolymer (Elvax-40). In the lower half of New Zealand white rabbit eye (Charles River Laboratories), anaesthetized by sodium pentothal (30 mg/kg), 2 adjacent micropockets were surgically produced using a pliable iris spatula. bFGF (200 ng/pellet) was implanted in one pocket, the compound or the control (Elvax-40 alone) in the other. Daily observations of the implants were made with a slit lamp stereomicroscope by 2 independent operators in a blinded manner. Angiogenic activity was expressed as the number of implants exhibiting neovascularization over the total implants. Data are expressed as angiogenic score (number of vessels × distance from the limbus).
Mouse model of oxygen induced retinopathy
C57Bl/6J mice were bred at the University of New Mexico Animal Research Facility in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Litters of 7-day-old C57Bl/6J mice were placed with their nursing mothers into an oxygen chamber at 75% oxygen until postnatal day P12. Mice were removed from the chamber at day 12 and maintained in room air until day 17, receiving a daily intraperitoneal injection of SR141716 at 0.7 mg/kg from day P12 to P16. By day 17, retinal neovascularization was present in 100% of the experimental untreated animals and they were killed. Newborn mice exposed only to room air served as controls.
Quantization of neovascular response in retinal whole mounts
New vessel tufts on the surface of the retina were stained using Isolectin GS-IB4 conjugated to Alexa fluor 488 (Invitrogen). Eyes (P17) were removed and fixed. The cornea was cut approximately 2 mm anterior to the limbus and removed. The lens was removed from the eye and any remaining hyaloid vessels were teased from the retina. The resulting eye cups were incubated for 30 minutes at room temperature with 5 μg/mL of lectin in PBS. The retina was removed, cut into eighths and mounted on glass slides. Images were obtained of the retinal segments and the percentage of the total retinal area occupied by new vessels was quantified using the MetaMorph 7.5 image analysis program.
Statistical analysis
All data were presented as means ± SD. Statistical analysis was performed using 1-way ANOVA followed by the Bonferroni posthoc analysis for multiple comparisons. The Student t test was used for dose-response curves. A P value < .05 was considered statistically significant.
Results
CB1 expression is regulated during the angiogenic process
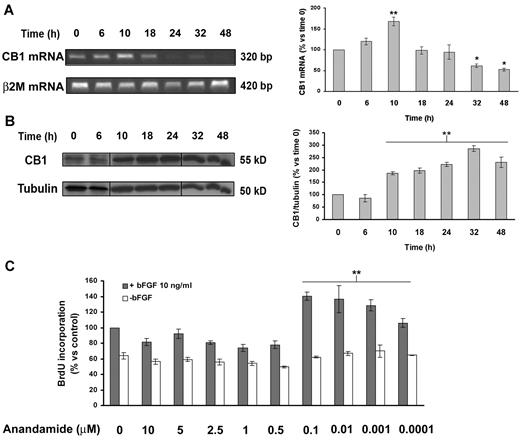
We first investigated whether CB1 receptor expression is modulated during angiogenesis in endothelial cells. Quiescent endothelial cells (time 0) were stimulated to proliferate with proangiogenic medium containing bFGF (10 ng/mL). CB1 mRNA and protein levels were analyzed at increasing time points. CB1 receptor mRNA levels reach a peak in proliferating endothelial cells at 10 hours (Figure 1A). CB1 receptor protein expression was faint or even absent in quiescent endothelial cells, contrasting with a gradual and progressive time-dependent induction in proliferating cells with a peak at 32 hours (Figure 1B). Then we assessed the functionality of CB1 in endothelial cells stimulated or not with bFGF (10 ng/mL), after a 24-hour treatment with anandamide. Anandamide stimulated bFGF-induced endothelial cells' proliferation in the nanomolar range, without affecting quiescent endothelial cells (Figure 1C). Therefore, CB1 receptor seems to be associated with angiogenesis.
CB1 expression is regulated during the angiogenic process. Expression levels of CB1 were determined by RT-PCR (A) and Western blotting (B) at increasing time points from angiogenic stimulation of quiescent cells. Vertical lines have been inserted to indicate a repositioned gel lane. The histograms report the quantification of the intensity bands expressed as mean ± SD of 3 independent experiments (ANOVA vs control, *P < .05, **P < .01). (C) BrdU incorporation levels expressed as mean ± SD (relative percentage vs control) of 3 independent experiments in triplicates.
CB1 expression is regulated during the angiogenic process. Expression levels of CB1 were determined by RT-PCR (A) and Western blotting (B) at increasing time points from angiogenic stimulation of quiescent cells. Vertical lines have been inserted to indicate a repositioned gel lane. The histograms report the quantification of the intensity bands expressed as mean ± SD of 3 independent experiments (ANOVA vs control, *P < .05, **P < .01). (C) BrdU incorporation levels expressed as mean ± SD (relative percentage vs control) of 3 independent experiments in triplicates.
CB1 inactivation inhibits bFGF-induced endothelial cell proliferation in vitro
We were prompted to analyze CB1 receptor functional involvement in angiogenesis by examining in vitro the consequences of its genetic or pharmacologic inactivation, respectively through siRNA silencing or by blocking CB1 receptor signaling with its selective antagonist SR141716.
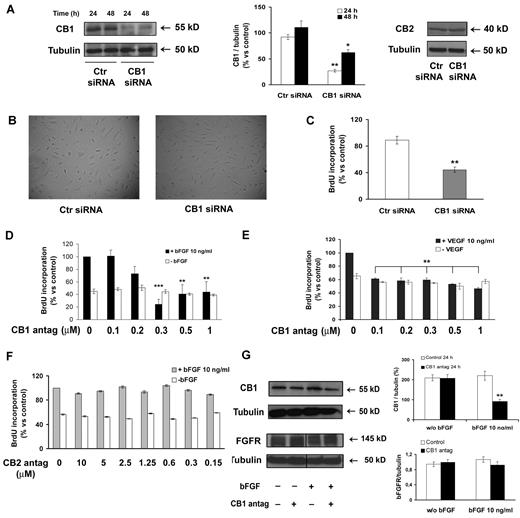
In siRNA transient transfection experiments, CB1 receptor protein expression was silenced up to 70% at 24 hours and the inhibition was maintained at a good level until 48 hours (Figure 2A). CB2 expression was not affected, thus ruling out off-target effect of the siRNA (Figure 2A). The morphology of endothelial cells following CB1 silencing (24 hours) was absolutely identical to that of control cells (Figure 2B).
CB1 inactivation inhibits endothelial cell proliferation. (A) HUVECs were transfected with either control siRNA or CB1-siRNA for 24 and 48 hours and the lysates were analyzed by immunoblot for CB1 and CB2 expression. (B) HUVECs transfected with either control-siRNA or CB1-siRNA for 24 hours were photographed. Magnification, ×10. (C) BrdU incorporation levels of bFGF-stimulated HUVECs expressed as mean ± SD (relative percentage vs control) of 3 independent experiments in triplicates. (D-F) HUVECs were pretreated or not with CB1 antagonist (30 minutes; D) or CB2 antagonist (30 minutes; F), then stimulated (black bars) or not (white bars) with bFGF (10 ng/mL, 24 hours; D, F) or VEGF (10 ng/mL, 24 hours; E). (G) Lysates were analyzed for CB1 and FGFR expression. Vertical lines have been inserted to indicate a repositioned gel lane. (A,G) Histograms report the quantification of the intensity of the bands calibrated to the intensity of tubulin bands (mean ± SD of 3 independent experiments; ANOVA vs control, *P < .05, **P < .01, ***P < .001).
CB1 inactivation inhibits endothelial cell proliferation. (A) HUVECs were transfected with either control siRNA or CB1-siRNA for 24 and 48 hours and the lysates were analyzed by immunoblot for CB1 and CB2 expression. (B) HUVECs transfected with either control-siRNA or CB1-siRNA for 24 hours were photographed. Magnification, ×10. (C) BrdU incorporation levels of bFGF-stimulated HUVECs expressed as mean ± SD (relative percentage vs control) of 3 independent experiments in triplicates. (D-F) HUVECs were pretreated or not with CB1 antagonist (30 minutes; D) or CB2 antagonist (30 minutes; F), then stimulated (black bars) or not (white bars) with bFGF (10 ng/mL, 24 hours; D, F) or VEGF (10 ng/mL, 24 hours; E). (G) Lysates were analyzed for CB1 and FGFR expression. Vertical lines have been inserted to indicate a repositioned gel lane. (A,G) Histograms report the quantification of the intensity of the bands calibrated to the intensity of tubulin bands (mean ± SD of 3 independent experiments; ANOVA vs control, *P < .05, **P < .01, ***P < .001).
Interestingly, CB1 silenced endothelial cells showed a strong inhibition of proliferation when stimulated by bFGF, compared with control siRNA-treated cells (Figure 2C).
The result was validated through a pharmacologic approach, blocking CB1 receptor signaling in endothelial cells with its antagonist SR141716. Cells were pretreated for 30 minutes with increasing concentrations of SR141716 to block CB1 receptors before exposure to bFGF (10 ng/mL, 24 hours). CB1 blockade inhibited endothelial proliferation (Figure 2D black bars). This inhibitory effect was not because of cytotoxicity because the proliferation of unstimulated endothelial cells did not change (Figure 2D white bars). The effect was dose-dependent up to 0.5μM. The EC50 dose was 0.24μM, therefore, the 0.3μM effective dose was chosen and used in the subsequent experiments. A comparable antiangiogenic effect was observed also stimulating endothelial cells with VEGF (10 ng/mL, 24 hours), even if it was less marked (Figure 2E). Similar results in the inhibition of proliferation were obtained also with AM251, a different CB1 antagonist, indicating that the effect is because of CB1 antagonism independently from the antagonist used (data not shown), whereas the effect was specifically because of CB1 blockade, because the selective CB2 antagonist SR144528 did not affect proliferation (Figure 2F). Neither CB1 knockdown nor its antagonism induced apoptosis or cell death in endothelial cells (data not shown). CB1 expression in endothelial cells was strongly reduced at 24 hours by antagonist treatment and this effect was observed uniquely in bFGF-stimulated cells and not in quiescent cells, suggesting a double action on CB1 receptor function and expression (Figure 2G). CB1 blockade did not induce any significant variation in the expression of FGF receptors (Figure 2G).
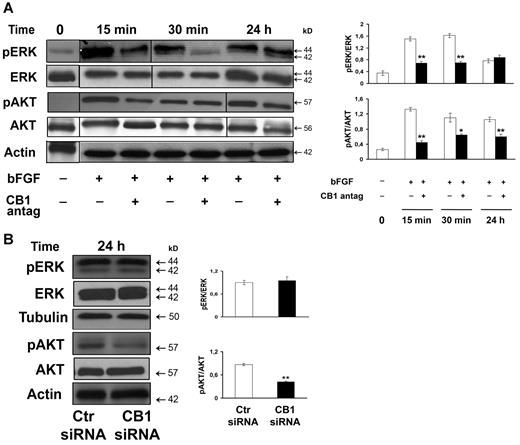
Thereafter, we evaluated the modification of cell signaling downstream CB1 receptor on bFGF-stimulated endothelial cells. We first probed the expression and phosphorylation of ERK, that is usually strongly activated both by growth factors, for example, bFGF, and by endocannabinoids and is an important early step for cell proliferation and motility during angiogenesis. Serum-starved endothelial cells were pretreated 30 minutes with SR141716 to block CB1 receptors, followed by the addiction of bFGF (10 ng/mL) for increasing time points. ERK phosphorylation (Thr202/Tyr204) was reduced by CB1 antagonism with highest inhibition at 30 minutes. At 24 hours ERK phosphorylation was restored to control levels; therefore, long-term ERK activity was not affected (Figure 3A). We next studied the effect of CB1 inactivation on the Akt pathway, because phosphorylated Akt is highly implicated in endothelial cell survival, proliferation, and angiogenesis. Akt phosphorylation (Ser473) was inhibited already at 15 minutes' treatment and maintained up to 24 hours (Figure 3A). These reductions are clearly related to a decrease in phosphorylation because the total amount of both ERK and Akt was not modified (Figure 3A). Similarly, in siRNA CB1 silenced cells, even if long-term ERK phosphorylation was not affected (24 hours), Akt phosphorylation and hence activation was considerably inhibited (Figure 3B).
CB1 inactivation interferes with ERK and Akt signaling pathways involved in angiogenesis. (A) Western blot of endothelial cells pretreated with CB1 antagonist SR141716 (30 minutes, 0.3μM) and stimulated with bFGF (10 ng/mL) for increasing time points (0, 15 minutes, 30 minutes, 24 hours). Vertical lines have been inserted to indicate a repositioned gel lane. (B) Western blot of endothelial cells transfected with either control siRNA or CB1-siRNA for 24 hours. (A-B) Quantification of the intensity of the bands calibrated to the intensity of total protein bands and loading control (means ± SD; ANOVA vs control, **P < .01).
CB1 inactivation interferes with ERK and Akt signaling pathways involved in angiogenesis. (A) Western blot of endothelial cells pretreated with CB1 antagonist SR141716 (30 minutes, 0.3μM) and stimulated with bFGF (10 ng/mL) for increasing time points (0, 15 minutes, 30 minutes, 24 hours). Vertical lines have been inserted to indicate a repositioned gel lane. (B) Western blot of endothelial cells transfected with either control siRNA or CB1-siRNA for 24 hours. (A-B) Quantification of the intensity of the bands calibrated to the intensity of total protein bands and loading control (means ± SD; ANOVA vs control, **P < .01).
CB1 inactivation inhibits bFGF-induced migration and capillary-like tube formation of endothelial cells
Sprouting angiogenesis includes successive phases of neovessel formation, growth, and stabilization, characterized by the migration of endothelial cells toward an angiogenic factor, proliferation, and organization into 3-dimensional capillary-like structures interconnected to form a polygonal network.35
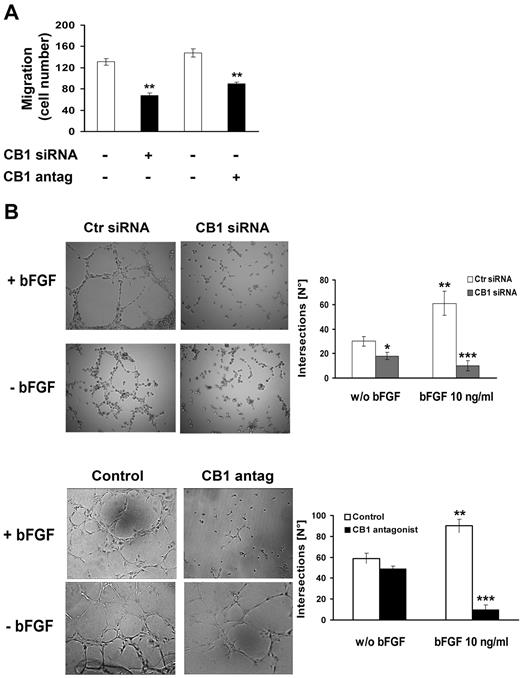
To examine whether knockdown of CB1 might have a functional impact on endothelial cell behavior, we carried out both migration and tube formation in vitro assays. CB1 silenced cells or cells preincubated with CB1 antagonist (0.3μM, 30 minutes) to block CB1 receptors, were plated in the upper compartment of Boyden chambers coated with Matrigel. In the lower compartment bFGF was used as chemoattractant (10 ng/mL). Basal migration (chemokinesis) of endothelial cells in the absence of bFGF stimulation was not affected, whereas both CB1 knockdown or its pharmacologic block inhibited bFGF-induced migration (Figure 4A).
CB1 inactivation inhibits migration and capillary-like tube formation of endothelial cells. (A) HUVECs transfected with either control siRNA or CB1-siRNA for 24 hours or pretreated or not with CB1 antagonist (0.3μM, 30 minutes) were assayed in the Transwell migration assay using bFGF as chemoattractant (10 ng/mL, 4 hours). The graph reports the mean ± SD of 3 independent experiments in triplicates expressed as percentage of migrated cells. Basal migration in the absence of bFGF (chemokinesis) was subtracted from each point (ANOVA, **P < .01 vs control). (B) HUVECs transfected with either control siRNA or CB1-siRNA for 24 hours or pretreated or not with CB1 antagonist (0.3μM, 30 minutes) were stimulated or not with bFGF (10 ng/mL, 6 hours). Magnification, ×10. Histograms represent the number of intersections for control (white bars) and CB1 antagonist-treatment (black bars; mean ± SD from 4 independent experiments; ANOVA, **P < .01, ***P < .001).
CB1 inactivation inhibits migration and capillary-like tube formation of endothelial cells. (A) HUVECs transfected with either control siRNA or CB1-siRNA for 24 hours or pretreated or not with CB1 antagonist (0.3μM, 30 minutes) were assayed in the Transwell migration assay using bFGF as chemoattractant (10 ng/mL, 4 hours). The graph reports the mean ± SD of 3 independent experiments in triplicates expressed as percentage of migrated cells. Basal migration in the absence of bFGF (chemokinesis) was subtracted from each point (ANOVA, **P < .01 vs control). (B) HUVECs transfected with either control siRNA or CB1-siRNA for 24 hours or pretreated or not with CB1 antagonist (0.3μM, 30 minutes) were stimulated or not with bFGF (10 ng/mL, 6 hours). Magnification, ×10. Histograms represent the number of intersections for control (white bars) and CB1 antagonist-treatment (black bars; mean ± SD from 4 independent experiments; ANOVA, **P < .01, ***P < .001).
Next, the effect on morphologic differentiation of endothelial cells into capillary-like tube structures was investigated using 2-dimensional Matrigel coat. Angiogenic response was measured by the extent and shape of the capillary like network originated in response to bFGF (10 ng/mL) after 6 hours in CB1 silenced cells or pretreated with CB1 antagonist (0.3μM, 30 minutes). CB1 silencing, as well as CB1 antagonism, seemed to be effective in inhibiting bFGF-induced capillary network formation by significantly reducing the number of tube-like structures' intersections (Figure 4B). Interestingly, the effect was strictly dependent on bFGF stimulation.
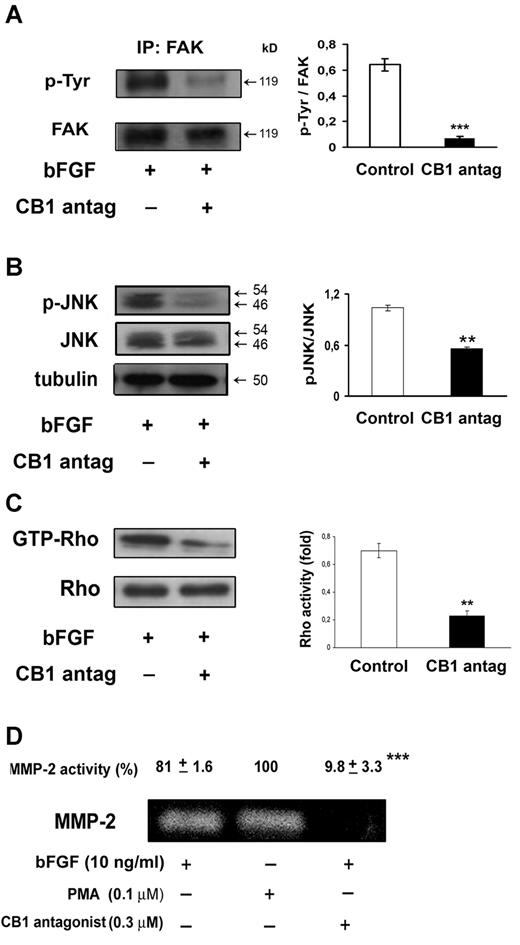
Involvement of FAK, JNK, Rho, and MMP-2 in the inhibition of angiogenesis by CB1 antagonism
Given the efficacy of CB1 antagonism to inhibit angiogenesis, we were prompted to investigate in more detail the molecular mechanism implicated. For the formation of new blood vessels, endothelial cell mobility and adhesion are required. In this regard, Focal Adhesion Kinase (FAK) plays a central role.36 FAK also recruits JUN N-terminal kinase (JNK) to focal adhesion sites promoting cell motility. Indeed, we found that CB1 receptor antagonism strongly inhibited tyrosine phosphorylation and hence activation of immunoprecipitated FAK (Figure 5A) and blocked the expression of phosphorylated JNK (Figure 5B). These observations, together with the reported inhibition of endothelial cell migration and morphogenesis, suggest that CB1 blockade may preferentially target cell motility.
CB1 antagonism inhibits FAK, JNK, Rho, and MMP-2 activation. Endothelial cells were pretreated with CB1 antagonist (30 minutes, 0.3μM) and stimulated with bFGF (10 ng/mL) for 24 hours. (A) Anti-FAK immunoprecipitates (IPs) were analyzed with anti-phosphotyrosine antibody. (B) Western blot analysis of extracts using phospho-JNK (Thr183/Tyr185) antibody. (C) Rho activity (level of GTP-bound Rho) was detected by Western blotting. (A-C) Quantification of the intensity of bands calibrated to the intensity of total protein bands (means ± SD; ANOVA vs control, **P < .01, ***P < .001). (D) MMP-2 activity was detected by gelatin zymography. HUVECs stimulated with PMA (0.1mM, 24 hours) were used as positive control for MMP-2 activity. The relative pixel density for the 72 kDa MMP-2 is shown. Data are presented as mean ± SE of 3 independent experiments (ANOVA, ***P < .001 vs control).
CB1 antagonism inhibits FAK, JNK, Rho, and MMP-2 activation. Endothelial cells were pretreated with CB1 antagonist (30 minutes, 0.3μM) and stimulated with bFGF (10 ng/mL) for 24 hours. (A) Anti-FAK immunoprecipitates (IPs) were analyzed with anti-phosphotyrosine antibody. (B) Western blot analysis of extracts using phospho-JNK (Thr183/Tyr185) antibody. (C) Rho activity (level of GTP-bound Rho) was detected by Western blotting. (A-C) Quantification of the intensity of bands calibrated to the intensity of total protein bands (means ± SD; ANOVA vs control, **P < .01, ***P < .001). (D) MMP-2 activity was detected by gelatin zymography. HUVECs stimulated with PMA (0.1mM, 24 hours) were used as positive control for MMP-2 activity. The relative pixel density for the 72 kDa MMP-2 is shown. Data are presented as mean ± SE of 3 independent experiments (ANOVA, ***P < .001 vs control).
The Rho family of small GTPases is involved in the regulation of several components of cell migration. Actually, Rho has been demonstrated to regulate endothelial organization critically during angiogenesis.37 To evaluate the effect of CB1 antagonism on bFGF-induced RhoA activity, we used a pull-down assay with the fusion protein GST-RBD, which recognizes only RhoA-GTP, its active form. We found a strong inhibition of RhoA activation. Total Rho levels were not affected (Figure 5C).
Matrix metalloproteinases (MMPs) are crucial for angiogenesis, being necessary for endothelial cell migration and tube formation. To assess whether CB1 antagonism also interfered with secreted MMP-2 (72 kDa) gelatinolytic activity, endothelial cells were serum-starved, pretreated with CB1 antagonist (0.3μM, 30 minutes) and then stimulated with bFGF (10 ng/mL) for 24 hours. As shown in Figure 5D, CB1 blockade led to a significant decrease of secreted MMP-2 gelatinolytic activity, as detected by gelatin zymography analysis.
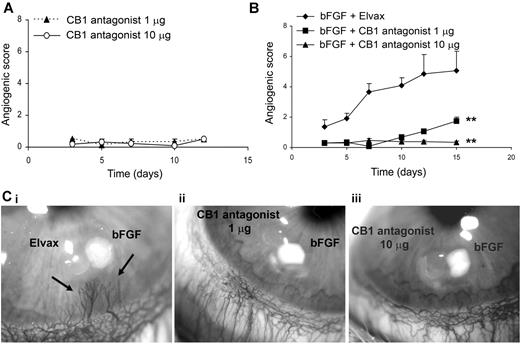
Corneal implantation of CB1 antagonist inhibits bFGF-stimulated angiogenesis in the rabbit cornea
The results shown in the preceding paragraphs suggest that CB1 receptor could be an excellent target for the development of a new antiangiogenic strategy, because only actively proliferating cells are affected and several crucial steps of the angiogenic process are inhibited. To investigate the pharmacologic potential of CB1 antagonism to inhibit angiogenesis also in vivo, we used the rabbit cornea assay. CB1 antagonist bearing pellets (1 and 10 μg/pellet), implanted in the cornea stroma, were devoid of any inflammatory and angiogenic activity (Figure 6A), and did not destabilize the underlying vasculature of the corneal limbus.
Corneal implantation of CB1 antagonist inhibits growth factor stimulated angiogenesis in the rabbit cornea. (A) Absence of angiogenic and inflammatory activity of CB1 antagonist (1 μg and 10 μg/pellet) implanted in the corneal stroma reported as angiogenic score (number of vessels × distance from the limbus; means ± SEM) during time (days; n = 6 implants). (B) Antiangiogenic activity on bFGF-induced neovascularization (200 ng/pellet), reported as angiogenic score (mean ± SEM; n = 6) during time (days). ANOVA, **P < .01 for CB1 antag.+bFGF vs Elvax+bFGF. (Ci-iii) bFGF+Elvax (i), CB1 antag 1 μg+bFGF (ii), CB1 antag 10 μg+bFGF (iii). Stereomicroscopic examination (18× original magnification) at day 12 after implantation. Arrows indicate the newly formed vessels.
Corneal implantation of CB1 antagonist inhibits growth factor stimulated angiogenesis in the rabbit cornea. (A) Absence of angiogenic and inflammatory activity of CB1 antagonist (1 μg and 10 μg/pellet) implanted in the corneal stroma reported as angiogenic score (number of vessels × distance from the limbus; means ± SEM) during time (days; n = 6 implants). (B) Antiangiogenic activity on bFGF-induced neovascularization (200 ng/pellet), reported as angiogenic score (mean ± SEM; n = 6) during time (days). ANOVA, **P < .01 for CB1 antag.+bFGF vs Elvax+bFGF. (Ci-iii) bFGF+Elvax (i), CB1 antag 1 μg+bFGF (ii), CB1 antag 10 μg+bFGF (iii). Stereomicroscopic examination (18× original magnification) at day 12 after implantation. Arrows indicate the newly formed vessels.
bFGF (200 ng/pellet) produced a potent neovascular growth, which progressed in the cornea stroma from the surrounding limbic vessels during time (Figure 6Ci arrows). CB1 blockade by SR141716, implanted adjacently to bFGF releasing pellets, inhibited bFGF induced angiogenesis (Figure 6ii and iii). The effect was not dose-dependent during the early stages (first 10 days of observation), but over the longer term, while 1 μg of CB1 antagonist was less potent and some capillaries grew, the higher concentration completely prevented bFGF-induced neovascular growth (Figure 6B).
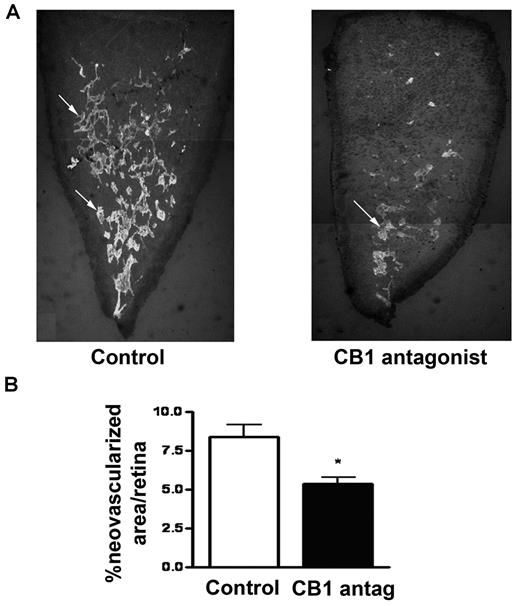
CB1 inactivation inhibits retinal pathologic neovascularization
To study the properties of CB1 peripheral blockade in a model of pathologic angiogenesis and confirm its potential use in angiogenesis-related diseases such as the ocular ones, we used the mouse model of oxygen-induced retinopathy (OIR), with consistent, quantifiable vascular changes (Figure 7A). In the OIR model, hyperoxia (75% oxygen from postnatal day P7 to P12) results in vascular obliteration. When mice are returned to normoxia (P12), retinas become hypoxic, resulting in pathologic neovascularization (P17). The mice received an intraperitoneal daily injection of either CB1 antagonist (0.7 mg/kg) or PBS from day P12 until P16. Mice were killed at day P17 and retinas were analyzed. Mice treated with the CB1 antagonist showed an evident and substantial decrease of the extent of retinal pathologic vascularization compared with vehicle-treated mice (Figure 7B).
CB1 antagonism inhibits ocular pathologic angiogenesis. (A) Mice were treated with a single intraperitoneal injection of CB1 antagonist (0.7 mg/kg) from day P12 to P16 and were killed on day P17. New vessel tufts on the surface of retinas were stained using Isolectin GS-IB4. Overlaping photographs of the retinal segments were taken at 4× magnification and the individual images were combined as montages to reconstruct the entire retinal segment. (B) Values represent the mean ± SEM for n = 6 animals for each group (ANOVA, *P < .05, **P < .01 vs control).
CB1 antagonism inhibits ocular pathologic angiogenesis. (A) Mice were treated with a single intraperitoneal injection of CB1 antagonist (0.7 mg/kg) from day P12 to P16 and were killed on day P17. New vessel tufts on the surface of retinas were stained using Isolectin GS-IB4. Overlaping photographs of the retinal segments were taken at 4× magnification and the individual images were combined as montages to reconstruct the entire retinal segment. (B) Values represent the mean ± SEM for n = 6 animals for each group (ANOVA, *P < .05, **P < .01 vs control).
Discussion
Starting from the discovery of the endocannabinoid system, a number of studies have pointed out that an altered endocannabinoid signaling and CB1 receptor expression could be involved in several pathophysiologic situations.3 In particular, CB1 receptor antagonism has been reported to display in vitro and in animal models beneficial properties, among these antifibrotic, anti-inflammatory, antiatherosclerotic, that extend beyond its central action on body weight reduction and improvement of cardiometabolic risk factors, therefore pointing out new biologic implications for the endocannabinoid signaling.9 As regard to angiogenesis, numerous studies have investigated the occurrence that drugs targeting the endocannabinoid system might be used to retard tumor growth, through the interference on key cell signaling pathways and also through the inhibition of neoangiogenesis.1,17,18
In the light of these observations, in the present study we investigated the functional involvement of CB1 receptor in angiogenesis, starting from the finding that it is faintly expressed in quiescent endothelial cells, whereas its expression is induced during the angiogenic process at both mRNA and protein levels. Anandamide significantly stimulated bFGF-induced proliferation in the nanomolar range, comparable with the concentrations endogenously reached following physiopathologic stimuli, suggesting basal growth-promoting effects of endocannabinoids, whereas μM doses did not affect proliferation, confirming what already observed in bovine aortic endothelial cells.38 We reported direct evidence that following siRNA-mediated knockdown or selective pharmacologic antagonism of CB1 receptors, angiogenesis was inhibited both in vitro and in vivo.
We found that, on the cellular level, CB1 receptors inactivation resulted in the inhibition of bFGF-induced endothelial cell proliferation, migration, capillary-like tube formation, all steps of the angiogenic process, through the prosurvival and migratory ERK and Akt pathways. Also VEGF-stimulated cell proliferation was inhibited by CB1 blockade. Interestingly, the proliferation of endothelial cells in the absence of stimulation by proangiogenic factors was not modified, ruling out nonspecific cytotoxic effects of both CB1 siRNA-mediated knockdown and pharmacologic antagonism.
To selectively block CB1 receptors we used the widely reported SR141716 antagonist.9 The EC50 dose was 0.3μM, very similar concentration able to exert antiproliferative effects also in human breast cancer cells.27 It is worth noting that this dose is in the range of low to high nanomolar, which is required to antagonize CB1 receptors and rules out the possibility of nonspecific effects on CB2 (ki > 1000nM) or other receptors.39 To better exclude non specific effects on CB2 receptors, we observed that blocking CB2 with its highly selective antagonist SR144528, did not affect endothelial proliferation induced by bFGF in the same experimental conditions used for CB1 antagonist.
The effect of CB1 inactivation on angiogenesis was further confirmed in vivo in the rabbit cornea assay and in the OIR model of pathologic angiogenesis. The inhibition of angiogenesis by CB1 blockade in vivo may be explained by its inhibitory action on proliferation, migration and morphogenic differentiation of endothelial cells in response to angiogenic growth factors like bFGF. In vivo results also indicate that the effect is conserved in several types of microvascular endothelial cells and not only in endothelial cell lines. Moreover, the finding that CB1 antagonism completely inhibited new blood vessel formation without effect on the underlying vasculature, rules out a nonspecific toxic effect on pre-existing mature capillaries, confirmed by the lack of cytotoxicity in cultured unstimulated endothelial cells. Consequently, low concentrations of CB1 antagonists may specifically target activated endothelial cells and block neoangiogenesis without affecting stable vasculature. Noteworthy, CB1 antagonism efficiently inhibits the development of abnormal new vessels in the retina of mice in the OIR model, being effective also on pathologic angiogenesis. The finding that CB1 inactivation inhibited, even if with a slightly different efficacy, both bFGF- and VEGF-induced angiogenesis in vitro, is not surprising and is concordant with the data obtained in vivo in the OIR model, where CB1 antagonism retains its efficacy to inhibit neovascularization, in a more complex setting where act several proangiogenic factors.
We do not know which downstream signaling pathways couple CB1 receptors to angiogenesis in vivo. However, our in vitro observations suggest the involvement of ERK, Akt, FAK, JNK, Rho, and MMP-2 in the antiangiogenic effects elicited by CB1 knockdown or antagonism. The binding of VEGF and bFGF to their receptors leads to receptor phosphorylation and activation of signaling cascades such MAPK and PI3K-Akt that regulate multiple critical steps in angiogenesis.40,41 FAK phosphorylation is important for endothelial cell migration and leads to its association with PI3K, required for Akt stimulation.36 Additional FAK-mediated events induce the expression of genes encoding matrix metalloproteinases through the activation of JNK, that promotes cell invasion.42 We found that CB1 receptors blockage strongly inhibits FAK, Akt, and JNK activation. The ability of CB1 antagonism to prevent FAK activation also would be anticipated to affect the Rho-mediated pathways entailed in angiogenesis.43 CB1 antagonism, by inhibiting RhoA activation, inhibits endothelial cell chemotaxis and also the matrix metalloproteinase-2 secretion, involved in cell invasion and capillary tube formation. Previous evidence reported by us and other groups demonstrated that several cannabinoid-related drugs, either CB1 agonists (Δ9 -THC, ACEA, Met-F-AEA), CB2 agonists (JWH-015, JWH133), mixed CB1/CB2 agonists (WIN-55 212-2), or even cannabinoids inactive at CB receptors (cannabidiol analogs) are able to inhibit angiogenesis at pharmacologic μM doses in animal models of cancer or granuloma inflammation.19,29,30,38,44 This antiangiogenic effect was mainly because of the inhibition of VEGF/VEGFR signaling and to the induction of endothelial cell apoptosis. In particular, the direct antiangiogenic effect of the anandamide analog Met-F-AEA (10μM), which has quite different binding and pharmacologic properties compared with anandamide, was because of the induction of apoptosis via p38MAPK and NFkB in a way almost completely independent from CB1 receptors, probably downstream vanilloid VR1 receptor or through receptor-independent mechanisms, as previously observed.31,45 We have to take into account that the pharmacologic effect of all these cannabinoid drugs does not necessarily reflect the action of endogenously produced endocannabinoid lipid mediators, that reach at most nanomolar concentrations.11 A biphasic action on CB receptors, with low endocannabinoid tone being proproliferative and high doses of exogenously added agonists being antiproliferative and proapoptotic, is very likely and already proposed.17 Indeed, proliferation-promoting functions of CB1 receptors have been already reported in neurogenesis, that is impaired in mice lacking CB1 and in wild-type mice administered with CB1 antagonist, implying that an endogenous signaling through this receptor promotes basal levels of neurogenesis in vivo.33,34 Furthermore, an antiproliferative action of CB1 antagonism in thyroid, mantle cell lymphoma, and breast cancers,25-27 as well as in adipocytes and in hepatic myofibroblasts has been demonstrated.10,46 As here showed, also CB1 receptor knockdown or pharmacologic antagonism inhibits angiogenesis in vitro and in vivo, suggesting that CB1 signaling could contribute to the proliferative response elicited by angiogenic factors, thus regulating the angiogenic process in physiologic and/or pathologic conditions.
In regard to CB1 antagonism, we have to take into account that the actually available CB1 antagonists, among these SR141716 and AM251, work not only as antagonists of cannabinoid-mediated effects but also as inverse agonists, blocking CB1 basal constitutive activity and transduction pathways downstream tyrosine-kinase receptors (RTK, eg, growth factor receptors) coupled to the CB1 receptor (G protein coupled receptor), through a transactivation mechanism involving ERK-MAPK cascade.47 Indeed, inhibition of the MAPK and Akt pathways is a common molecular mechanism for the antiproliferative effect observed in consequence of CB1 blockade in preadipocytes and hepatic myofibroblasts.10,46 It is well known that angiogenic factors like bFGF stimulate their respective thyrosine-kinase receptors inducing ERK-MAPK and Akt signaling pathways.40 Therefore, inverse agonism could be an alternative mechanism by which SR141716 could switch off ERK and Akt activated by angiogenic factors, thus inducing biologic responses, in this instance inhibition of angiogenesis, that negatively interfere with particular RTK pathways. As a matter of fact, CB1 antagonists have been already shown to act at a step downstream from the FGF receptor, inhibiting the neurite outgrowth stimulated by bFGF.48 In support of our hypothesis it has been very recently reported a CB1-dependent transactivation of FGFR in embryonic cortical neurons.49
In the present work we demonstrate for the first time that CB1 inactivation inhibits angiogenesis, suggesting a role for the endocannabinoid system in the angiogenic process. Collectively, our data suggest that a strategy aimed at blocking CB1 receptors may function as a brake to angiogenesis under pathologic conditions. Obviously, further studies are required to ensure the efficacy of CB1 antagonists and their potential application in angiogenesis-related diseases. Moreover, the development of CB1 antagonists unable to pass the blood-brain barrier is needed, to avoid the psychotropic side-effects limitations that recently led to discontinue the antiobesity strategy aimed at blocking central nervous system CB1 receptors. The discovery of peripheral beneficial properties displayed by CB1 antagonists should redirect research efforts to a noncentral CB1 blocking strategy.
In conclusion, our findings provide new insight into the physiopathology of the endocannabinoid system and in particular of CB1 receptor signaling, strengthening the new concept of endocannabinoids as positive modulators of cell proliferation, because CB1 receptor signaling contributes to the proliferative response induced by proangiogenic growth factors. Headways in the knowledge of the endocannabinoid system biology in the field of vascular biology led to the evidence that peripheral CB1 antagonism could be a promising strategy for pharmacotherapy in a wide range of diseases, like those angiogenesis-related.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Endocannabinoid Research Group.
This study was supported by Associazione Educazione e Ricerca Medica Salernitana (ERMES) and by Sanofi-Aventis (grant to M.B.). Simona Pisanti was supported by a fellowship from FIRC (Italian Foundation for Cancer Research).
Authorship
Contribution: S.P. designed and performed research, analyzed data, and wrote the paper; P.P., L.P., M.C.P., and C.L. performed research; P.G.M. and L.M. performed in vivo experiments; P.G. analyzed data; M.Z. and A.D. contributed in vivo tools; and M.B. designed research and supervised all the aspects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maurizio Bifulco, Dip di Scienze Farmaceutiche e Biomediche, Università degli Studi di Salerno, 84084 Fisciano (Salerno) Italy; e-mail: maubiful@unisa.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal