Abstract
Administration of a single dose of anti-CD40L mAb at the time of allogeneic BM transplantation tolerizes peripheral alloreactive T cells and permits establishment of mixed hematopoietic chimerism in mice. Once engrafted, mixed chimeras are systemically tolerant to donor Ags through a central deletion mechanism and will accept any donor organ indefinitely. We previously found that the PD-1/PD-L1 pathway is required for CD8 T-cell tolerance in this model. However, the cell population that must express PD-1 and the role of other inhibitory molecules were unknown. Here, we report that LAG-3 is required for long-term peripheral CD8 but not CD4 T-cell tolerance and that this requirement is CD8 cell-extrinsic. In contrast, adoptive transfer studies revealed a CD8 T cell–intrinsic requirement for CTLA4/B7.1/B7.2 and for PD-1 for CD8 T-cell tolerance induction. We also observed that both PD-L1 and PD-L2 are independently required on donor cells to achieve T-cell tolerance. Finally, we uncovered a requirement for TGF-β signaling into T cells to achieve peripheral CD8 but not CD4 T-cell tolerance in this in vivo system.
Introduction
A long-standing goal of immunologists has been to develop ways of controlling the robust immunologic response against allogeneic Ags to prevent destruction of tissue allografts while maintaining the ability to protect from pathogens. An effective approach to inducing solid organ transplantation tolerance involves establishment of mixed hematopoietic chimerism, a state in which both recipient and donor BM cells coexist in one individual.1,2 Dual hematopoiesis from both recipient and donor hematopoietic stem cells ensures that the recipient's thymus is seeded with APCs from both sources. Any newly developing T cells with strong TCR reactivity to either host or donor Ags will be negatively selected on these APCs in the thymus, thereby conferring lifelong, systemic, donor-specific tolerance in mixed chimeras.3 Clinically a protocol using combined BM and organ graft transplantation has necessitated extensive T-cell depletion and thymic irradiation of the recipient to overcome the barrier posed by preexisting alloreactive T cells.4 This nonselective T-cell ablation leaves the patient immunocompromised for a period of time. Thus, we are using a mouse BM transplantation (BMT) model to develop and dissect mechanisms of peripheral T-cell tolerance for induction of mixed chimerism using approaches that avoid lymphoablation of the recipient.
One of the most effective experimental approaches to tolerance induction involves blockade of the CD40:CD40L pathway,5-7 which reduces expression of costimulatory ligands and inflammatory cytokines by APCs. When blocking anti-CD40L is given together with allogeneic BMT, preexisting donor-reactive T cells in the periphery are rapidly rendered unresponsive8 and specifically deleted.1,2,9 Unfortunately, use of anti-CD40L in nonhuman primates and humans has been associated with thromboembolic complications.10 The studies described here were designed to gain insight into the molecular interactions necessary for achievement of peripheral T-cell tolerance in the murine anti-CD40L allo-BMT model with the broader goal of informing development of alternative therapeutic approaches to establishing mixed chimerism without lymphoablation or toxicity.
To evaluate mechanisms of peripheral T-cell tolerance, we use development of mixed hematopoietic chimerism as a readout because both peripheral CD4 and CD8 T cells must be tolerized to achieve donor BM engraftment.11,12 We have previously demonstrated that donor-reactive CD4 and CD8 T cells very rapidly become unresponsive and disappear from the periphery in recipients of allogeneic BMT with anti-CD40L.9,13 The mechanisms governing tolerance of the CD8 T-cell compartment are quite complex and distinct from those governing CD4 T-cell tolerance. In fact, we have found that recipient DCs, B cells, MHC class II, and CD4 T cells are all specifically required for CD8 but not CD4 T-cell tolerance to be achieved.9,14 More recently, a requirement for recipient programmed death-1 (PD-1) for CD8 but not CD4 T-cell tolerance has been demonstrated,8 though the cell population that must express this inhibitory receptor was not identified.
PD-1 is an immunoreceptor tyrosine-based switch motif (ITSM)–containing inhibitory molecule15 that has 2 known ligands, PD-L1 (expressed ubiquitously) and PD-L2 (expressed on APCs). PD-1 KO animals spontaneously develop autoimmune disease (glomerulonephritis16 and dilated cardiomyopathy17 ). Moreover, PD-1 is a required mediator of CD8 exhaustion during chronic viral infection18,19 and is essential for control of peripheral autoreactive CD8 T cells.20-23 Other molecules have been described to work in synergy with PD-1 to promote and maintain peripheral T-cell tolerance. LAG-3 is an inhibitory molecule and a homolog of CD4 that binds to MHC class II. LAG-3 KO mice have no overt autoimmunity or hyperresponsiveness24 ; however, LAG-3 has been reported to regulate CD8 T-cell homeostasis and expansion following Ag-driven activation in vivo,25,26 inhibit accumulation of self-reactive CD8 T cells in an autoimmunity model,27 and synergize with PD-1 in maintaining CD8 exhaustion.28 CTLA4 is a widely studied coinhibitory molecule that competes with CD28 for binding to B7.1 (CD80) and B7.2 (CD86). CTLA4 KO animals succumb to severe lymphoproliferative and autoimmune disease.29 Another inhibitory molecule that is critical for immune homeostasis is the pleiotropic cytokine TGF-β, and T cells that are unresponsive to this cytokine promote autoimmunity.30
Although a role for recipient PD-1 in CD8 T-cell tolerance has been demonstrated in our model, whether PD-1 is CD8 T-cell intrinsically required and the contribution of other inhibitory molecules had not been investigated. Here, we describe a role for CD8 T cell–extrinsic LAG-3 and CD8 T cell–intrinsic CTLA4/B7.1/B7.2 combined with CD8 T cell–intrinsic PD-1 signaling induced by ligation of PD-L1 and PD-L2 on donor hematopoietic cells in the induction of peripheral CD8 T-cell tolerance by treatment with anti-CD40L and allo-BMT. These pathways, together with signaling via the TGFbRII molecule in T cells, are all shown to be specifically required for achievement of CD8 but not CD4 T-cell tolerance, further delineating the distinct pathways controlling tolerization of these 2 cell subsets. These results are novel and significant because they demonstrate that multiple inhibitory pathways work together to promote CD8 T-cell tolerance in this clinically relevant model of allogeneic tolerance induction. These molecules, together with the NFAT1 transcription factor,31 induce early unresponsiveness and rapid peripheral deletion of donor-reactive CD8 T cells.8,31
Methods
Mice
Female C57BL/6 (B6: H-2b), B10.A (H-2a), and dnTGFbRII Tg mice were purchased from The Jackson Laboratory or Taconic Farms. KbDb double-deficient mice were purchased from Taconic Farms. 2C TCR Tg mice,32 PD-1−/−, PD-L1−/−, and PD-L2−/− mice (Genentech), and B7.1/B7.2/CTLA4 triple-deficient mice (from Dr A. Sharpe, Brigham and Women's Hospital/Harvard Medical School) used here as the source of CTLA4−/− CD8 T cells) were bred in our animal facility. All mice were housed in a specific pathogen-free microisolator environment. Animal experimental protocols were reviewed and approved by the institutional committee at Massachusetts General Hospital.
Conditioning and BMT
Six- to 12-week-old mice received 3 Gy total body irradiation (TBI) from a 137Cesium irradiator on day −1. Anti-CD40L mAb (clone MR1, 2 mg; National Cell Culture Center) was administered intraperitoneally on day 0, before intravenous injection of 20-25 × 106 T cell–depleted, MHC-mismatched BM cells (BMCs). CD8-depleting mAbs (2.43, 0.72-1.44 mg), where mentioned, was administered intraperitoneally on day −1. Where indicated, a 2- to 4-week treatment course with blocking anti-LAG-3 mAb33 (clone C9B7w) was given intraperitoneally at a dose of 100 μg on day 0, followed by 50 μg on days 2, 4, 6, 8, and 10 for a total of 350 μg per mouse.
Preparation of B6 mice with a traceable population of transgenic CD8 cells specific for the Ld MHC class I alloantigen (2C.B6 chimeras)
Mice were prepared as previously described.9 Briefly, 5 million BMCs from 2C-TCR transgenic B6 mice were transplanted into naive B6 mice treated with 3 Gy total body irradiation (TBI) on the same day. 2C CD8 cells were identified by staining with the unconjugated 1B2 clonotypic mAb and allophycocyanin-conjugated anti–mouse IgG1 secondary Ab and counterstained with CD8-PE. After 8 weeks, the percentage of 2C CD8 cells among PBLs ranged from 12.4% to 47.0%. 2C.B6 synchimeras were used as recipients of 3Gy TBI/anti-CD40L with B10.A allo-BMT 6-9 weeks after syngeneic BMT.
Analysis of cell-surface protein expression by flow cytometry (FCM)
To analyze the expression of exhaustion-associated molecules on 2C+ and 2C− CD8 T cells, 2C.B6 synchimeras were used as recipients of T cell–depleted B10.A Ld+ BMT with or without anti-CD40L (clone MR1). On day 4 post-BMT, the animals were killed and their splenocytes stained using 1B2 (clonotypic for the 2C TCR) with anti–mouse IgG1 FITC secondary, anti–LAG-3 PE, anti-CTLA4 PE, anti-2B4 PE, anti-CD122 PE, or anti-CD127 PE with anti-CD8 allophycocyanin (all from eBioscience, BD Biosciences, or BioLegend).
Adoptive transfer of CD8 T cells
Six- to 8-week-old female KbDb double-deficient animals on the C57BL/6 background, which lack all classical MHC class I molecules and, therefore, lack CD8 T cells, were used as recipients. These animals received the 3 Gy TBI/anti-CD40L conditioning regimen. At the time of intravenous injection of 25 × 106 T cell–depleted allogeneic B10.A BMCs, the recipients were also infused with 6-9 × 106 purified recipient-type CD8 T cells. CD8 T cells were isolated from WT C57BL/6, CTLA4/B7.1/B7.2−/−, or LAG-3−/− C57BL/6 spleens using mouse CD8 T-cell isolation kits and columns for MACS separation (Miltenyi Biotec). For LAG-3 KO CD8 T cells, spleens were kindly provided by C.J.W. and D.A.A.V. (mice cared for in accordance with the St Jude Institutional Animal Care and Use Committee).
FCM analysis of multilineage chimerism and 2C+ cells in the peripheral blood
Four-color FCM analysis was used to determine multilineage chimerism. Donor-derived cells were identified with FITC-conjugated anti-MHC class I mAb (anti-H-2Dd mAb 34-2-12). The cells were counterstained with PE- or allophycocyanin-conjugated anti-CD4, -CD8, -B220 (BD PharMingen), and -MAC1 (Caltag Laboratories) mAbs. The percentage of donor chimerism in the peripheral blood was followed over time in the CD11b+, B220+, CD4+, and CD8+ lineages in all experiments. For simplicity, only B-cell chimerism or CD11b+ cell chimerism, which are each broadly representative of chimerism in all of these lineages, is presented in the figures. Propidium iodide staining was used to exclude dead cells. A cell lineage was defined as chimeric when ≥ 5% 34-2-12+ cells were found within that lineage.
Statistical analysis
Expression analysis and chimerism were compared using the Mann-Whitney U test.
Results
Phenotypic characterization of tolerized versus nontolerized CD8 T cells
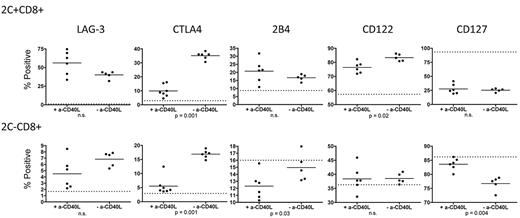
To generate animals with a small, traceable population of donor-reactive CD8 T cells within the polyclonal T-cell pool, we gave 3Gy TBI to wild-type (WT) B6 mice followed by 5 × 106 syngeneic B6 2C TCR transgenic BMCs intravenously. The 2C TCR directly recognizes the Ld MHC class I molecule32 and, therefore, is donor-reactive when Ld+ B10.A BM is transplanted. We previously demonstrated equivalent up-regulation of PD-1 on donor-reactive 2C+ CD8 T cells from tolerized (+anti-CD40L) and nontolerized (−anti-CD40L) animals receiving B10.A BMT.8 We have now performed a more extensive phenotypic comparison of tolerized and rejecting 2C cells on day 4 post-BMT in this model. Cell surface LAG-3 expression was equivalently up-regulated (relative to naive 2C/B6 animals indicated by the dotted line in Figure 1) in tolerant (+anti-CD40L) versus rejecting (−anti-CD40L) donor-reactive 2C+ and polyclonal 2C− CD8 T cells. Intracellular CTLA4 expression, however, was significantly greater in rejecting compared with tolerized 2C+ and 2C− CD8 T cells.
Expression of exhaustion-associated molecules on CD8 T cells tolerized by 3Gy TBI/anti-CD40L/allo-BMT. Splenocytes from 2C/B6 recipients of Ld+ allo-BMT with or without anti-CD40L were analyzed on day 4 post-BMT for expression of exhaustion-associated LAG-3, CTLA4 (intracellular), and 2B4 and the cytokine receptor molecules CD122 and CD127. The dotted line indicates expression level in conditioned control 2C.B6 mice that did not receive BMT. N = 5-7 mice per group.
Expression of exhaustion-associated molecules on CD8 T cells tolerized by 3Gy TBI/anti-CD40L/allo-BMT. Splenocytes from 2C/B6 recipients of Ld+ allo-BMT with or without anti-CD40L were analyzed on day 4 post-BMT for expression of exhaustion-associated LAG-3, CTLA4 (intracellular), and 2B4 and the cytokine receptor molecules CD122 and CD127. The dotted line indicates expression level in conditioned control 2C.B6 mice that did not receive BMT. N = 5-7 mice per group.
To further characterize tolerized donor-reactive CD8 T cells in this model, we also evaluated expression of the inhibitory receptor 2B4, the IL-2 receptor β chain (shared by the IL-15 receptor) CD122, and the IL-7 receptor α chain CD127. Cell-surface 2B4 was equivalently up-regulated in tolerized and rejecting donor-reactive 2C+ CD8 T cells; however, among the polyclonal 2C− CD8 T cells, there was significantly greater down-regulation of 2B4 in the tolerized versus rejecting animals (Figure 1). Surface CD122 was up-regulated to a greater extent on rejecting compared with tolerized 2C+ CD8 T cells, but no change in expression was observed in the polyclonal 2C− population. CD127 was down-regulated on donor-reactive 2C+ cells to a similar extent in tolerized and rejecting animals. However, within the polyclonal CD8 T-cell pool, down-regulation only occurred in the rejecting animals, which had significantly less expression of CD127 compared with the polyclonal CD8 T cells from tolerant animals (Figure 1).
LAG-3 is required for CD8 but not CD4 T-cell tolerance
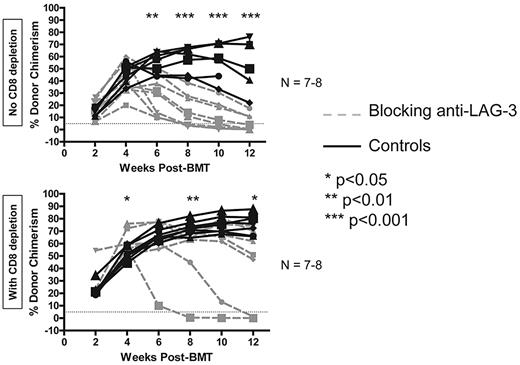
To determine whether LAG-3 is required for CD8 T-cell tolerance in our model using 3 Gy TBI/anti-CD40L with allo-BMT, we first made use of the blocking anti–murine LAG-3 mAb, clone C9B7w.33 WT B6 recipients of B10.A BMT with the regimen were given 100 μg of anti–LAG-3 on day 0, followed by 50 μg on days 2, 4, 6, 8, and 10 for a total of 350 μg per mouse. The percentage of donor chimerism in the peripheral blood was followed over time in the CD11b+, B220+, CD4+, and CD8+ lineages. Gradual rejection of donor marrow was observed, beginning between 4 and 6 weeks post-BMT in animals receiving blocking anti–LAG-3, unless recipient CD8 T cells were depleted, in which case most (5 of 7) animals remained chimeric for the duration of follow-up (Figure 2). B-cell (B220+) chimerism is depicted as a representative lineage in animals achieving multilineage hematopoietic chimerism. Among the experiments presented in this manuscript, groups of animals that accept the donor graft have donor T-cell chimerism levels averaging 5.1%-47.7% and 10.1%-77.5% for the CD4+ and CD8+ lineages, respectively. The data presented in Figure 2 demonstrate that blockade of LAG-3 prevents the complete tolerization of CD8 T cells with this regimen.
LAG-3 blockade prevents development of long-term CD8 T-cell tolerance. WT B6 animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with or without administration of blocking anti–LAG-3 mAb. CD8 depletion was performed where indicated by giving 0.72-1.44 mg of anti-CD8 (clone 2.43) intraperitoneally on day −1. Dashed gray lines represent animals receiving blocking anti–LAG-3, while solid black lines represent control animals not treated with anti–LAG-3. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 7-8 mice per group.
LAG-3 blockade prevents development of long-term CD8 T-cell tolerance. WT B6 animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with or without administration of blocking anti–LAG-3 mAb. CD8 depletion was performed where indicated by giving 0.72-1.44 mg of anti-CD8 (clone 2.43) intraperitoneally on day −1. Dashed gray lines represent animals receiving blocking anti–LAG-3, while solid black lines represent control animals not treated with anti–LAG-3. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 7-8 mice per group.
LAG-3 is CD8 T-cell extrinsically required for tolerance and is expressed at very low levels on recipient CD4 T cells and B cells
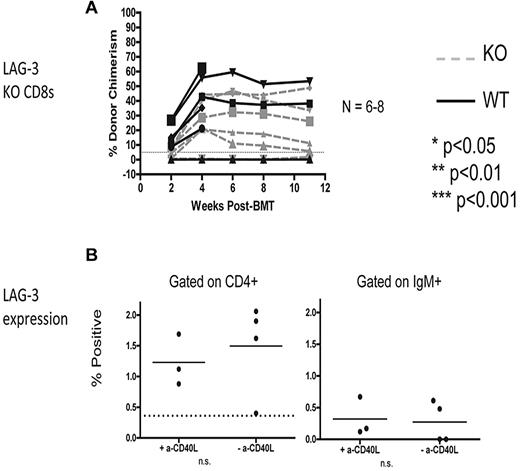
Next, we sought to determine whether the requirement for LAG-3 in the recipient is CD8 T cell–intrinsic because it was possible that LAG-3 is needed indirectly on recipient CD4 T cells, B cells, or DCs, all of which promote CD8 T-cell tolerance in this model. To test whether there is a CD8 T cell–intrinsic requirement for LAG-3, we used an adoptive transfer model that we have previously described.31 Briefly, MHC class I–deficient (KbDb KO) animals lacking CD8 T cells were given 3 Gy TBI on day −1, followed by 2 mg of anti-CD40L and allogeneic B10.A BMCs on day 0. At the time of allo-BM infusion, 6-9 × 106 recipient-type CD8 T cells were transferred intravenously. Using this model, we found that LAG-3 KO CD8 T cells were tolerized equally as well as the WT CD8 T cells, indicating that LAG-3 is dispensable on CD8 T cells to promote their tolerance (Figure 3A). Expression of surface LAG-3 on CD4 T cells and B cells was examined and found to be expressed at very low levels (< 2% LAG-3+) on day 4 postBMT (Figure 3B).
LAG-3 is required CD8 T-cell extrinsically for achievement of peripheral T-cell tolerance and is expressed at low levels on recipient CD4 T cells and B cells. (A) KbDb MHC class I–deficient animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with B10.A BMCs. Adoptive transfer of 6-9 × 106 purified recipient-type CD8 T cells was performed by intravenous injection at the time of allo-BMT. Dashed gray lines represent LAG-3 KO CD8 T cells, while solid black lines represent WT CD8 T cells. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 6-8 mice per group. (B) Expression of surface LAG-3 was detected using flow cytometry to examine gated populations of CD4+ T cells (left) and IgM+ B cells (right). N = 3-4 mice per group.
LAG-3 is required CD8 T-cell extrinsically for achievement of peripheral T-cell tolerance and is expressed at low levels on recipient CD4 T cells and B cells. (A) KbDb MHC class I–deficient animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with B10.A BMCs. Adoptive transfer of 6-9 × 106 purified recipient-type CD8 T cells was performed by intravenous injection at the time of allo-BMT. Dashed gray lines represent LAG-3 KO CD8 T cells, while solid black lines represent WT CD8 T cells. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 6-8 mice per group. (B) Expression of surface LAG-3 was detected using flow cytometry to examine gated populations of CD4+ T cells (left) and IgM+ B cells (right). N = 3-4 mice per group.
The CTLA4 and PD-1 pathways are required CD8 T-cell intrinsically for tolerance
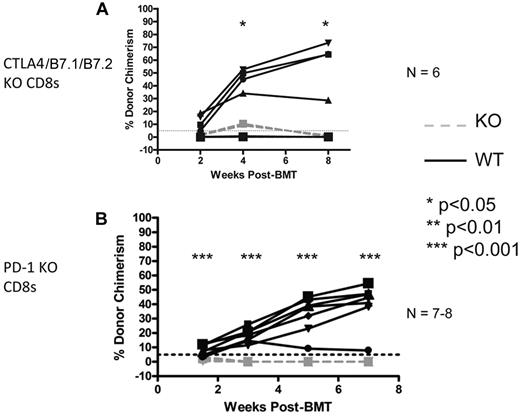
We have previously demonstrated that CTLA4 is required for tolerance of CD4 T cells in mice receiving allo-BMT with anti-CD40L and CD8 T-cell depletion.34 Because CD8 T cells depend on CD4 T cells for tolerance induction in this model, it would not be informative to use anti-CTLA4 mAb to assess the requirement for CTLA4 on CD8 T cells. To assess the role of CD8 T cell–intrinsic CTLA4 or its B7 ligands, we used adoptive transfer of CD8 T cells isolated from CTLA4/B7.1/B7.2 triple-deficient donors into KbDb KO recipients of the regimen at the time of allogeneic BMT. Control animals received WT B6 CD8 T cells. As shown in Figure 4A, CTLA4/B7.1/B7.2-deficient CD8 T cells promptly rejected donor BM, indicating that this pathway is required on CD8 T cells to achieve tolerance.
CTLA4/B7.1/B7.2 and PD-1 are CD8 T cell-intrinsically required for peripheral tolerance induced by anti-CD40L and allogeneic BMT. KbDb MHC class I–deficient animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with B10.A BMCs. Adoptive transfer of 6-9 × 106 purified recipient-type CD8 T cells was performed by intravenous injection at the time of allo-BMT. Dashed gray lines represent CTLA4/B7.1/B7.2 KO (A) or PD-1 KO (B) CD8 T cells, while solid black lines represent WT CD8 T cells. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 6-8 mice per group.
CTLA4/B7.1/B7.2 and PD-1 are CD8 T cell-intrinsically required for peripheral tolerance induced by anti-CD40L and allogeneic BMT. KbDb MHC class I–deficient animals were used as recipients of 3 Gy TBI/anti-CD40L/allo-BMT with B10.A BMCs. Adoptive transfer of 6-9 × 106 purified recipient-type CD8 T cells was performed by intravenous injection at the time of allo-BMT. Dashed gray lines represent CTLA4/B7.1/B7.2 KO (A) or PD-1 KO (B) CD8 T cells, while solid black lines represent WT CD8 T cells. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 6-8 mice per group.
Previous studies demonstrated a requisite role for recipient PD-1 in induction of CD8 T-cell tolerance in this model8 ; however, whether PD-1 is necessary CD8 T-cell intrinsically or on another required cell type in the recipient (eg, CD4 T cells or B cells) that has been shown9,14 to indirectly promote CD8 T-cell tolerance was not determined. To address this issue, we again used the CD8 T-cell adoptive transfer model and found that PD-1–deficient CD8 T cells rapidly rejected the donor BM, while wild-type (WT) CD8 T cells were readily tolerized (Figure 4B). We conclude that both CTLA4/B7.1/B7.2 and PD-1 signaling into CD8 T cells is required to tolerize them with anti-CD40L and allogeneic BMT.
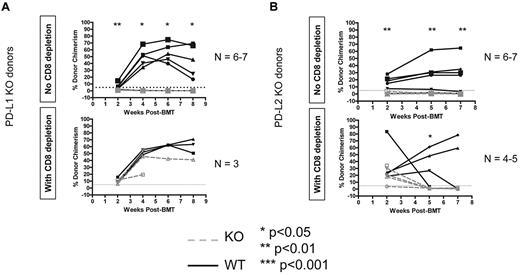
Both donor PD-L1 and PD-L2 are required for CD8 T-cell tolerance, but PD-L1 is not required for CD4 tolerance
Because we knew from previous studies that PD-1 is required in the recipient,8 we next tested whether PD-L1 and/or PD-L2 were required on the donor to achieve CD8 T-cell tolerance because we found that neither ligand was required in the recipient (data not shown). Using WT B10.S recipients of the 3Gy TBI/anti-CD40L regimen and PD-L1 KO B6 donors, we found that PD-L1 is indeed required on donor hematopoietic cells (Figure 5A). Consistent with previous studies showing that this pathway is not required for CD4 T-cell tolerance,8 donor PD-L1 was not required if recipient CD8 T cells were depleted (Figure 5A). When PD-L2 KO B6 BM was transplanted into B10.S animals receiving 3Gy TBI/anti-CD40L, we again observed rejection (Figure 5B). However, recipients of PD-L2 KO allo-BMT rejected the donor graft even when CD8-depleted, indicating a possible role for PD-L2 in CD4 T-cell tolerance. Hence, both PD-L1 and PD-L2 are required on donor BMCs to achieve tolerance in this model.
PD-1 ligands must be expressed on donor BMCs for recipient CD8 T-cell tolerance. WT or KO B6 animals were used as BMT donors with B10.S animals used as recipients of the 3 Gy/anti-CD40L/allo-BMT regimen. Dashed gray lines represent PD-L1 KO donors (A) or PD-L2 KO donors (B), while solid black lines with closed symbols represent WT control donors. CD8 depletion was performed where indicated by giving 0.72-1.44 mg of anti-CD8 (clone 2.43) intraperitoneally on day −1. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 4-8 mice per group.
PD-1 ligands must be expressed on donor BMCs for recipient CD8 T-cell tolerance. WT or KO B6 animals were used as BMT donors with B10.S animals used as recipients of the 3 Gy/anti-CD40L/allo-BMT regimen. Dashed gray lines represent PD-L1 KO donors (A) or PD-L2 KO donors (B), while solid black lines with closed symbols represent WT control donors. CD8 depletion was performed where indicated by giving 0.72-1.44 mg of anti-CD8 (clone 2.43) intraperitoneally on day −1. The percentage of donor chimerism in the B-cell lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 4-8 mice per group.
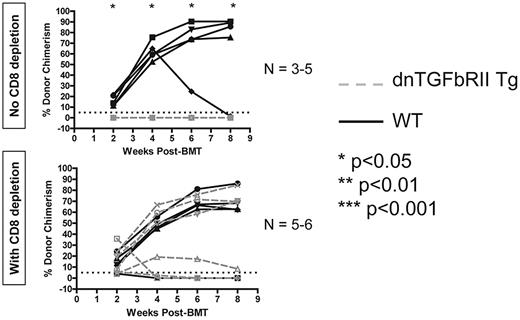
TGF-β signaling into T cells is required to achieve peripheral CD8 but not CD4 T-cell tolerance with anti-CD40L and allogeneic BMT
Finally, we examined the role of the inhibitory cytokine TGF-β in peripheral T-cell tolerance induced by anti-CD40L and allogeneic BMT. Using recipients in which expression of a dominant-negative form of the TGFbRII molecule was restricted to T cells (controlled by the CD4 promoter lacking the CD8 silencer),30 we performed allogeneic BMT with 3 Gy TBI and anti-CD40L to determine whether tolerance could be achieved. Recipients expressing dominant-negative TGFbRII in T cells all rejected unless their peripheral CD8 T cells were depleted (Figure 6). We conclude that TGF-β–mediated signaling into T cells is specifically required for achievement of peripheral tolerance of CD8 but not CD4 T cells.
TGF-β signaling into T cells is required for achievement of peripheral CD8 T-cell tolerance using anti-CD40L and allogeneic BMT. Recipients expressing a dominant-negative form of the TGFbRII specifically in T cells were given 3 Gy TBI, anti-CD40L, and allogeneic BMT and followed for development of chimerism as a readout for peripheral T-cell tolerance induction. The percentage of donor chimerism in the CD11b+ lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 3-6 mice per group.
TGF-β signaling into T cells is required for achievement of peripheral CD8 T-cell tolerance using anti-CD40L and allogeneic BMT. Recipients expressing a dominant-negative form of the TGFbRII specifically in T cells were given 3 Gy TBI, anti-CD40L, and allogeneic BMT and followed for development of chimerism as a readout for peripheral T-cell tolerance induction. The percentage of donor chimerism in the CD11b+ lineage of the peripheral blood is shown. The dotted line at 5% donor chimerism represents our cutoff for calling an animal a chimera. N = 3-6 mice per group.
Discussion
Here, we show that, as seen for unresponsive CD8 T cells in chronic viral infection,35 donor-reactive 2C+ CD8 T cells from tolerized animals receiving 3 Gy TBI/anti-CD40L with Ld+ allo-BMT also up-regulate LAG-3, CD122, and 2B4 and down-regulate CD127 relative to 2C+ CD8 T cells from naive controls. These data indicate that, before their deletion, tolerized donor-reactive CD8 T cells cannot respond to IL-7. Up-regulation of CD122 is significantly less pronounced in tolerized versus rejecting donor-reactive CD8 T cells, suggesting that T-cell activation occurs but is attenuated in tolerant mice. Down-regulation of CD127 is consistent with T-cell activation and the short-lived effector cell (SLEC) phenotype.36 2B4 can transduce an inhibitory signal when expressed at high levels and ligated by CD48,37 and it is up-regulated equivalently in tolerized and rejecting 2C+ CD8 cells but down-regulated in tolerant compared with rejecting polyclonal CD8 T cells. It is possible that 2B4 is functionally involved in CD8 T-cell tolerance, and future studies are warranted to determine whether this is the case. The polyclonal CD8 T-cell pool in tolerant and rejecting mice showed a less pronounced activation phenotype than their 2C counterparts, consistent with prior findings.8 This is likely because the 2C population analyzed consists of residual donor-reactive cells that have not yet been deleted, whereas in the polyclonal pool there are many cells that are not donor-reactive. Moreover, polyclonal CD8 T cells that are donor-reactive and partly activated are deleted in tolerized mice and expanded in rejecting mice, as indicated by previous kinetic analyses of donor-reactive CD8 cells in this model.8
LAG-3 is a homolog of CD4 and was an interesting candidate to investigate in CD8 T-cell tolerance in our model since, in addition to being involved in CD8 exhaustion,28 it binds to MHC class II and transduces a negative signal.38 Previous studies in our model have demonstrated that recipient MHC class II14 and donor MHC class II39 are essential for development of CD8 but not CD4 T-cell tolerance. Using a blocking anti–LAG-3 mAb, we now show that LAG-3 is required for long-term mixed chimerism because rejection is observed starting between 4 and 6 weeks post-BMT unless CD8 T cells are depleted. However, we found that adoptively transferred LAG-3–deficient CD8 T cells were readily tolerized by the 3 Gy TBI/anti-CD40L allogeneic BMT regimen, indicating that this molecule is not intrinsically required by CD8 cells to undergo tolerance. NK cells are dispensable for tolerance in this model, so their expression of LAG-333 is likely not a factor in promoting CD8 T-cell tolerance. The possibility that LAG-3 is needed on recipient CD4 T cells, which are required to promote CD8 T-cell tolerance, was considered. Indeed, in a Treg-dependent tolerance induction protocol, administration of a depleting anti-LAG-3 mAb prevented tolerance because of clearance of suppressive CD4+ Tregs.40 However, Tregs are not required for tolerance in our BMT model.9 Moreover, less than 2% of the CD4 cells (and B cells) in tolerized mice expressed LAG-3, making it unlikely that CD4 T cells (or B cells) provide a necessary source of LAG-3 for peripheral CD8 T-cell tolerance induction. Instead, we hypothesize that plasmacytoid DCs (pDCs), which express high levels of LAG-3,33 may be the requisite source of LAG-3 for CD8 T-cell tolerance in this model.
When we tested whether there is a CD8 T cell–intrinsic requirement for the CTLA4/B7 pathway using a system in which all recipient hematopoietic cells were WT except for CD8 T cells, we found that CTLA4/B7.1/B7.2 triple-deficient CD8 T cells could not be tolerized by anti-CD40L and allo-BMT. This is consistent with the recent finding in our model that B7.1/B7.2 is needed on recipient DCs to achieve CD8 T-cell tolerance in this costimulation blockade-based allo-BMT regimen, presumably by ligating CTLA4 on recipient CD8 T cells.41 This finding is the first demonstration, to our knowledge, of an in vivo system in which the inhibitory CTLA4/B7.1/B7.2 pathway has been shown to be CD8 T-cell intrinsically required for tolerance.
We further investigated the role of PD-1 in CD8 T-cell tolerance in this model. PD-1 signaling is an important mediator of CD8 exhaustion42 and contributes to CD8 T-cell tolerance established during initial Ag encounter in an autoimmunity model 43 . We demonstrate here that PD-1 must be expressed on peripheral CD8 T cells themselves and that both PD-L1 and PD-L2 on the donor are independently required for peripheral T-cell tolerance. It is possible that both ligands must ligate the PD-1 receptor for CD8 T-cell tolerance but, perhaps more likely, there could instead be distinct signaling events initiated by each ligand on the donor BM. A dichotomy between the effects of PD-L1 and PD-L2 was previously described in a GVH model, but only PD-L2 and not PD-L1 was found to affect CD8 cell expansion.44 In contrast to our demonstration that PD-L1 was not involved in the tolerization of CD4 T cells (8 and Figure 5A), only PD-L1 and not PD-L2 was found to inhibit CD4-dependent skin allograft rejection.45 In a model of cardiac allograft acceptance induced with CTLA4-Ig, recipient and donor PD-L1, but not PD-L2, was required to achieve graft acceptance.46 The differences between our model and the cardiac allograft acceptance model reflect the markedly differing pathways involved in achieving tolerance, which has only been shown to be systemic in the mixed chimerism model 12 . However, when PD-L1 or PD-L2 was blocked at the time of allogeneic BMT (B6 into Balb/c) in a model involving nonmyeloablative conditioning, sirolimus (mTOR inhibitor), and anti-CD40L, no effect on BM engraftment or subsequent skin graft tolerance was observed.46 This is in contrast to our results demonstrating the requirement for donor PD-L1 and PD-L2 to achieve allogeneic BM engraftment with 3 Gy TBI/anti-CD40L. The differences may be because of the additional immunosuppressive effects of sirolimus, which are absent in our model. The inhibitory signaling induced by PD-L1 and PD-L2 may only be necessary in minimal regimens that lack global immunosuppression as part of the chimerism induction approach. One possibility in our model is that donor PD-L2 initiates PD-1 signaling in the recipient while donor PD-L1 initiates inhibitory signaling through B7.1.47 Future studies will dissect the nonredundant functions of the PD-L molecules in this model.
Finally, we examined the role of TGF-β in peripheral CD8 T-cell tolerance induced by this regimen. Previous studies had suggested a role for a secreted factor in promotion of CD8 T-cell tolerance by recipient B cells, though both IDO and IL-10 had been excluded.41 Thus, we examined whether TGF-β signaling into T cells is a requirement for tolerance by using recipients that express a dominant-negative form of the receptor specifically on T cells. Our finding that these recipients reject the allogeneic BM graft only if peripheral CD8 T cells are present reveals that TGF-β is a critical cytokine for control of donor-reactive CD8 but not CD4 T cells. This result, combined with the observation that recipient B cells are required for CD8 but not CD4 T-cell tolerance,14 is consistent with the hypothesis that B cells are the source of TGF-β that promotes CD8 T-cell tolerance.
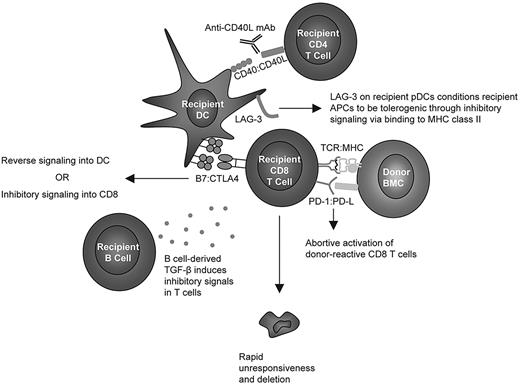
In light of the findings described here, we conclude with a hypothetical, unifying model depicted in Figure 7. PD-1 signaling has been reported to block TCR-induced “stop” signals that are necessary for prolonged interaction with APCs,48 and we hypothesize that CD8 T cells require an early signal43 through PD-1 ligation to abort their activation program and enter the state of unresponsiveness8 observed before deletion of peripheral, donor-reactive CD8 T cells. Moreover, we hypothesize that CTLA4 on CD8 T cells must ligate B7 molecules on recipient DCs41 either to directly inhibit TCR signaling in CD8 T cells or to transduce inhibitory reverse signaling through B7 into the DC, as described recently.41 Studies in an in vitro tolerance model using resting DCs have also demonstrated CD8 cell intrinsic requirements for both PD-1 and CTLA4,49 while others have found a role for PD-1 but not CTLA4.48 The observation that LAG-3 is CD8 T-cell extrinsically required but expressed at very low levels on recipient CD4 T cells and B cells is consistent with the hypothesis that this molecule expressed on DCs (possibly pDCs50 ) interacts with MHC class II on APCs and thereby conditions them through inhibitory signaling to become tolerogenic for peripheral CD8 T cells. This hypothesis may explain the requisite role for MHC class II14 in the recipient to achieve CD8 T-cell tolerance. Because MHC and B7 molecules are not required on recipient B cells, we hypothesize that the role for recipient B cells involves secretion of soluble factors, although our studies have ruled out IDO or IL-10 as required factors.41 We now describe a critical role for TGF-β signaling into T cells, and we hypothesize that recipient B cells may promote CD8 T-cell tolerance by secreting this inhibitory cytokine.
Hypothetical model of cellular and molecular interactions required for CD8 T-cell tolerance induced by anti-CD40L and allogeneic BMT.
Hypothetical model of cellular and molecular interactions required for CD8 T-cell tolerance induced by anti-CD40L and allogeneic BMT.
In summary, we have described mechanistic insights in an in vivo model of peripheral CD8 T-cell tolerance to allogeneic BMCs that highlight the importance of multiple inhibitory pathways, including those involving LAG-3, CTLA4/B7.1/B7.2, PD-1, and TGF-β, in controlling the robust alloresponse. Of these 4 pathways, 3 (LAG-3, PD-1, and TGF-β) are not required for CD4 T-cell tolerance, highlighting the disparate mechanisms involved in tolerizing the 2 T-cell subsets in the same mice, despite the common end point of deletion of peripheral donor-specific CD413 and CD89 T cells. Both CD434 and CD8 T-cell subsets (Figure 4) require the CTLA4/B7.1/B7.2 pathway cell-intrinsically to be tolerized. Together with previously published data demonstrating a requisite role for the NFAT1 transcription factor31 in CD8 but not CD4 T-cell tolerance, these data will promote the development of approaches to using pathway-specific immune therapies for tolerance induction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Fabienne Haspot and Raphael Clynes for critical review of the manuscript, Mr Orlando Moreno for outstanding animal husbandry and technical assistance, and Ms Shavree Washington for expert assistance with the manuscript.
This work was supported by National Institutes of Health (NIH) grant RO1HL49915. C.L.L. was supported by government funds awarded by the Department of Defense, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a. D.A.A.V. was supported by NIH R01 AI39480, National Cancer Institute (NCI) Comprehensive Cancer Center Support CORE grant (CA21765), and the American Lebanese Syrian Associated Charities (ALSAC).
National Institutes of Health
Authorship
Contribution: C.L.L. planned, performed, and analyzed experiments, and wrote the manuscript; C.J.W. provided LAG-3 KO spleens and reviewed the manuscript; S.B., S.L., and G.Z. performed experiments; D.A.A.V. provided LAG-3 KO spleens, contributed important intellectual content, and reviewed the manuscript; and M.S. provided overall direction and oversaw all experimental studies, and edited and revised the manuscript.
Conflict-of-interest disclosure: D.A.A.V. and C.J.W. have submitted patents that are pending and are entitled to a share in net income generated from licensing of these patent rights for commercial development. The remaining authors declare no competing financial interests.
Correspondence: Megan Sykes, MD, Columbia Center for Translational Immunology, Columbia University College of Physicians and Surgeons, 622 West 168th St, PH 17-111, New York, NY 10032; e-mail: Megan.Sykes@columbia.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal