Abstract
The anti-CD20 mAb rituximab has substantially improved the clinical outcome of patients with a wide range of B-cell malignancies. However, many patients relapse or fail to respond to rituximab, and thus there is intense investigation into the development of novel anti-CD20 mAbs with improved therapeutic efficacy. Although Fc-FcγR interactions appear to underlie much of the therapeutic success with rituximab, certain type II anti-CD20 mAbs efficiently induce programmed cell death (PCD), whereas rituximab-like type I anti-CD20 mAbs do not. Here, we show that the humanized, glycoengineered anti-CD20 mAb GA101 and derivatives harboring non-glycoengineered Fc regions are type II mAb that trigger nonapoptotic PCD in a range of B-lymphoma cell lines and primary B-cell malignancies. We demonstrate that GA101-induced cell death is dependent on actin reorganization, can be abrogated by inhibitors of actin polymerization, and is independent of BCL-2 overexpression and caspase activation. GA101-induced PCD is executed by lysosomes which disperse their contents into the cytoplasm and surrounding environment. Taken together, these findings reveal that GA101 is able to potently elicit actin-dependent, lysosomal cell death, which may potentially lead to improved clearance of B-cell malignancies in vivo.
Introduction
The addition of the anti-CD20 mAb rituximab to chemotherapy has substantially improved the clinical outcome for many patients with a wide range of B-cell malignancies.1-3 However, despite the unprecedented success of rituximab, a substantial proportion of patients with CD20-positive B-cell malignancies fail to achieve a complete remission or relapse after receiving rituximab-containing immunochemotherapy.4 These areas of unmet clinical need highlight the requirement to develop improved treatments for these patients. Given both the success with rituximab and the rapid development of mAb engineering technology, there is currently intense investigation into the development of novel anti-CD20 mAbs aimed at improving therapeutic efficacy. Central to this challenge, is an enhanced understanding of the mechanism of action of anti-CD20 mAbs.
Anti-CD20 mAbs can activate a range of potential tumor cell killing pathways (reviewed in Lim et al5 ) including Fc-Fcγ receptor (FcγR) interactions (namely Ab-dependent cellular cytotoxicity [ADCC] and phagocytosis mediated by FcγR-expressing immune effector cells such as macrophages and/or NK cells), complement-dependent cytotoxicity (CDC), or the direct induction of programmed cell death (PCD). Although it is well established that Fc-FcγR interactions are critical for the in vivo efficacy of anti-CD20 mAbs,6-8 the role of complement remains disputed as to whether it is beneficial,9,10 inconsequential,7,11,12 or even detrimental to anti-CD20 mAb efficacy.13,14 However, the potential importance of PCD in enhancing anti-CD20 mAb potency remains largely underinvestigated, perhaps because it does not appear to play a major role in the therapeutic efficacy of rituximab.15 We have characterized anti-CD20 mAbs into 2 subtypes based on their Ag engagement properties and effector function profiles. Type I (rituximab-like) anti-CD20 mAbs redistribute CD20 into membrane lipid rafts and potently activate complement, whereas type II (tositumomab-like) anti-CD20 mAbs do not activate complement, but more potently evoke direct PCD.16 Importantly, type II anti-CD20 mAbs showed superior efficacy in vivo,9,11 with F(ab)′2 fragments providing substantial immunotherapy in lymphoma xenograft models, suggesting that direct PCD contributes toward the superior efficacy of type II anti-CD20 mAbs.9
Despite the apparent efficacy of type II anti-CD20 mAbs in preclinical studies, there has been little focus on their clinical development. The majority of next-generation anti-CD20 mAbs in clinical trials are type I, developed with an emphasis on enhancing Fc-mediated effects such as ADCC or CDC, and their clinical efficacy has not as yet been compared with rituximab. In contrast, GA101 is a novel anti-CD20 mAb, which in addition to its glycoengineered Fc to enhance ADCC, harbors a modified elbow-hinge region resulting in superior PCD induction.17 Currently, the mechanisms underlying this improved PCD remain undefined. GA101 (like tositumomab) has, however, demonstrated superior therapeutic efficacy over rituximab in preclinical studies,17 and phase 1 clinical studies in patients with relapsed/refractory B-cell malignancies have demonstrated promising clinical activity.18,19 Furthermore, a variant of GA101 with a non-glycoengineered, wild-type Fc-domain mediated superior in vivo efficacy compared with rituximab in xenograft models, suggesting that mechanisms independent of Fc glycoengineering contribute to the superior efficacy of GA101.20 Therefore, a clearer understanding of the biologic mechanisms underlying PCD evoked by GA101 is important in providing new insights into its mechanism of action, as well as informing the development of anti-CD20 mAbs with improved clinical efficacy.
Previously, we observed that the type II anti-CD20 mAb tositumomab induced caspase-independent cell death which correlated with its ability to induce homotypic adhesion (HA),16 and this correlation was also reported for GA101.17 Given the importance of actin cytoskeleton remodeling in lymphocyte activation and signal transduction,21 we have recently investigated its role in cell death and HA induced by tositumomab and an anti-HLA DR mAb, demonstrating that the actin cytoskeleton is critical for HA and cell death evoked by these mAbs. Furthermore, cell death was mediated by lysosomes which swell and disperse their contents into the cytosol.22 These findings contribute to a growing body of evidence supporting the role of lysosomes in triggering nonapoptotic cell death, through the release of their contents into the cytosol, a process known as lysosomal membrane permeabilization (LMP).23
In this study, we confirm that GA101 is a type II anti-CD20 mAb that in contrast to rituximab induces high levels of PCD in B-lymphoma cell lines, and extend these findings to primary B-cell malignancies. Furthermore, we reveal that GA101 evokes this characteristic nonapoptotic mode of cell death, dependent on actin reorganization and mediated by lysosomes.22 Importantly, cell death was independent of BCL-2 and caspase activation, potentially circumventing resistance to chemotherapy-induced apoptosis. Thus, GA101 is the first humanized anti-CD20 mAb to enter the clinic, which potently elicits this novel mode of direct cell death.
Methods
Cell lines
Human B-lymphoma cell lines (Raji, Daudi, Granta 519, and SU-DHL4) were obtained from the European Collection of Cell Cultures (ECACC). Raji cells overexpressing the antiapoptotic protein BCL-2 and Raji cells expressing pAcGFP-actin have been described previously.22,24 Cells were maintained in RPMI 1640 or DMEM media supplemented with 10% FCS and 2mM glutamine (Invitrogen) at 37°C, 5% CO2.
Abs and reagents
GA101 was kindly provided by Roche Glycart AG. Rituximab was obtained from the Christie Hospital pharmacy (Manchester, United Kingdom). The anti-HER2 mAb trastuzumab was purchased commercially from Hoffmann La Roche AG. Ofatumumab and a GA101 derivative with a wild-type, non-glycomodified huIgG1 Fc region, GA101 (NG), were produced in-house based on published sequences. mAbs were produced in either the 293F Freestyle system (Invitrogen) or CHO-K1 cells. (NB: These mAb may differ for example, in their carbohydrate structures, from mAb produced for clinical use). F(ab)′2 fragments of IgG were produced by standard pepsin digestion as previously described.25 The actin inhibitors latrunculin B and cytochalasin D, the vacuolar ATPase inhibitor concanamycin A and the cytotoxic drugs mitoxantrone and doxorubicin were purchased from Sigma-Aldrich. The caspase inhibitor N-(2-Quinolyl)valyl-aspartyl-(2,6-difluorophenoxy)methyl ketone (Q-VD-OPH) and cathepsin inhibitor III (Z-FG-NHO-BzOME) were purchased from Calbiochem. Abs against the following were used: cleaved caspase-3 (Cell Signaling Technology), BCL-2 (DAKO), cathepsin B (Abcam), lysosome-associated membrane protein-1 (Calbiochem), β-actin (Sigma-Aldrich), and BSA (Abcam).
Primary tumor samples
Ethical approval was obtained from the Manchester Cancer Research Center Biobank Ethics Committee or the Southampton and South West Hampshire Research Ethics Committee, in accordance with the declaration of Helsinki. For B-cell chronic lymphocytic leukemia (B-CLL), PBMCs were isolated from whole blood by Ficoll gradient centrifugation, washed, cryopreserved in FCS with 10% DMSO for subsequent analysis. No further enrichment for B cells was performed as all samples contained at least 85% CD20-positive B cells as detected by flow cytometry. For lymphoma samples, single-cell suspensions were isolated from tissue biopsies, Ficoll-purified and cryopreserved as described for B-CLL cells.
Cell death and cell viability assays
Cells were seeded at 5 × 105/mL, treated with anti-CD20 mAbs at 5 or 10 μg/mL and incubated at 37°C, 5% CO2 in plastic 24-well plates. Trastuzumab was used as an isotype control. Inhibitors were added for 30 minutes before treatment with mAb. Before cell death analysis, cells were gently pipetted to disaggregate clumps and form a single-cell suspension. To quantify cell death, cells were washed and resuspended in binding buffer (10mM HEPES, 140mM NaCl, and 2.5mM CaCl2) and labeled with annexin V–FITC or annexin V–Cy5.5 (BD Biosciences) to detect phosphatidylserine exposure and propidium iodide (PI; Sigma-Aldrich) or 7-Aminoactinomycin D (7-AAD; eBioscience) to detect loss of plasma membrane integrity, and analyzed by dual color flow cytometry. For 51Cr-release cytotoxicity assays, cells were prelabeled with 51Cr for 1 hour at 37°C then treated as described for flow cytometric cell death analysis. Cells were then centrifuged at 200g for 5 minutes and 50-μL quintuplicate samples of supernatant were harvested and 51Cr release, which occurs when dead cells lose their membrane integrity, was quantified using a TopCount NXT (PerkinElmer). Maximal release of 51Cr was obtained using Raji cells treated with 0.5% Triton X-100 in normal media for 5 minutes. Background release was determined from untreated cells. The percentage of specific 51Cr release was calculated using the formula: ([sample release − background release]/[maximum release − background release]) × 100. Cell viability was assessed using the XTT Cell Proliferation kit II (Roche) according to the manufacturer's instructions. The XTT assay is a colorimetric assay based on the ability of viable, metabolically active cells to reduce the yellow tetrazolium salt XTT to an orange formazan dye. Cells were treated as above and 100-μL quintuplicate samples were harvested and incubated with 50 μL of XTT reagent for 4 hours at 37°C in 96-well plates, then absorbance was detected at 485 nm (reference wavelength: 680 nm) using a GENios Multi-Detection Plate Reader (Tecan) and normalized relative to untreated controls.
Live cell isolation and re-treatment
Cells were treated with mAbs (10 μg/mL) for 4 hours, and the remaining live cell subpopulation was isolated by negative selection using a MACS-based Dead Cell Removal Kit (Miltenyi Biotec), according to manufacturer's instructions. Isolated cells were rested in culture overnight then re-treated with mAb for a further 4 hours. Cell death was quantified by annexin V/PI staining and compared with previously untreated cells as controls.
Microscopic analysis of HA
Cells were treated with anti-CD20 mAbs (5-10 μg/mL) in flat-bottomed plastic plates (Corning) for 4 hours at 37°C and HA was assessed semi-quantitatively using an Olympus IX50 inverted microscope with a 4× or 20× Ph lens. Images were acquired using a CCL2 digital cooled camera (Olympus) and were analyzed with Metamorph (Molecular Devices) and Adobe Photoshop software.
Transmission electron microscopy
Raji cells were treated with mAb 10 μg/mL or staurosporine (2μM) for 24 hours and samples were processed and analyzed as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Time lapse microscopy
Cells were plated on glass-based dishes (Iwaki) in a humidified atmosphere at 37°C, 5% CO2. Images were captured every 5 minutes using a Zeiss Axiovert 200M microscope equipped with a Roper Cascade EMCCD-cooled camera or an Olympus IX81 spinning disk confocal with a Roper quant EM camera both equipped with Chroma Sedat excitation filters (406/488/555/647 nm), using a 60× lens. Images were acquired using Metamorph software, and videos were generated using Imaris software (Bitplane).
Immunofluorescence microscopy
Immunofluorescence staining protocol was performed as described previously.26 Cells were then imaged using an Olympus BX51 microscope equipped with a CoolSnap HQ camera and Sedat excitation filters using an 40× or 100× lens, and images were processed using Metamorph and Imaris software.
DNA and nuclear fragmentation assays
DNA breaks were detected by TUNEL using the APO-BrdU TUNEL assay kit (Invitrogen) according to manufacturer's instructions, and analyzed by flow cytometry. Nuclear fragmentation was analyzed by resuspending cells in hypotonic PI solution (50 μg/mL PI, 0.1% (wt/vol) sodium citrate, 0.1% [vol/vol] Triton X-100) to stain nuclei, incubated overnight at 4°C, and analyzed by flow cytometry. Nuclear fragmentation was detected as the hypodiploid (sub-G1) fraction.
Western blotting
Cell lysates were prepared in lysis buffer supplemented with protease inhibitor cocktail (Cell Signaling) and cell supernatants were harvested from cell suspensions and filtered. Samples were separated by SDS-PAGE and proteins transferred to PVDF membranes using the Surelock Western blot Module (Invitrogen). Membranes were blocked with 5% nonfat dried milk, incubated with appropriately diluted primary antibodies according to the manufacturer's instructions, washed, and incubated with HRP-conjugated secondary antibodies (1:2500; DAKO). Membranes were treated with ECL reagent (Amersham) and visualized using a Fujifilm image analyzer.
Lysosome volume and permeability assays
To assess total lysosomal volume, cells were labeled with 75nM Lysotracker green (Invitrogen) for 1 hour before analysis by flow cytometry. For double Lysotracker/annexin V staining, cells were incubated with Lysotracker then resuspended in binding buffer containing annexin V–Cy5.5 and samples were analyzed by dual-color flow cytometry. To assess lysosomal membrane permeabilization, a well-established method was used.27,28 Cells were labeled with 5μM acridine orange (AO) for 15 minutes at 37°C, washed and treated with mAb. AO is a metachromatic fluorochrome that emits red fluorescence when highly concentrated in acidic lysosomes, and green fluorescence in the more pH neutral cytosol. Thus, leakage of lysosomal contents into the cytosol can be detected as an increase in green fluorescence by flow cytometry.
Flow cytometry
Flow cytometric data were acquired using a FACSCalibur cytometer (BD Biosciences) operated by CellQuest software and 10 000 events were collected per sample. Data were analyzed using WinMDI software (Version 2.8).
Statistical analysis
Error bars represent the SEM of 3 independent experiments unless otherwise stated. To compare the difference between the experimental groups, an unpaired, 2-tailed t test was calculated.
Results
GA101 induces superior Fc-independent PCD and HA
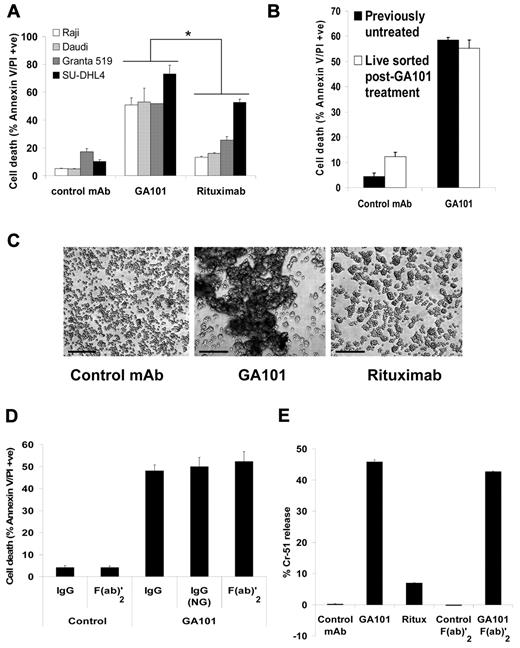
The ability of GA101 to induce direct PCD was tested in a panel of CD20-positive B-lymphoma cell lines of different origins (Raji and Daudi: Burkitt lymphoma, Granta 519: mantle cell lymphoma and SU-DHL4: diffuse large B-cell lymphoma) using annexin V/PI staining. GA101 induced significantly higher cell death compared with rituximab in the B-lymphoma cell lines tested (Raji and Granta 519 P < .001, Daudi P < .01, SUDHL4 P < .03, Figure 1A). A kinetic analysis of cell death in Raji cells revealed that GA101-induced killing peaked at 50%-60%, with no further death observed up to 72 hours after treatment (data not shown). Therefore, to determine whether the remaining viable cell population acquires resistance to GA101-induced killing, we have isolated this viable cell population using MACS, and re-treated them with GA101. Strikingly, the amount of cell death induced in this population was equivalent to that induced in previously untreated cells (Figure 1B). In addition, we allowed GA101-treated cells to recover for 15 days in culture until cell death returned to background levels and re-treated them with GA101. Similarly, these cells were equally sensitive to GA101-induced killing compared with previously untreated cells (data not shown). Therefore, these data strongly suggest that although a subpopulation of cells remains viable following GA101 treatment, they do not acquire resistance to direct PCD induced by GA101. Importantly, the overall ability of anti-CD20 mAbs to induce PCD generally correlated with the extent of HA as assessed by light microscopy, with cells undergoing more pronounced HA following treatment with GA101 than with rituximab (Figure 1C).
GA101 induces Fc-independent PCD and HA. (A) A panel of CD20-positive, human B-lymphoma cell lines was treated with 10 μg/mL mAb and cell death was analyzed after 24 hours using the annexin V–FITC/PI assay. The anti-HER2 mAb trastuzumab was used as a human IgG1 isotype control. GA101 induced significantly higher cell death than rituximab in all the cell lines tested (for Raji and Granta 519, P < .001; Daudi, P < .01; SU-DHL4, P < .03). (B) Raji cells were treated with GA101 (10 μg/mL) for 4 hours and the viable cell population was isolated, rested overnight, then re-treated with GA101 for 4 hours. Subsequently, cell death was analyzed as in panel A. Mean and SEM of 2 independent experiments are shown. Cell death induced in viable isolated cells was equivalent to that induced in previously untreated cells. (C) The extent of HA induced by mAbs (10 μg/mL) was assessed by low-magnification light microscopy 4 hours after treatment, and HA in Daudi cells is shown as an example (scale bar, 500 μm). GA101 induced superior HA than rituximab. (D) Cell death induced by GA101 in Raji cells was directly compared with that induced by a non-glycoengineered, wild-type human IgG1-bearing derivative of GA101, GA101 (NG), and F(ab)′2 fragments of GA101. All induced equivalent amounts of cell death confirming that GA101-induced cell death is Fc independent. (E) 51Cr-release cell death assay. Cells were prelabeled with 51Cr for 1 hour at 37°C before treatment with mAb and after 4 hours, 51Cr release was measured as described in “Cell death and cell viability assays.” Mean + SEM of quintuplicate samples representative of 2 independent experiments are shown. GA101 induced significantly greater 51Cr release than rituximab, and F(ab)′2 fragments of GA101 were sufficient for the induction of 51Cr release.
GA101 induces Fc-independent PCD and HA. (A) A panel of CD20-positive, human B-lymphoma cell lines was treated with 10 μg/mL mAb and cell death was analyzed after 24 hours using the annexin V–FITC/PI assay. The anti-HER2 mAb trastuzumab was used as a human IgG1 isotype control. GA101 induced significantly higher cell death than rituximab in all the cell lines tested (for Raji and Granta 519, P < .001; Daudi, P < .01; SU-DHL4, P < .03). (B) Raji cells were treated with GA101 (10 μg/mL) for 4 hours and the viable cell population was isolated, rested overnight, then re-treated with GA101 for 4 hours. Subsequently, cell death was analyzed as in panel A. Mean and SEM of 2 independent experiments are shown. Cell death induced in viable isolated cells was equivalent to that induced in previously untreated cells. (C) The extent of HA induced by mAbs (10 μg/mL) was assessed by low-magnification light microscopy 4 hours after treatment, and HA in Daudi cells is shown as an example (scale bar, 500 μm). GA101 induced superior HA than rituximab. (D) Cell death induced by GA101 in Raji cells was directly compared with that induced by a non-glycoengineered, wild-type human IgG1-bearing derivative of GA101, GA101 (NG), and F(ab)′2 fragments of GA101. All induced equivalent amounts of cell death confirming that GA101-induced cell death is Fc independent. (E) 51Cr-release cell death assay. Cells were prelabeled with 51Cr for 1 hour at 37°C before treatment with mAb and after 4 hours, 51Cr release was measured as described in “Cell death and cell viability assays.” Mean + SEM of quintuplicate samples representative of 2 independent experiments are shown. GA101 induced significantly greater 51Cr release than rituximab, and F(ab)′2 fragments of GA101 were sufficient for the induction of 51Cr release.
Previously, we and others showed that direct PCD induced by first-generation anti-CD20 mAbs (such as tositumomab) is independent of the Ab Fc domain.9,29 However, Fc-FcγR interactions can potentially influence signaling pathways and affect the induction of direct PCD. GA101 harbors a glycoengineered, afucosylated, Fc domain with enhanced binding to FcγR. Therefore, to investigate whether the superior cell death observed with GA101 is independent of the glycosylation differences in its Fc domain we generated a derivative of GA101 with a wild-type, non-glycomodified huIgG1 Fc domain, GA101 (NG), and compared the ability of this derivative to induce PCD with GA101. GA101 (NG) evoked similar levels of cell death (Figure 1D) and HA (not shown) to that induced by GA101, confirming that cell death is independent of the altered Fc glycosylation in GA101. Furthermore, we also showed that F(ab)′2 fragments of GA101 were sufficient for the induction of cell death (Figure 1D) and HA (not shown), confirming that these processes are Fc independent. These findings were further validated using a 51Cr-release assay. In this assay, the magnitude of GA101-induced cell death was similar to that observed in the annexin V/PI flow cytometry assay, and GA101 F(ab)′2 fragments were shown to be sufficient to evoke cell death (Figure 1E).
The increased cell death evoked by GA101 translated into longer-term consequences, including superior cell growth inhibition 72 hours after treatment (supplemental Figure 1a). Furthermore, GA101 induced greater inhibition of colony growth after 14 days compared with rituximab, independent of disaggregation (supplemental Figure 1b).
This efficient induction of cell death is in keeping with the type II nature of GA101 previously reported based on its weak ability to translocate CD20 into membrane lipid rafts or induce CDC (supplemental Figure 2a-b and Mossner et al17 ). We have extended these findings to demonstrate that GA101 also triggers intracellular calcium flux less efficiently than rituximab, which is another defining feature of type II anti-CD20 mAbs30 (supplemental Figure 2c).
Finally, cell death induced by GA101 was compared with type I anti-CD20 mAbs in the presence of serum complement in Raji cells. GA101 induced greater cell death than rituximab and ofatumumab, a next-generation fully human type I anti-CD20 mAb which is highly potent in inducing CDC,31 in the presence of complement (supplemental Figure 3).
Actin reorganization is critical for cell death and HA induced by GA101
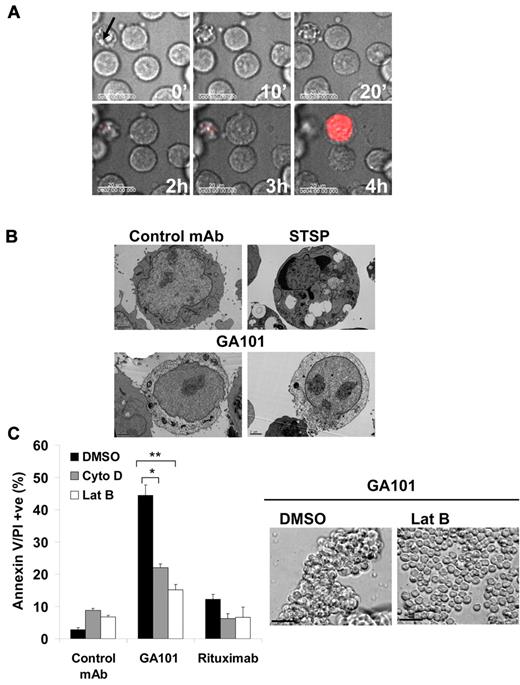
The correlation between mAb-induced HA and cell death has been reported for mAbs targeting not only the CD20 Ag but also other cell-surface Ags16,32 and thus we aimed to determine whether these 2 processes are indeed functionally linked. First, analysis of GA101-treated cells using time-lapse microscopy showed that HA occurs rapidly, within 20 minutes, and is subsequently followed by cell swelling and cell death as detected by 7-AAD staining because of loss of plasma membrane integrity (Figure 2A and supplemental Video 1). Furthermore, ultrastructural analysis of GA101-treated cells using transmission electron microscopy revealed that cells undergoing HA show signs of cell death including cytoplasmic decomposition and loss of cell membrane integrity (Figure 2B). Because HA requires the rearrangement of the actin cytoskeleton, we investigated the importance of this process in GA101-induced PCD. Indeed, disruption of the actin cytoskeleton using inhibitors of actin polymerization (cytochalasin D and latrunculin B) significantly inhibited cell death (P < .02 for cytochalasin D and P < .01 for latrunculin B) and HA induced by GA101 (Figure 2C). Similarly, GA101-induced cell growth inhibition in the absence of disaggregation was attenuated by latrunculin B (supplemental Figure 4). Furthermore, confocal time-lapse analysis of Raji cells expressing GFP-labeled actin showed that GA101 triggers rapid actin relocalization toward points of cell-cell contact (supplemental Figure 5 and supplemental Video 2).
Cell death and HA induced by GA101 is dependent on actin polymerization. (A) Time-lapse microscopy of Raji cells following treatment with GA101. Cells were suspended in phenol red-free media containing 7-aminoactinomycin D (7-AAD, 5 μg/mL) as a marker for cell death, and treated with GA101 (10 μg/mL) at time 0. Phase-contrast and fluorescence images were captured every 5 minutes and overlaid, with a sample of images captured at different time points shown (scale bar, 20 μm). Cellular adhesion was followed by cell swelling, loss of plasma membrane integrity, and cell death. Arrow marks a morphological control for apoptosis shown on the top left of the image. (B) Morphological pattern of cell death induced by GA101. Images show adhering cells showing gross cytoplasmic disintegration and loss of plasma membrane integrity after treatment with GA101. Staurosporine (STSP) was used as a morphological control for apoptosis. (C) Cells were incubated with inhibitors of actin polymerization (cytochalasin D and latrunculin B, 10μM) before treatment with mAb. Cell death and homotypic adhesion were analyzed 4 hours after treatment. Disruption of the actin cytoskeleton significantly inhibited cell death induced by GA101 and HA as shown in the example with latrunculin B (scale bar, 100 μm; *P < .02 **P < .01).
Cell death and HA induced by GA101 is dependent on actin polymerization. (A) Time-lapse microscopy of Raji cells following treatment with GA101. Cells were suspended in phenol red-free media containing 7-aminoactinomycin D (7-AAD, 5 μg/mL) as a marker for cell death, and treated with GA101 (10 μg/mL) at time 0. Phase-contrast and fluorescence images were captured every 5 minutes and overlaid, with a sample of images captured at different time points shown (scale bar, 20 μm). Cellular adhesion was followed by cell swelling, loss of plasma membrane integrity, and cell death. Arrow marks a morphological control for apoptosis shown on the top left of the image. (B) Morphological pattern of cell death induced by GA101. Images show adhering cells showing gross cytoplasmic disintegration and loss of plasma membrane integrity after treatment with GA101. Staurosporine (STSP) was used as a morphological control for apoptosis. (C) Cells were incubated with inhibitors of actin polymerization (cytochalasin D and latrunculin B, 10μM) before treatment with mAb. Cell death and homotypic adhesion were analyzed 4 hours after treatment. Disruption of the actin cytoskeleton significantly inhibited cell death induced by GA101 and HA as shown in the example with latrunculin B (scale bar, 100 μm; *P < .02 **P < .01).
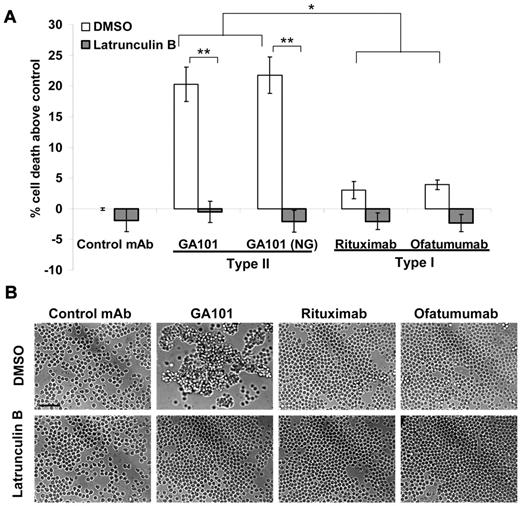
We next investigated the ability of GA101 to elicit this mode of direct PCD in a panel of primary B-cell malignancies. GA101 induced superior PCD compared with rituximab, and ofatumumab in B-CLL (P < .005, Figure 3A and Table 1), which correlated with an increase in HA (Figure 3B). Cell death induced by GA101 in B-CLL was independent of Fc glycoengineering as both GA101 and GA101 (NG) evoked equivalent amounts of death. Importantly, the actin inhibitor latrunculin B, significantly inhibited cell death (P < .0001) and HA induced by GA101 in B-CLL (Figure 3). A similar trend toward increased cell death (supplemental Table 1) and HA (not shown) was observed with GA101 (NG) compared with rituximab in primary MCL and DLBCL.
GA101 induces actin-dependent PCD and HA in primary B-CLL. (A) Primary B-CLL cells were isolated from patient blood samples as described in “Primary tumor samples,” preincubated with vehicle control (DMSO) or latrunculin B (Lat B, 10μM) then treated with anti-CD20 mAbs (5 μg/mL) for 4 hours, and cell death was assessed using annexin V-Cy5.5/7-AAD staining. Mean ± SEM of 4 independent patient samples are shown. Because of the heterogeneous levels of background cell death in individual patient samples, death was expressed as percentage above control. GA101 and GA101 (NG), the non-glycoengineered derivative of GA101, both induced significantly higher cell death than rituximab and ofatumumab. Cell death was completely ablated by latrunculin B (*P < .005, **P < .0001). (B) Primary B-CLL cells were treated as above and assessed for HA using light microscopy. Figure shows representative images from patient CLL31 (scale bar, 50 μm). GA101-induced HA correlates with PCD, and is blocked by the disruption of the actin cytoskeleton with latrunculin B.
GA101 induces actin-dependent PCD and HA in primary B-CLL. (A) Primary B-CLL cells were isolated from patient blood samples as described in “Primary tumor samples,” preincubated with vehicle control (DMSO) or latrunculin B (Lat B, 10μM) then treated with anti-CD20 mAbs (5 μg/mL) for 4 hours, and cell death was assessed using annexin V-Cy5.5/7-AAD staining. Mean ± SEM of 4 independent patient samples are shown. Because of the heterogeneous levels of background cell death in individual patient samples, death was expressed as percentage above control. GA101 and GA101 (NG), the non-glycoengineered derivative of GA101, both induced significantly higher cell death than rituximab and ofatumumab. Cell death was completely ablated by latrunculin B (*P < .005, **P < .0001). (B) Primary B-CLL cells were treated as above and assessed for HA using light microscopy. Figure shows representative images from patient CLL31 (scale bar, 50 μm). GA101-induced HA correlates with PCD, and is blocked by the disruption of the actin cytoskeleton with latrunculin B.
B-CLL patient information
| Clinical characteristics . | % Cell death above control* . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID . | Age/sex . | Binet stage . | Previous therapy . | TP53 (Del17p) analysis . | WBC, ×109/L . | CD20+ cells, % . | CD20 expression, MFI† . | GA101 . | GA101 (NG)‡ . | Rituximab . | Ofatumumab . |
| CLL31 | 64/M | Relapsed B | Chlorambucil, fludarabine, cyclophosphamide | Negative | 288.5 | 96.8 | 151 | 20 | 22.1 | −2.3 | 3.4 |
| CLL35 | 79/F | A | No prior therapy | Negative | 46.7 | 88.5 | 160 | 14 | 15 | 1.6 | 5.6 |
| CLL39 | 63/M | B | No prior therapy | Negative | 20 | 86.8 | 122 | 20.7 | 17.7 | 5.5 | 2 |
| CLL42 | 74/F | Relapsed C | Chlorambucil and steroids | Negative | 66.2 | 96.1 | 134 | 27.7 | 28.6 | 3.9 | 4.7 |
| Clinical characteristics . | % Cell death above control* . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID . | Age/sex . | Binet stage . | Previous therapy . | TP53 (Del17p) analysis . | WBC, ×109/L . | CD20+ cells, % . | CD20 expression, MFI† . | GA101 . | GA101 (NG)‡ . | Rituximab . | Ofatumumab . |
| CLL31 | 64/M | Relapsed B | Chlorambucil, fludarabine, cyclophosphamide | Negative | 288.5 | 96.8 | 151 | 20 | 22.1 | −2.3 | 3.4 |
| CLL35 | 79/F | A | No prior therapy | Negative | 46.7 | 88.5 | 160 | 14 | 15 | 1.6 | 5.6 |
| CLL39 | 63/M | B | No prior therapy | Negative | 20 | 86.8 | 122 | 20.7 | 17.7 | 5.5 | 2 |
| CLL42 | 74/F | Relapsed C | Chlorambucil and steroids | Negative | 66.2 | 96.1 | 134 | 27.7 | 28.6 | 3.9 | 4.7 |
WBC indicates white blood cells; MFI, median fluorescence intensity; M, male; and F, female.
Direct programmed cell death induced by anti-CD20 mAbs (as depicted in Figure 3A).
MFI above isotype control detected by treatment of cells with rituximab (5 μg/mL) followed by PE-conjugated anti-human Fc (1:100) and analyzed by flow cytometry.
GA101 (NG) is a variant of GA101 with a wild-type, non-glycoengineered human IgG1 Fc domain.
Together, these data demonstrate that GA101-induced PCD is dependent on HA, secondary to actin relocalization toward cell-cell junctions.
GA101 induces nonapoptotic cell death that can circumvent resistance to apoptosis
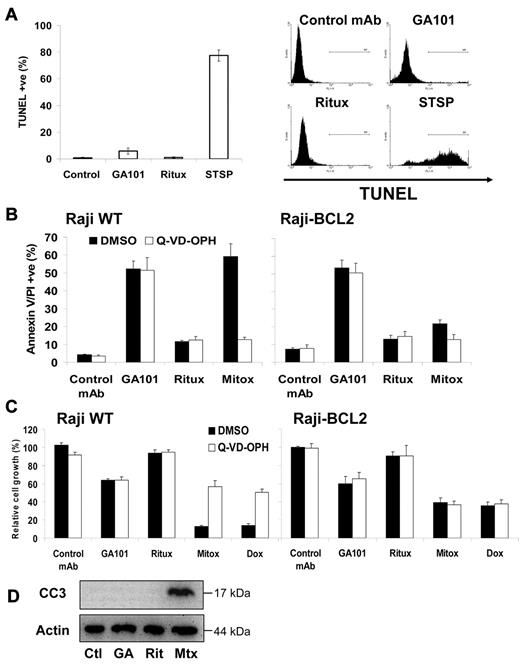
To further explore whether the actin-dependent cell death induced by GA101 was related to apoptosis, we performed the TUNEL assay to detect the presence of DNA breaks. Only very low levels of DNA breaks were detected after treatment of Raji cells with GA101 (∼ 5%), which was marginally higher than background (Figure 4A), despite cells being effectively killed by GA101 (Figure 1A,E). These findings were corroborated using hypotonic PI staining to detect nuclear fragmentation, as only low levels of nuclear fragmentation (sub-G1/hypodiploid cells) were detected up to 72 hours after treatment (supplemental Figure 6). The lack of DNA/nuclear fragmentation observed is consistent with the morphologic appearance of GA101-induced cell death, which showed no features of apoptosis such as chromatin condensation or apoptotic body formation (Figure 2B), implying a nonapoptotic mode of cell death.
GA101-induced cell death is independent of DNA fragmentation, caspase activation, and BCL-2 overexpression. (A) Raji cells were treated with anti-CD20 mAb (10 μg/mL) or staurosporine (STSP, 2μM), a positive control for apoptosis, for 24 hours and DNA fragmentation was assessed using TUNEL staining analyzed by flow cytometry. Mean ± SEM of 3 independent experiments is shown on the left, with representative plots on the right. GA101 does not induce significant DNA fragmentation. (B) Wild-type Raji (left) and Raji cells that overexpress the antiapoptotic protein BCL-2, (Raji-BCL2; right), were preincubated with DMSO or Q-VD-OPH (20μM) for 30 minutes, then treated with anti-CD20 mAbs (10 μg/mL) or mitoxantrone (1 μg/mL), and cell death measured 48 hours later. Mean + SEM of 4 independent experiments are shown. Neither BCL-2 overexpression, caspase inhibition, nor a combination of both, had any impact on cell death induced by GA101, despite inhibiting mitoxantrone-induced apoptosis (P < .008). (C) Raji and Raji-BCL2 cells were treated as in panel B and growth inhibition assessed using the XTT assay as described in “Cell death and cell viability assays” 48 hours after treatment, with absorbance normalized relative to untreated cells. Mean + SEM of 4 independent experiments are shown. Caspase inhibition and BCL-2 overexpression had no impact on GA101-induced growth inhibition, both of which significantly attenuated growth inhibition induced by chemotherapy (mitoxantrone and doxorubicin, 1 μg/mL; P < .01). (D) Raji cells were treated with anti-CD20 mAbs or mitoxantrone as described above and Western blot analysis was performed for cleaved caspase 3 (CC3). No CC3 was observed following anti-CD20 mAb treatment (Ctl indicates control mAb; GA, GA101; Rit, rituximab; and Mtx, mitoxantrone).
GA101-induced cell death is independent of DNA fragmentation, caspase activation, and BCL-2 overexpression. (A) Raji cells were treated with anti-CD20 mAb (10 μg/mL) or staurosporine (STSP, 2μM), a positive control for apoptosis, for 24 hours and DNA fragmentation was assessed using TUNEL staining analyzed by flow cytometry. Mean ± SEM of 3 independent experiments is shown on the left, with representative plots on the right. GA101 does not induce significant DNA fragmentation. (B) Wild-type Raji (left) and Raji cells that overexpress the antiapoptotic protein BCL-2, (Raji-BCL2; right), were preincubated with DMSO or Q-VD-OPH (20μM) for 30 minutes, then treated with anti-CD20 mAbs (10 μg/mL) or mitoxantrone (1 μg/mL), and cell death measured 48 hours later. Mean + SEM of 4 independent experiments are shown. Neither BCL-2 overexpression, caspase inhibition, nor a combination of both, had any impact on cell death induced by GA101, despite inhibiting mitoxantrone-induced apoptosis (P < .008). (C) Raji and Raji-BCL2 cells were treated as in panel B and growth inhibition assessed using the XTT assay as described in “Cell death and cell viability assays” 48 hours after treatment, with absorbance normalized relative to untreated cells. Mean + SEM of 4 independent experiments are shown. Caspase inhibition and BCL-2 overexpression had no impact on GA101-induced growth inhibition, both of which significantly attenuated growth inhibition induced by chemotherapy (mitoxantrone and doxorubicin, 1 μg/mL; P < .01). (D) Raji cells were treated with anti-CD20 mAbs or mitoxantrone as described above and Western blot analysis was performed for cleaved caspase 3 (CC3). No CC3 was observed following anti-CD20 mAb treatment (Ctl indicates control mAb; GA, GA101; Rit, rituximab; and Mtx, mitoxantrone).
Therefore, we next investigated whether GA101-induced death was independent of typical apoptotic regulation. First, we assessed the sensitivity of Raji cells transfected to overexpress the antiapoptotic protein BCL-2,24 and showed that BCL-2 overexpression had no effect on cell death evoked by GA101, despite potently inhibiting apoptosis induced by mitoxantrone (P < .008). Second, caspase activity was blocked using the pan-caspase inhibitor Q-VD-OPH, which inhibited mitoxantrone-induced apoptosis (P < .005) but had no impact on GA101-induced cell death (Figure 4B). Furthermore, the combination of caspase inhibition (using Q-VD-OPH) and BCL-2 overexpression was unable to block cell death induced by GA101 (Figure 4B). Similarly, neither BCL-2 overexpression, nor caspase inhibition, nor both combined, had any effect on GA101-induced growth inhibition as detected by the XTT assay, despite partially rescuing growth of cells treated with mitoxantrone or doxorubicin (P < .01, Figure 4C). Finally, Western blot analysis confirmed the absence of caspase-3 processing, one of the terminal executioner caspases, following treatment with GA101 (Figure 4D). Taken together, these data show that GA101 induces a nonapoptotic form of cell death, even in cells rendered resistant to chemotherapy-induced apoptosis.
The role of lysosomes in GA101-induced cell death
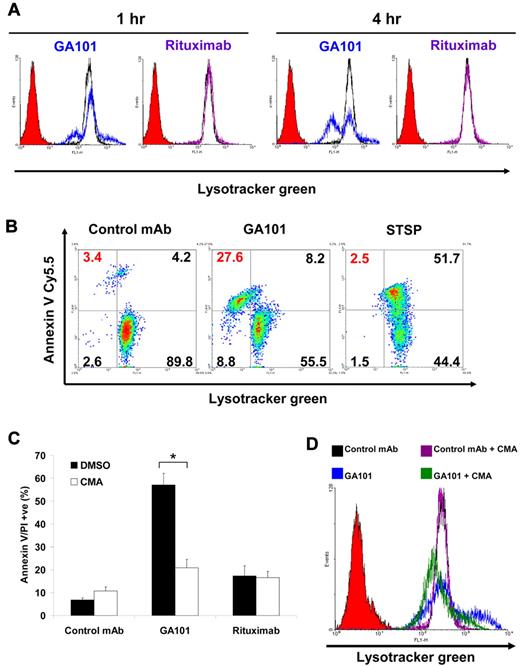
It is now well established that lysosomes play a vital role in inducing nonapoptotic cell death, triggered through LMP.23 Therefore, we examined the role of lysosomes in GA101-induced cell death by labeling Raji cells with the lysosome-specific dye, Lysotracker green. GA101 induced a small increase in Lysotracker staining within 1 hour of treatment, followed by a decrease in staining in a subpopulation of cells at 4 hours. These findings suggest that GA101 induces an initial enlargement of lysosomes followed by subsequent collapse. In contrast, rituximab induced no changes in Lysotracker staining (Figure 5A). To correlate the changes in the lysosomal compartment with cell death, cells were labeled with Lysotracker green then stained with annexin V–Cy5.5 to label dead cells. These experiments revealed that the loss of Lysotracker staining coincides with cell death, suggesting that lysosomal collapse and cell death are linked (Figure 5B). In contrast, cells that have undergone apoptosis after staurosporine treatment showed no decrease in Lysotracker staining confirming that lysosomal collapse is a characteristic feature of GA101-induced cell death. Because vacuolar ATPases are proton pumps that activate and acidify intracellular membrane-bound vesicles including lysosomes, we next examined their role in GA101-induced cell death. Pretreatment of Raji cells with concanamycin A, a potent and specific inhibitor of vacuolar ATPases, significantly attenuated the cell death (P < .001) and the increase in Lysotracker staining induced by GA101 (Figure 5C-D). Similarly, GA101-induced cell growth inhibition in the absence of disaggregation was attenuated by concanamycin A (supplemental Figure 7). Collectively, these findings indicate that GA101-induced cell death is associated with a collapse of the lysosomal compartment and is dependent on vacuolar ATPases.
Lysosomal changes associated with GA101-induced cell death. (A) To determine changes in lysosomal volume, Raji cells were treated with mAbs (10 μg/mL) for 1 or 4 hours, labeled with Lysotracker green (75nM) and analyzed by flow cytometry. Histograms represent cells treated with control mAb (black), GA101 (blue), rituximab (purple), and unlabeled cells to set the background (solid red). GA101 induced an enlargement of the lysosomal compartment at 1 hour and a subsequent collapse in a subpopulation of the cells at 4 hours, whereas rituximab induced no changes in lysosomal volume. (B) To directly correlate cell death with lysosomal volume, cells were treated with mAbs. After 24 hours, cells were costained with Lysotracker green and annexin V Cy5.5 to label the dead cell population and analyzed by flow cytometry. Staurosporine (STSP) was used as a positive control for apoptosis. Cell death evoked by GA101 was associated with a collapse of the lysosomal compartment (upper left quadrant, in red). (C) Cells were preincubated with the V-ATPase inhibitor concanamycin A (CMA, 100nM) before treatment with mAbs and death was analyzed 24 hours after treatment. CMA significantly inhibited cell death induced by GA101 (*P < .001). (D) Cells were treated as above and lysosomal volume was quantified using Lysotracker green staining 1 hour after treatment. Histograms represent cells treated with control mAb (black), control mAb + CMA (purple), GA101 (blue), GA101 + CMA (green), and background (solid red). CMA prevents the increase in lysosomal volume induced by GA101.
Lysosomal changes associated with GA101-induced cell death. (A) To determine changes in lysosomal volume, Raji cells were treated with mAbs (10 μg/mL) for 1 or 4 hours, labeled with Lysotracker green (75nM) and analyzed by flow cytometry. Histograms represent cells treated with control mAb (black), GA101 (blue), rituximab (purple), and unlabeled cells to set the background (solid red). GA101 induced an enlargement of the lysosomal compartment at 1 hour and a subsequent collapse in a subpopulation of the cells at 4 hours, whereas rituximab induced no changes in lysosomal volume. (B) To directly correlate cell death with lysosomal volume, cells were treated with mAbs. After 24 hours, cells were costained with Lysotracker green and annexin V Cy5.5 to label the dead cell population and analyzed by flow cytometry. Staurosporine (STSP) was used as a positive control for apoptosis. Cell death evoked by GA101 was associated with a collapse of the lysosomal compartment (upper left quadrant, in red). (C) Cells were preincubated with the V-ATPase inhibitor concanamycin A (CMA, 100nM) before treatment with mAbs and death was analyzed 24 hours after treatment. CMA significantly inhibited cell death induced by GA101 (*P < .001). (D) Cells were treated as above and lysosomal volume was quantified using Lysotracker green staining 1 hour after treatment. Histograms represent cells treated with control mAb (black), control mAb + CMA (purple), GA101 (blue), GA101 + CMA (green), and background (solid red). CMA prevents the increase in lysosomal volume induced by GA101.
GA101 induces lysosomal membrane permeabilization and cathepsin-mediated cell death
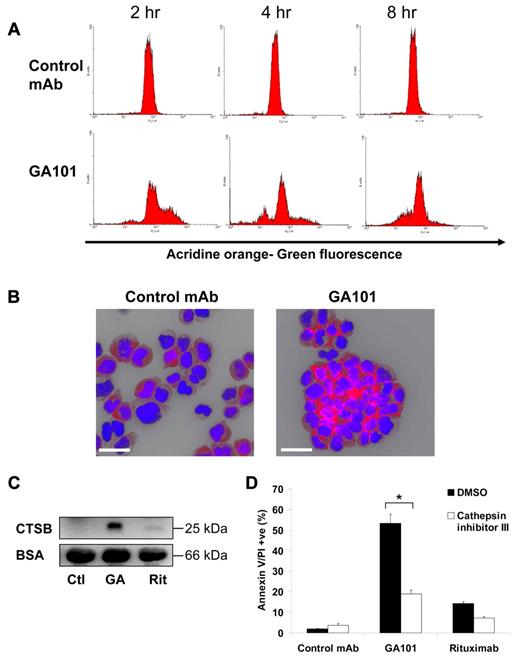
Having established that GA101-induced cell death is associated with lysosomal changes, we investigated the ability of GA101 to trigger LMP and release of lysosomal contents into the cytosol, which is the hallmark feature of lysosomal cell death. Lysosomes were labeled with AO before treatment of cells with mAb according to a well-established method.27,28 When AO leaks into the more pH-neutral cytosol, it results in an increase in green fluorescence which can be detected using flow cytometry. GA101 induced an increase in AO green fluorescence within 2 hours after treatment indicating LMP. This was followed by a subsequent decrease in green fluorescence at 4 and 8 hours, likely because of the extracellular release of lysosomal contents secondary to plasma membrane permeabilization (Figure 6A). To confirm the release of lysosomal contents into the cytosol, immunofluorescence staining of cathepsin B, a classic lysosomal protease, was performed on GA101-treated cells. Large amounts of cathepsin B were deposited into the cytosol and surrounding points of cellular adhesion (Figure 6B). Interestingly, immunofluorescence staining of lysosome-associated membrane protein-1 (LAMP-1) revealed a similar staining pattern, suggesting relocalization of lysosomes toward points of cellular adhesion (supplemental Figure 8). Furthermore, marked levels of cathepsin B were detected in the supernatant of GA101-treated Raji cells (Figure 6C), consistent with the decrease in AO green fluorescence, confirming the extracellular release of lysosomal contents following plasma membrane permeabilization. Finally, the importance of cathepsins in the cell death process was assessed using a specific inhibitor, which markedly inhibited cell death by GA101 (P < .001, Figure 6D). Altogether, these data demonstrate that GA101 induces LMP and cathepsin B release, which is required for GA101-induced cell death.
The role of lysosomal membrane permeabilization and lysosomal cathepsins in GA101-induced cell death. (A) Raji cells were incubated with acridine orange (AO) to label lysosomes, washed twice, treated with mAbs, and analyzed at different time points. Leakage of lysosomal contents into the cytoplasm was measured as an increase in green fluorescence detected by FACS. GA101 induced an increase in green fluorescence at 2 hours, followed by a subsequent loss of fluorescence at 4 and 8 hours. (B) Fluorescence microscopy of the lysosomal protease cathepsin B staining (red) of Raji cells 4 hours after treatment with mAbs. DNA was counterstained with DAPI (blue; scale bar, 40 μm). GA101 induces marked cathepsin B release into the cytosol and surrounding points of cellular adhesion. (C) Western blot of the active 25-kDa subunit of cathepsin B (CTSB) in cell supernatants 4 hours after treatment with mAbs. BSA was used as a loading control on the same supernatants diluted 1:5. GA101 evokes extracellular cathepsin B release (Ctl indicates control mAb; GA, GA101; and Rit, rituximab). (D) Cells were preincubated with cathepsin inhibitor III (100μM) before treatment with mAbs and cell death was analyzed 4 hours after treatment. Figure shows mean + SEM of triplicates, representative of 2 independent experiments. Cathepsin inhibitor III inhibits GA101-induced cell death (*P < .001).
The role of lysosomal membrane permeabilization and lysosomal cathepsins in GA101-induced cell death. (A) Raji cells were incubated with acridine orange (AO) to label lysosomes, washed twice, treated with mAbs, and analyzed at different time points. Leakage of lysosomal contents into the cytoplasm was measured as an increase in green fluorescence detected by FACS. GA101 induced an increase in green fluorescence at 2 hours, followed by a subsequent loss of fluorescence at 4 and 8 hours. (B) Fluorescence microscopy of the lysosomal protease cathepsin B staining (red) of Raji cells 4 hours after treatment with mAbs. DNA was counterstained with DAPI (blue; scale bar, 40 μm). GA101 induces marked cathepsin B release into the cytosol and surrounding points of cellular adhesion. (C) Western blot of the active 25-kDa subunit of cathepsin B (CTSB) in cell supernatants 4 hours after treatment with mAbs. BSA was used as a loading control on the same supernatants diluted 1:5. GA101 evokes extracellular cathepsin B release (Ctl indicates control mAb; GA, GA101; and Rit, rituximab). (D) Cells were preincubated with cathepsin inhibitor III (100μM) before treatment with mAbs and cell death was analyzed 4 hours after treatment. Figure shows mean + SEM of triplicates, representative of 2 independent experiments. Cathepsin inhibitor III inhibits GA101-induced cell death (*P < .001).
Discussion
We have examined the extent and the mode of PCD elicited by the next-generation humanized, glycoengineered type II anti-CD20 mAb GA101. Several important observations have arisen from this work. First, GA101 induced significantly larger amounts of direct PCD than rituximab and ofatumumab in both cell lines and primary B-cell tumors. Second, actin reorganization was critical for GA101-induced cell death. Third, cell death evoked by GA101 was independent of BCL-2 and caspases, and could circumvent resistance to apoptosis. Finally, GA101-induced cell death was mediated by lysosomes undergoing LMP, confirming that this mode of death is a defining feature of type II anti-CD20 mAbs.22
Our finding of the superiority of GA101 in inducing direct cell death is in agreement with the recently published study by Mossner et al.17 Furthermore, we have extended this observation to primary B-cell malignancies, including B-CLL, in which GA101 was more potent in its specific ability to elicit direct PCD than rituximab and also ofatumumab, a fully human next-generation anti-CD20 mAb now licensed for the treatment of fludarabine and alemtuzumab-refractory B-CLL.33 However, the contribution of the enhanced PCD ability of GA101 to overall anti-CD20 mAb efficacy compared with other anti-CD20 mAb effector mechanisms, such as CDC (enhanced in ofatumumab), has not yet been determined in primary B-cell malignancies, and is likely to vary depending on the disease, the host, the location and nature of malignant B cells. Such assessment of the relative contribution of the different anti-CD20 mAb effector mechanisms to overall mAb therapeutic efficacy can only be made in vivo and ultimately will be determined in randomized clinical trials.
Interestingly, the differences in the ability of anti-CD20 mAbs to evoke PCD cannot be explained by the epitope of CD20 they recognize because both GA101 and rituximab bind closely overlapping epitopes within the large extracellular loop, whereas ofatumumab binds a different region.34 This suggests that it is more likely that the orientation and kinetics of binding dictate the ability of anti-CD20 mAbs to induce PCD. In this regard, recently published crystal structure studies demonstrated that GA101 binds CD20 in a completely different orientation than rituximab or ofatumumab.35
Although GA101 induced substantially higher levels of direct PCD compared with the type I anti-CD20 mAbs, a subpopulation of cells remains unaffected. This observation may reflect the nature of this novel cell death phenomenon, as it is consistent with previous studies investigating direct PCD induced by anti-CD20 mAbs.16,29,36,37 Here, we have demonstrated that this subpopulation of viable cells, on isolation or recovery, is equally sensitive to GA101-induced PCD compared with previously untreated cells, suggesting that this subpopulation does not acquire resistance to GA101 killing.
Our study provides further insights into the mechanism of PCD evoked by GA101, showing that HA plays an important role in the process. Importantly, HA is not a consequence of spontaneous mAb aggregation because of Fc-Fc binding as F(ab)′2 fragments of GA101 were equally effective as GA101 IgG at inducing HA and PCD. However, bivalency appears to be the minimum requirement for this process as previous studies have demonstrated that univalent Fab′ fragments of type II anti-CD20 mAbs do not induce PCD in contrast to F(ab)′2 fragments,29,38 which is likely to be the case with GA101. Anti-CD20 mAb-induced HA was previously shown to be independent of lymphocyte function-associated Ag-1 and integrins,39 and therefore, the cell-surface adhesion receptor that mediates this phenomenon is yet to be determined.
We have demonstrated that the peripheral relocalization of actin toward cell-cell junctions was critical for both HA and cell death induced by GA101. The actin cytoskeleton was shown to be essential for HA and PCD triggered by mAbs targeting various Ags including anti-HLA DR in our system,22 anti-CD99 in Ewing sarcoma32 and anti-CD47 in B-CLL.40 Interestingly, cell death induced by these mAbs was caspase independent, implying that actin-mediated HA triggers nonapoptotic cell death. Consistent with these findings, GA101-induced cell death was independent of BCL-2 overexpression and caspase activation, and was not associated with DNA fragmentation, a hallmark feature of apoptosis. However, it remains unknown why the engagement of certain cell-surface Ags by mAb triggers such profound actin relocalization. Nevertheless, we have observed marked relocalization of CD20 and HLA DR to cell-cell junctions during HA induced by GA101 and anti-HLA DR mAb, respectively (W.A., unpublished observations, May 2010). The rapid relocalization of actin along with cell-surface Ags observed resembles the organization of supramolecular activation clusters (SMACs) at the site of Ag presentation during heterotypic adhesion between lymphocytes and APCs.21 In B cells, the formation of this “immunologic synapse” after B-cell receptor activation was shown to be mediated by Rho-family kinases, which are important regulators of the actin cytoskeleton.41 Therefore, it is plausible that CD20 ligation induces its cytoskeletal rearrangements through the activation of Rho-family kinases to trigger HA and cell death, and this hypothesis forms the subject of ongoing investigation.
An important finding to emerge from this work is that lysosomes play a critical role in triggering GA101-induced cell death. Lysosomes function as cellular recycling and waste disposal units by degrading organelles and macromolecules delivered to the lysosomal compartment by autophagy, endocytosis, or phagocytosis using over 50 lysosomal hydrolases.42 However, on disruption of their membranes during LMP, the release of lysosomal hydrolases into the cytosol, particularly cathepsin proteases, can trigger nonapoptotic cell death.43 Our findings demonstrate that GA101 induced an enlargement of the lysosomal compartment followed by LMP associated with cathepsin B release into the cytosol and toward points of cell-cell contact, causing cathepsin-dependent cell death. Furthermore, inhibition of vacuolar ATPases, which serve to activate acidic vacuoles including lysosomes, significantly attenuated cell death. Although the role of autophagy in GA101-induced cell death cannot be excluded by these experiments, we have previously demonstrated cell death induced by the type II anti-CD20 mAb tositumomab was independent of the autophagy regulatory genes atg12 and beclin-1,22 and thus it seems unlikely that autophagy is required for this mode of cell death. Further work is required to establish the mechanism through which HA and actin redistribution trigger LMP. However, because the actin cytoskeleton is required for lysosomal trafficking, it is conceivable that the rapid relocalization of actin toward points of cell-cell adhesion rapidly drives lysosomes to this region, damaging their membranes in the process. In support of this hypothesis, we have observed the relocalization of lysosomes toward cell-cell junctions as detected by LAMP-1 staining, along with diffuse cathepsin B staining at the cell-cell interface, indicative of LMP.44
Our results suggest that by using type II anti-CD20 mAb such as GA101 it may be possible to exploit this direct, actin-dependent, lysosomal-mediated PCD as an additional tumor cell death pathway in mAb therapy. Applying this type of mAb-induced cell death in B-cell malignancies has potentially important advantages to translate to the clinic. First, because of its Fc-independent nature, it can provide a new therapeutic option in patients in which Fc-FcγR–dependent mechanisms are impaired, such as patients with low-affinity FcγRIIIa, or patients with immune effector cell failure that is intrinsic or secondary to depletion by chemotherapy regimes. In particular, the latter subset of patients may not benefit from mAb design focused exclusively on enhancing Fc-FcγR interactions if their FcγR-expressing cells are deficient. Second, through its ability to bypass the apoptotic machinery it may help to eradicate tumors which are resistant to apoptosis. Therefore, GA101 may provide a potential therapeutic opportunity for patients suffering from B-cell malignancies with defects in apoptosis, which have been shown to be predictive of a poor response or relapse to not only chemotherapy, but also immunochemotherapy. Such defects in the apoptotic machinery leading to impaired responses to rituximab-containing immunochemotherapy include overexpression of the transcriptional repressor BCL-6 in DLBCL,45 as well as overexpression of the BCL-2 family member MCL-146 and loss of the tumor suppressor TP53 in B-CLL.3,47,48 Indeed, recent studies have shown that the activation of nonapoptotic cell death programs can eradicate various apoptosis-resistant malignancies including hormone-refractory prostate cancer49 and glucocorticoid-resistant acute lymphoblastic leukemia.50 Furthermore, because LMP is central to cell death induced by GA101, it will be interesting to examine whether agents that induce lysosomal destabilization will be able to further sensitize tumor cells to GA101-induced cell death, through novel combination therapies. The most clinically relevant of these “lysosomal-destabilizing” agents is vincristine,51 which forms a component of numerous routine chemotherapy regimens used in lymphomas, with or without rituximab.
In conclusion, we have demonstrated that the type II anti-CD20 mAb GA101 can induce direct actin-dependent, lysosomal cell death. This additional mechanism of action for anti-CD20 mAb offers hope not only for those patients with rituximab-resistant disease but that the efficacy of anti-CD20 mAb can be improved further and encourages clinical development and investigation in exploiting this direct death pathway.
Part of this work was presented orally at the 51st Annual Meeting of the American Society of Hematology, New Orleans, LA, December 7, 2009.52
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to C. Klein and P. Umana (Roche, Glycart AG) for providing GA101. We thank Steven Bagley, Achille Dunne, and Aleksandr Mironov (University of Manchester) for their assistance and expertise in imaging studies. We also thank the Manchester Cancer Research Center Biobank, Mrs Deepti Wilks and Dr Adrian Bloor (Christie Hospital NHS Trust) for facilitating the provision and preparation of patient samples. Furthermore, we thank Mrs Debbie Burt (Clinical Immunotherapy laboratory, Paterson Institute for Cancer Research, University of Manchester) for her assistance with the chromium assays, and Michael Lim for critical reading of the manuscript.
This work was supported by fellowships and grants from Cancer Research UK (CRUK), the Ministry of Higher Education of Kuwait, the Medical Research Council (MRC), the Sankyo Foundation for Life Sciences, and Leukemia & Lymphoma Research UK.
Authorship
Contribution: W.A., A.I., M.S.C., and T.M.I. initiated the studies; W.A., A.I., J.H., E.C., S.P., S.H.L., K.S., A.T., and S.A.B. performed experiments; C.H.T.C. and A.T. provided vital novel reagents; W.A., A.I., J.H., S.A.B., M.J.G., M.S.C., and T.M.I. analyzed the data; W.A. wrote the paper; and A.I., J.H., E.C., S.A.B., M.J.G., M.S.C., and T.M.I. edited the paper.
Conflict-of-interest disclosure: T.M.I. has received honorarium for consultancy work with Hoffman La Roche. The remaining authors declare no competing financial interests.
Correspondence: Tim Illidge, School of Cancer and Enabling Sciences, Manchester Academic Health Science Centre, University of Manchester, Manchester, M20 4BX, United Kingdom; e-mail: tmi@manchester.ac.uk.