Abstract
Acute promyelocytic leukemia (APL) is a distinct subtype of acute myeloid leukemia that results from the expression of the promyelocytic leukemia–retinoic acid receptor α (PML-RAR-α) oncoprotein. It is characterized by severe hemorrhagic complications due in part to excessive fibrinolysis, resulting from the excessive generation of the fibrinolytic enzyme, plasmin, at the cell surface of the PML cells. The treatment of patients with all-trans retinoic acid (ATRA) effectively ameliorates the disease by promoting the destruction of the PML-RAR-α oncoprotein. In the present study we show for the first time that the plasminogen receptor, S100A10, is present on the extracellular surface of APL cells and is rapidly down-regulated in response to all-trans retinoic acid. The loss of S100A10 is concomitant with a loss in fibrinolytic activity. Furthermore, the induced expression of the PML-RAR-α oncoprotein increased the expression of cell surface S100A10 and also caused a dramatic increase in fibrinolytic activity. Depletion of S100A10 by RNA interference effectively blocked the enhanced fibrinolytic activity observed after induction of the PML-RAR-α oncoprotein. These experiments show that S100A10 plays a crucial role in the generation of plasmin leading to fibrinolysis, thus providing a link to the clinical hemorrhagic phenotype of APL.
Introduction
Acute promyelocytic leukemia (APL) is a disease characterized by a single genetic defect, namely fusion of the retinoic acid receptor gene (RARA) (located on 17q21) with the promyelocytic leukemia (PML) gene (located on 15q21) via a t(15;17) translocation, resulting in the expression of a PML-RAR-α fusion protein and clonal expansion of immature promyelocytes.1-4 APL accounts for ∼ 10% of acute myeloid leukemia in children.5-7 Peak incidence of the disease occurs in young adults and is characterized by the accumulation of abnormal (leukemic) promyelocytes in the bone marrow.
A defining characteristic of APL is the life-threatening coagulopathy associated with the disease. Before the introduction of all-trans retinoic acid (ATRA) therapy, APL was associated with a high incidence of hemorrhagic death, specifically because of intracranial and pulmonary hemorrhage. Since the introduction of ATRA therapy, disease-free survival and overall survival has improved; however, induction mortality remains a problem and hemorrhage still accounts for most early deaths.8 For example, a recent study reported that among 134 patients treated with induction chemotherapy with ATRA and anthracyclines, 32% died early, with hemorrhagic death accounting for 21% of all patients.8 The hemorrhagic diathesis associated with APL is thought to result from excessive fibrinolysis through up-regulation of proteins that activate the generation of the fibrinolytic enzyme, plasmin, at the cell surface of the PML cells.9,10
Plasminogen, the precursor of plasmin, circulates in the blood in an inactive form but, when converted by its activators, tissue plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA), to plasmin, acquires the ability to digest fibrin clots, a process called fibrinolysis. Overproduction of this fibrinolytic enzyme may cause abnormal bleeding. Clinical evidence, such as increased levels of circulating thrombin-antithrombin III complexes, prothrombin fragments 1 and 2, D-Dimer complexes, low plasma levels of plasminogen, α2-plasmin inhibitor, and plasmin-activator inhibitor 1, is consistent with excessive fibrinolysis.11-15
The assembly of plasminogen and its activators on the cell surface is facilitated by the protein, S100A10 (also referred to as p11). S100A10 is a member of the S100 family of calcium-binding proteins and is typically found in most cells bound to its annexin A2 (p36) ligand as the heterotetrameric (S100A10)2-(annexin A2)2 complex, AIIt.16,17 The S100A10 subunit of AIIt possesses a carboxyl-terminal lysine residue that binds tPA and plasminogen, resulting in the conversion of plasminogen to plasmin.18 The binding of plasmin to AIIt protects plasmin from inactivation by α2-antiplasmin.19 Removal of the carboxyl-terminal lysines from S100A10 by carboxypeptidase B results in the loss of plasminogen binding and plasmin generation.20 Loss of S100A10 from the extracellular surface of cancer cells significantly reduces plasmin generation, thus dramatically effecting the cells' capacity to degrade extracellular matrix and infiltrate into surrounding tissue.21 In contrast, increased extracellular levels of S100A10 result in increased plasminogen binding and increased production of plasmin.21
In this current study we investigated the role that S100A10 plays in the regulation of fibrinolysis at the surface of APL cells. Our studies indicate that S100A10 is the main plasminogen regulator on the surface of APL cells and that treatment of APL cells with ATRA results in the loss of cell surface S100A10 and a reduction in the fibrinolytic activity of APL cells. Furthermore, we show for the first time that the induced expression of the PML-RAR-α fusion protein results in a rapid increase in the cell surface levels of annexin A2 and S100A10 concomitant with increased fibrinolysis. Interestingly, when S100A10 expression is selectively blocked in PML-RAR-α–expressing cells, fibrinolysis is dramatically decreased despite the presence of elevated annexin A2 levels.
Methods
A detailed description of the routine methods is presented in the supplemental Data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article. Only nonroutine procedures and specialized materials are described here.
Cell culture
NB4 cells (DSMZ) were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% FCS. The engineered U937/PR9 cells, which express PML-RAR-α under the control of the zinc-inducible promoter (kindly provided by Miller et al, Lady Davis Institute for Medical Research, Sir Mortimer Davis Jewish General Hospital, Montreal, QC)22 , U937, and SN4 cells were maintained in DMEM (Invitrogen) supplemented with 10% FCS. Cells were maintained at a cell density of 0.3-1.0 × 106 cells/mL.
Plasmids
pSUPER plasmids are described in detail in the supplemental Methods.
Transfections
To establish S100A10 and annexin A2 knockdown cell lines we first transfected Phoenix packaging cells plated in 25-cm3 flasks with 4 μg of the pSUPER-retro plasmids with 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection the target cell lines (NB4 and PR9) were infected with the Phoenix supernatants. Forty-eight hours after infection the S100A10 knockdown cells were selected with 2 μg/mL puromycin for ≥ 1 week.
Treatment with ATRA
Cells in the exponential growth phase were used to start the suspension culture. Before exposure to ATRA, cell density was adjusted to 3-5 × 105/mL. Stock solutions of ATRA (Sigma-Aldrich) were diluted in ethanol (Sigma-Aldrich) and added to medium to reach the final working concentration (1μM). Cells were grown in medium containing 1μM ATRA for 120 hours with samples taken at various times. Control medium had the same amount of ethanol added. Cell density was kept at < 1 × 106/mL throughout the experiment.
Induction of PR9 cells
PR9 and SN4 cells (3-5 × 105 cells/mL) were treated with 100μM ZnSO4 (Sigma-Aldrich) daily until PML-RAR-α protein expression was confirmed by Western blotting. Cell density was maintained at < 1 × 106 cells/mL for the duration of the experiment.
Analysis of protein expression
Proteins were analyzed by Western immunoblot, flow cytometry, and cell surface biotinylation as described in detail in the supplemental Methods.
Analysis of gene expression
S100A10 and annexin A2 gene expression was analyzed by reverse transcription and quantitative real-time PCR as described in the supplemental Methods.
Matrigel invasion and cell migration
Cells were loaded (1 × 105) into the upper portion of Transwell chambers, coated with Matrigel or Fibrin (BD Biosciences). Plasminogen (0.5μM; American Diagnostica) in serum-free RPMI was added to the upper chamber. As a chemoattractant, complete RPMI was added to the lower chamber. After 48 hours, cells on both the underside of the membrane and in the media in the lower chamber were collected and counted with a hemocytometer.
Plasminogen activation
Cells (1 × 106) were plated in 96-well plates. Cells were incubated with or without uPA (50nM) for 10 minutes at room temperature, washed with incubation buffer (HBSS containing 3mM CaCl2 and 1mM MgCl2) and incubated with 0.5μM glu-plasminogen for 10 minutes before adding 500μM plasmin substrate S2251 (Chromogenix; Diapharma Group). The rate of plasmin generation was measured at 405 nm.
Plasminogen binding assays
Preparation of FITC-plasminogen is described in the supplemental Methods. Cells were washed and cultured in the absence of serum for 2 hours before assay. Cells were incubated with 200nM FITC Glu-plasminogen, either with or without ϵ-aminocaproic acid (ϵ-ACA; 100mM; a synthetic inhibitor of the plasminogen system), for 1 hour at 4°C in HBSS (1mM MgCl2 and 3mM CaCl2). Plasminogen binding was measured by FACS, excluding cells that were positive for propidium iodide (Sigma-Aldrich). Percent-binding refers to the percentage of specific plasminogen binding.
Statistics
Statistical significance was determined by Student t test or 1-way ANOVA with Tukey multiple comparisons. Results were regarded as significant if 2-tailed P values were < .05. All data are expressed as mean ± SD.
Results
ATRA treatment of NB4 cells results in down-regulation of S100A10
Blood from patients with APL showed increased fibrinolysis that is ameliorated by ATRA treatment. It was originally proposed that the ATRA-dependent down-regulation of cell surface annexin A2 was responsible for the reversal of abnormal fibrinolysis in APL.23 Because the fibrinolytic regulatory protein, S100A10, is anchored to the cell surface through its tight binding to annexin A2, we examined whether ATRA treatment also affected the levels of S100A10. For these experiments we used the well-characterized NB4 APL cell line. NB4 cells were treated with 1μM ATRA for ≤ 5 days. Cell lysates were collected at various times (0-96 hours), and protein expression of annexin A2 and S100A10 were examined by immunoblotting. ATRA treatment of NB4 cells resulted in a loss in expression of both annexin A2 and S100A10 protein by 48 hours, whereas vehicle-treated cells were unaffected (Figure 1A). Interestingly, the loss of total cellular S100A10 occurred earlier than annexin A2. Expression of S100A10 and annexin A2 mRNA was unaffected by ATRA treatment (supplemental Figure 1), suggesting that ATRA did not affect the transcription of these proteins. Flow cytometric analysis showed that after ATRA treatment for 72 hours S100A10 cell surface expression was decreased 80% (P < .001) (Figure 1B), whereas the cell surface expression of annexin A2 was only reduced by 20% (P < .01) (Figure 1C). After 5 days of treatment with ATRA, cell surface proteins were biotinylated and precipitated with streptavidin. Western blot analysis confirmed that S100A10 and annexin A2 were dramatically reduced (Figure 1D). In contrast, 2 other well-established myeloid cell fibrinolytic regulatory proteins, α-enolase and histone H2B, were unchanged or not detectable, respectively, after ATRA treatment (Figure 1D).
ATRA decreases cell surface S100A10 levels. NB4 cells were treated with 1μM ATRA or a vehicle control for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, or α-enolase were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 (B) and annexin A2 (C) in ATRA-treated NB4 cells. Cells were incubated with anti-S100A10 and anti–annexin A2 antibodies. Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in NB4 cells was calculated with WinMDI software (**P < .01 and ***P < .001). NB4 cells treated with ATRA for 5 days were incubated with Sulfo-NHS-SS-biotin and lysed, and the biotinylated (cell surface) proteins were collected with streptavidin beads and subjected to SDS-PAGE and Western blotting for S100A10, annexin A2 (D).
ATRA decreases cell surface S100A10 levels. NB4 cells were treated with 1μM ATRA or a vehicle control for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, or α-enolase were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 (B) and annexin A2 (C) in ATRA-treated NB4 cells. Cells were incubated with anti-S100A10 and anti–annexin A2 antibodies. Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in NB4 cells was calculated with WinMDI software (**P < .01 and ***P < .001). NB4 cells treated with ATRA for 5 days were incubated with Sulfo-NHS-SS-biotin and lysed, and the biotinylated (cell surface) proteins were collected with streptavidin beads and subjected to SDS-PAGE and Western blotting for S100A10, annexin A2 (D).
NB4 cells exhibit decreased plasminogen binding, plasmin generation, and fibrinolysis after treatment with ATRA
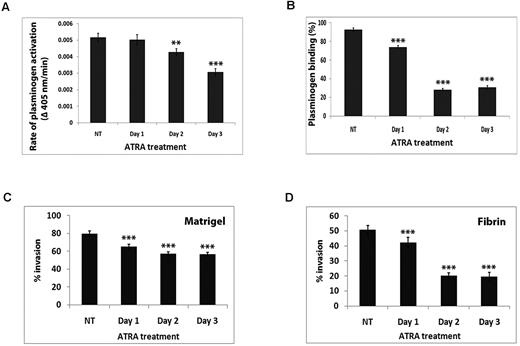
S100A10 can account for the generation of ≤ 90% of the cell surface plasmin generation of some cells.21 Therefore, we compared the rates of plasmin generation by NB4 cells before and after ATRA treatment. After 24 hours there was no significant reduction in plasmin generation, but after 48 hours we observed a 20% reduction (P < .001; Figure 2A) in plasmin generation by the NB4 cells treated with ATRA compared with vehicle treatment. Furthermore, the rate of plasmin generation was reduced by 40% after 72 hours of treatment (Figure 2A).
ATRA-dependent loss of cellular S100A10 corresponds to decreased cellular plasminogen binding, plasmin generation, and invasive capability of APL cells. NB4 cells were treated with ATRA for the indicated times then incubated with uPA (50nM) for 10 minutes at room temperature. Cells were washed and incubated with plasminogen (0.5μM) followed by the addition of the plasmin substrate, S2251. The rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (A). ATRA-treated NB4 cells were incubated with 200nM FITC plasminogen for 1 hour at 4°C, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (B). ATRA-treated NB4 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (C) or fibrin-coated (D) chambers in the absence of FBS that also contained plasminogen (0.5μM). The lower chamber contained FBS as chemoattractant. Data are expressed as the mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by one-way ANOVA (with Tukey multiple comparisons) in comparison to untreated NB4 cells (NT; ***P < .001).
ATRA-dependent loss of cellular S100A10 corresponds to decreased cellular plasminogen binding, plasmin generation, and invasive capability of APL cells. NB4 cells were treated with ATRA for the indicated times then incubated with uPA (50nM) for 10 minutes at room temperature. Cells were washed and incubated with plasminogen (0.5μM) followed by the addition of the plasmin substrate, S2251. The rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (A). ATRA-treated NB4 cells were incubated with 200nM FITC plasminogen for 1 hour at 4°C, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (B). ATRA-treated NB4 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (C) or fibrin-coated (D) chambers in the absence of FBS that also contained plasminogen (0.5μM). The lower chamber contained FBS as chemoattractant. Data are expressed as the mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by one-way ANOVA (with Tukey multiple comparisons) in comparison to untreated NB4 cells (NT; ***P < .001).
S100A10 binds plasminogen (Kd, 1.8μM) by a mechanism that involves the interaction of the carboxyl-terminal lysine residue of S100A10 with the kringle domains of plasminogen.21 The contribution of S100A10 to the plasminogen binding capacity NB4 cells was evaluated by FACS by comparing the amount of plasminogen bound with the surface of vehicle-treated and ATRA-treated cells. ATRA treatment for 48 hours resulted in a 69% decrease (P < .01) in plasminogen binding (Figure 2B). The loss in plasminogen binding was maximal after 48 hours, and further loss in plasminogen binding was not observed after 72 hours of ATRA treatment (Figure 2B).
To assess the effect of ATRA on the proteolytic activity of NB4 cells, we performed in vitro invasion assays with the use of a modified Boyden chamber. ATRA-treated NB4 cells were placed in the upper chamber, the insert between chambers was coated with Matrigel or fibrin, and a chemoattractant stimulus, FBS, was added to the lower chamber. Because the cells must proteolyze the Matrigel or fibrin barrier to reach the lower chamber, this assay gives a measure of the proteolytic (Matrigel barrier) or fibrinolytic (fibrin barrier) activity of the cells. We observed that 18% fewer NB4 cells (P < .001) migrated across the Matrigel barrier after treatment with ATRA for 1 day in comparison to vehicle-treated NB4 cells (65% ± 2.75% after 1 day of ATRA treatment, and 79.8% ± 2.71% in vehicle-treated NB4 cells; n = 3; Figure 2C). Maximal effect was observed after treatment for 2 days, at which time 29% fewer ATRA-treated NB4 cells migrated across a Matrigel barrier in comparison to vehicle-treated NB4 cells (57% ± 2.45% after 1 day of treatment, and 79.8% ± 2.71% in vehicle-treated NB4 cells; n = 3; Figure 2C). ATRA treatment had a more dramatic effect on migration across the fibrin barrier. We observed that in comparison to vehicle-treated NB4 cells, 60% fewer NB4 cells migrated across the fibrin barrier after 2 days of treatment (50.8% ± 2.86% in vehicle-treated NB4 cells and 20.2% ± 1.94% after day 2; n = 3; Figure 2D). In contrast, the movement of NB4 cells to the lower chamber in the absence of the Matrigel or fibrin barrier was identical, suggesting that the migration of cells to the chemoattractant was unaffected by ATRA treatment. These results show that treatment of APL cells with ATRA results in a loss of fibrinolytic activity concomitant with a loss in cell surface S100A10.
Depletion of S100A10 in NB4 cells results in decreased plasminogen binding and plasmin generation
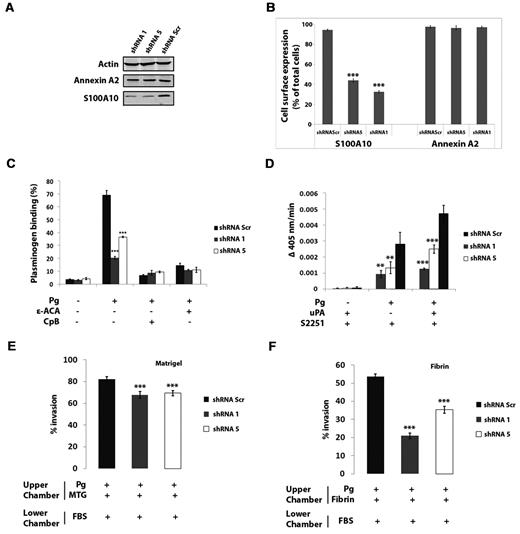
To elucidate the role that S100A10 plays in plasminogen binding and plasmin generation of APL cells, NB4 cells were transduced with a retroviral shRNA system that used shRNA specific for 2 sequences of S100A10 (shRNA-1 and shRNA-5) and a shRNA scramble sequence (shRNA-Scr). Compared with NB4 cells transduced with the shRNA-Scr, the total cellular levels of S100A10 were depleted by 80%-90% in the transduced shRNA-1 and shRNA-5 cells (Figure 3A). Moreover, cell surface levels of S100A10 were decreased by 55% in shRNA-5–expressing NB4 cells and 65% in shRNA-1–expressing NB4 cells (Figure 3B). We also observed that total and cell surface annexin A2 protein levels were unaffected by S100A10 depletion (Figure 3A-B).
Depletion of S100A10 by shRNA in NB4 cells. NB4 cells were transduced with a retroviral shRNA system with the use of shRNA specific for 2 sequences of S100A10 (shRNA-1 and shRNA-5) and a shRNA scramble sequence (shRNA-Scr). Cell lysates were prepared, and total levels of S100A10 and annexin A2 were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 and annexin A2 (B). Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in NB4 cells was calculated with WinMDI software (**P < .01 and ***P < .001). S100A10-depleted NB4 cells were incubated with 200nM FITC plasminogen, either with or without ϵ-ACA (100mM) or CpB (5 U/mL), for 1 hour at 4°C, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (C). S100A10-depleted NB4 cells were incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (D). ATRA-treated NB4 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (E) or fibrin-coated (F) chambers in the absence of FBS which also contained plasminogen (0.5μM). Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as the mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by 1-way ANOVA (with Tukey multiple comparisons) in comparison to untreated NB4 cells (NT; ***P < .001).
Depletion of S100A10 by shRNA in NB4 cells. NB4 cells were transduced with a retroviral shRNA system with the use of shRNA specific for 2 sequences of S100A10 (shRNA-1 and shRNA-5) and a shRNA scramble sequence (shRNA-Scr). Cell lysates were prepared, and total levels of S100A10 and annexin A2 were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 and annexin A2 (B). Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in NB4 cells was calculated with WinMDI software (**P < .01 and ***P < .001). S100A10-depleted NB4 cells were incubated with 200nM FITC plasminogen, either with or without ϵ-ACA (100mM) or CpB (5 U/mL), for 1 hour at 4°C, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (C). S100A10-depleted NB4 cells were incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (D). ATRA-treated NB4 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (E) or fibrin-coated (F) chambers in the absence of FBS which also contained plasminogen (0.5μM). Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as the mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by 1-way ANOVA (with Tukey multiple comparisons) in comparison to untreated NB4 cells (NT; ***P < .001).
S100A10 depletion resulted in a 70% decrease (P < .001) in plasminogen binding in NB4 shRNA-1 cells in comparison to NB4 shRNA-Scr cells (69% ± 0.46%, n = 3, by NB4 shRNA-Scr cells; and 20.4% ± 3.64%, n = 3, by NB4 shRNA-1 cells; Figure 3C) and a 50% decrease (P < .001) for NB4 shRNA-5 cells (36.5% ± 1.24%, n = 3, for NB4 shRNA-5 cells). In the presence of ϵ-ACA, plasminogen binding was reduced 80%-90% (10.9% ± 1.76%, n = 3, in shRNA-1 cells; 11.1% ± 0.53%, n = 3, in shRNA-5 cells; and 14.6% ± 2.0%, n = 3, in shRNA-Scr cells) (Figure 3C), and pretreatment of cells with carboxypeptidase B, which cleaves carboxy-terminal lysines,20 resulted in a 90% reduction in plasminogen binding (8.9% ± 0.5%, n = 3, in shRNA-1 cells; 9.5% ± 1.9%, n = 3, in shRNA-5 cells; and 6.9% ± .66%, n = 3, in shRNA-Scr cells). Because the binding of plasminogen to the carboxyl-terminal lysines of its receptor proteins is essential for both plasminogen binding and plasmin generation, this indicates that S100A10 is responsible for most of this carboxy-terminal lysine-dependent plasminogen binding (Figure 3C).
In the presence of plasminogen alone, we observed a 70% and 55% reduction (Figure 3D) in plasmin generation by NB4 shRNA-1 cells and NB4 shRNA-5 cells, respectively, in comparison to NB4 shRNA-Scr cells. In the presence of both plasminogen and uPA similar differences in the rate of plasmin generation were observed between shRNA-1 and -5, and shRNA-Scr (Figure 3D).
S100A10 deficiency results in decreased proteolytic activity of NB4 cells
Next, we examined the proteolytic activity of the control and S100A10-depleted NB4 cells with the use of the modified Boyden chambers. We observed that in response to the chemoattractant, 18% fewer NB4 shRNA-1 cells and 16% fewer NB4 shRNA-5 cells (P < .001) migrated across the Matrigel barrier compared with the NB4 shRNA-Scr cells (66.7% ± 2.5% in shRNA-1 cells, 69.3% ± 3.1% in shRNA-5 cells, and 82.3% ± 2.1% in shRNA-Scr cells, n = 3; Figure 3E). Loss of S100A10 had a dramatic effect on migration across a fibrin barrier. We observed 60% or 35% fewer NB4 shRNA-1 cells and NB4 shRNA-5 cells, respectively, had migrated across the fibrin barrier in comparison to NB4 shRNA-Scr cells (21% ± 2% in shRNA-1 cells, 35.3% ± 1.5% in shRNA-5 cells, and 53.7% ± 1.5% in shRNA-Scr cells; n = 3; Figure 3F). Thus, our results suggest that S100A10 plays an important role in the regulation of the fibrinolytic activity of the NB4 cells.
Induction of PML-RAR-α fusion protein up-regulates S100A10 and annexin A2 cell surface levels concomitant with increased plasmin generation
NB4 cells constitutively express the oncogenic PML-RAR-α fusion protein. The loss of S100A10 concomitant with the loss of PML-RAR-α fusion protein during ATRA treatment suggested but did not prove that S100A10 was regulated by this oncoprotein. To directly address the relationship between S100A10 and the PML-RAR-α fusion protein, we used the U937/PR9 cell line that expresses PML-RAR-α under the control of a zinc-inducible promoter. We observed that expression of PML-RAR-α occurred within 24 hours of induction with 100μM ZnSO4 (Figure 4A).24 Interestingly, expression of both S100A10 and annexin A2 protein (Figure 4A) but not mRNA (supplemental Figure 1C) was dramatically increased in the PR9 cells on treatment with ZnSO4. To rule out the possibility that increases in cellular Zn2+ levels affected the levels of S100A10, SN4 cells, which were transfected with the empty inducible vector alone, were incubated with ZnSO4, and changes in total cellular expression of S100A10 or annexin A2 were not observed. Similarly, FACS analysis indicated that the cell surface expression of S100A10 (Figure 4B) and annexin A2 (Figure 4C) were increased after Zn2+ induction, whereas levels of these proteins on the surface of Zn2+-treated SN4 cells were unaffected. These data show for the first time that S100A10 and annexin A2 protein levels are regulated by the oncogenic PML-RAR-α fusion protein.
Expression of PML-RAR-α oncoprotein up-regulates cell surface expression of S100A10. PR9 and mock-transfected SN4 cells were treated with ZnSO4 for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, α-enolase, and PML-RAR were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 (B) and annexin A2 (C). Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in ZnSO4-treated PR9 and SN4 cells was calculated with the use of WinMDI software (**P < .01 and ***P < .001). Zinc-induced PR9 cells were incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by 1-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (D).
Expression of PML-RAR-α oncoprotein up-regulates cell surface expression of S100A10. PR9 and mock-transfected SN4 cells were treated with ZnSO4 for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, α-enolase, and PML-RAR were examined by Western blotting with the use of actin as a loading control (A). Flow cytometric analysis of cell surface S100A10 (B) and annexin A2 (C). Quantification of flow cytometric analysis of cell surface S100A10 and annexin A2 in ZnSO4-treated PR9 and SN4 cells was calculated with the use of WinMDI software (**P < .01 and ***P < .001). Zinc-induced PR9 cells were incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by 1-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (D).
Next, we examined whether the induced increased expression of S100A10 and annexin A2 affected the rate of plasmin generation. We observed that plasmin generation increased concomitant with the increased surface expression of S100A10 and annexin A2 (Figure 4D). After 24 hours of induction, PR9 cells exhibited a 15% increase in plasmin generation compared with vehicle-treated PR9 cells (Figure 4D). By 48 hours the rate of plasmin generation had increased by 35% (Figure 4D). Further induction of cells with Zn2+ for 72 and 96 hours resulted in an increased plasmin generation of 37% and 43%, respectively, in comparison to vehicle-treated PR9 cells (Figure 4D).
Depletion of S100A10 in PML-RAR-α–expressing PR9 cells decreases plasminogen binding, plasmin generation, and fibrinolytic activity
PR9 cells were depleted of S100A10 with the use of RNA interference. Under these conditions the total levels of S100A10 were depleted by 80%-90% by expression of shRNA-1 and shRNA-5, respectively (Figure 5A-B) as determined by densitometry comparisons. Importantly, the total and cell surface annexin A2 protein levels were unaffected by S100A10 depletion (Figure 5A,D). Moreover, cell surface levels of S100A10 were decreased by 70% in shRNA-5–expressing PR9 cells and by 65% in shRNA-1–expressing PR9 cells (Figure 5C).
Zn2+ induction of S100A10-depleted PR9 cells up-regulates cell surface expression of S100A10. S100A10-depleted PR9 cells were treated with ZnSO4 for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, and PML-RAR were examined by Western blotting with the use of actin as a loading control (A). S100A10 up-regulation was quantified by determining the ratio between S100A10 and actin (B). Flow cytometric analysis of cell surface S100A10 (C) and annexin A2 (D) in S100A10 shRNA-transduced PR9 cells before and after induction with ZnSO4.
Zn2+ induction of S100A10-depleted PR9 cells up-regulates cell surface expression of S100A10. S100A10-depleted PR9 cells were treated with ZnSO4 for the indicated times. Cell lysates were prepared, and total levels of S100A10, annexin A2, and PML-RAR were examined by Western blotting with the use of actin as a loading control (A). S100A10 up-regulation was quantified by determining the ratio between S100A10 and actin (B). Flow cytometric analysis of cell surface S100A10 (C) and annexin A2 (D) in S100A10 shRNA-transduced PR9 cells before and after induction with ZnSO4.
Next, we induced the expression of the PML-RAR-α fusion protein and examined the S100A10 and annexin A2 levels in the anti-S100A10 shRNA and control (shRNA-Scr–expressing) PR9 cells. We observed that the Zn2+-induced expression of PML-RAR-α did not affect the annexin A2 levels (Figure 5A,D). Interestingly, the levels of total and cell surface S100A10 in the anti-S100A10 shRNA-expressing cells were modestly increased by Zn2+ induction (Figure 5A-C) but were still much lower than the levels observed for Zn2+-induced shRNA-Scr cells, suggesting that expression of the oncogenic PML-RAR-α fusion protein partially restored the cellular levels of S100A10 in the anti-S100A10 shRNA-expressing cells. FACS analysis showed that Zn2+-induced increased annexin A2 cell surface expression was comparable in shRNA-Scr– and anti-S100A10 shRNA-expressing cells (Figure 5D).
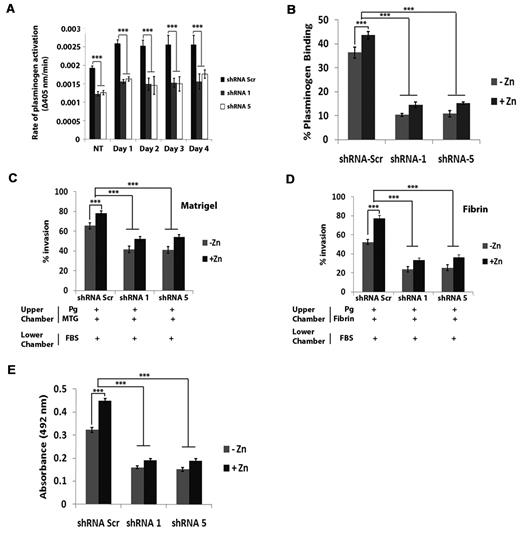
PR9 cells expressing shRNA-Scr, shRNA-5, and shRNA-1 exhibited a maximal increase in plasmin generation after 24 hours of Zn2+ induction (Figure 6A). However, the rate of plasmin generation by PR9 shRNA-1 and shRNA-5 cells was 40% less (P < .001) than that of PR9 shRNA-Scr cells (Figure 6A). Because annexin A2 levels were comparable between shRNA-Scr, shRNA-5, and shRNA-1 cells, these data suggest that a significant proportion of the increased plasmin generation observed after induction of the oncogenic PML-RAR-α fusion protein is because of S100A10. Differences observed in the rate of plasmin generation between S100A10-depleted PR9 cells and annexin A2–depleted PR9 cells depended on the degree of expression of S100A10 (supplemental Figure 3). Similarly, after 24 hours of Zn2+ induction, plasminogen binding was ∼ 65% less in shRNA-1– and shRNA-5–expressing cells in comparison to shRNA-Scr PR9 cells (43.6% ± 1.55%, n = 3, by shRNA-Scr PR9 cells; 14.6% ± 1.25%, n = 3, by shRNA-1 PR9 cells; and 15.3% ± 0.5%, n = 3, by shRNA-5 PR9 cells; Figure 6B).
Up-regulation of cell surface S100A10 expression increases plasmin generation and invasive capability of PR9 cells. S100A10-depleted PR9 cells were treated with ZnSO4 for the indicated times and then incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (A). S100A10-depleted NB4 cells, induced and noninduced, were incubated with 200nM FITC plasminogen for 1 hour, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (B). ZnSO4-treated S100A10 shRNA-transduced PR9 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (C) or fibrin-coated (D) chambers in the absence of FBS which also contained plasminogen (0.5μM). The lower chamber contained FBS as chemoattractant. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by one-way ANOVA (with Tukey multiple comparisons; ***P < .001). S100A10-depleted PR9 cells (5 × 106), in the absence or presence of Zn2+, were added on top of a fibrin bed, along with 0.5μM plasminogen, and incubated overnight at 37°C. Conditioned media was collected and used for the TintElize D-dimer ELISA kit (Biopool) as per manufacturer's instructions (E).
Up-regulation of cell surface S100A10 expression increases plasmin generation and invasive capability of PR9 cells. S100A10-depleted PR9 cells were treated with ZnSO4 for the indicated times and then incubated with uPA (50nM) and plasminogen (0.5μM), and the rate of plasmin generation was measured at 405 nm. Statistical analysis was performed by Student t test (***P < .001), and data are expressed as Δ405 nm/s ± SD of 3 independent experiments (A). S100A10-depleted NB4 cells, induced and noninduced, were incubated with 200nM FITC plasminogen for 1 hour, and plasminogen binding was measured by FACS. Statistical analysis was performed by one-way ANOVA with Tukey multiple comparisons, and data are expressed as the percentage of specific binding ± SD of 3 independent experiments (B). ZnSO4-treated S100A10 shRNA-transduced PR9 cells (1 × 105 cells) were added to the upper chamber of Matrigel-coated (C) or fibrin-coated (D) chambers in the absence of FBS which also contained plasminogen (0.5μM). The lower chamber contained FBS as chemoattractant. Invading cells were quantified as described in “Matrigel invasion and cell migration.” Data are expressed as mean number of cells per 40× field ± SD of 3 independent experiments. Statistical analysis was performed by one-way ANOVA (with Tukey multiple comparisons; ***P < .001). S100A10-depleted PR9 cells (5 × 106), in the absence or presence of Zn2+, were added on top of a fibrin bed, along with 0.5μM plasminogen, and incubated overnight at 37°C. Conditioned media was collected and used for the TintElize D-dimer ELISA kit (Biopool) as per manufacturer's instructions (E).
To examine the effect of S100A10 on the fibrinolytic activity of the PR9 cells before and after expression of PML-RAR-α fusion protein we performed in vitro invasion assays with Boyden chambers in which the inserts were coated with Matrigel or fibrin as described earlier. We observed that 36% fewer PR9 shRNA-1 cells and 37% fewer PR9 shRNA-5 cells (P < .001) migrated across the Matrigel barrier than PR9 shRNA-Scr (41.7% ± 3.27% in shRNA-1 cells, 41% ± 3.46% in shRNA-5 cells, and 65.5% ± 3.08% in shRNA-Scr cells; n = 3; Figure 6C). Furthermore, 55% fewer PR9 shRNA-1 cells and 51% fewer PR9 shRNA-5 cells (P < .001) migrated through the fibrin barrier compared with PR9 shRNA-Scr–expressing cells (23.8% ± 2.86% in shRNA-1 cells, 25.5% ± 3.27% in shRNA-5 cells, and 52.3% ± 2.65% in shRNA-Scr cells; n = 3; Figure 6D). Thus, S100A10 regulates the fibrinolytic activity of the PR9 cells.
Next, we observed that the induction of the PML-RAR-α fusion protein resulted in an increase in migration of the PR9 cells through the Matrigel and fibrin barrier. Specifically, 16% and 30% more PR9 cells (shRNA-Scr) migrated through Matrigel and fibrin, respectively, after zinc induction in comparison to the noninduced PR9 cells (65.5% ± 3.08% in noninduced shRNA-Scr cells and 78.2% ± 2.32% in zinc-induced shRNA-Scr cells through Matrigel; 52.3% ± 2.66% in noninduced shRNA-Scr cells and 77% ± 3.46% in zinc-induced shRNA-Scr cells fibrin; Figure 6C-D). However, when S100A10 was depleted, there was a loss of 34% and 50%, respectively, in the number of cells that migrated through the Matrigel and fibrin barrier.
As an additional measure of the fibrinolytic activity of the PR9 cells, we performed a D-dimer ELISA. D-dimer is a fibrin-derived fragment that is released when cross-linked fibrin is broken down by the fibrinolytic system. To measure fibrin breakdown products, cells were placed on a fibrin layer for 24 hours in the presence or absence of Zn2+, and the conditioned media was collected and assayed for D-dimer. As shown in Figure 6E, induction of the PML-RAR-α fusion protein resulted in a 30% increase in D-dimer production by control PR9 cells. We also observed that depletion of S100A10 reduced the D-dimer production of PR9 cells and PR9 cells expressing the PML-RAR-α fusion protein by ∼ 50% (Figure 6E). These data further confirm that induction of the PML-RAR-α fusion protein results in an increase in the fibrinolytic activity of the PR9 cells and that much of this increased fibrinolytic activity is blocked by depletion of S100A10.
Discussion
APL represents a special subtype of acute myeloid leukemia whose characteristic biologic and clinical features include the presence of the specific t(15;17) chromosomal translocation in leukemic blasts and the frequent existence of severe hemorrhagic diathesis at the time of diagnosis. ATRA has greatly improved the management of APL, but it has not significantly changed the rate of early hemorrhagic deaths. The mechanism for the hemorrhagic deaths partially involves hyperfibrinolysis which occurs because of the excessive production of the fibrinolytic enzyme, plasmin at the surface of the PML cells. Attempts to prevent the thrombohemorrhagic risk associated with APL with the use of drugs such as heparin, tranexamic acid, or other anticoagulant or antifibrinolytic therapy have to date not been successful, suggesting the need for drug design that is based on an understanding of the disease at a molecular level.25 Therefore, understanding the mechanism of plasmin regulation at the cell surface of the PML cells is critical to elucidate the cause of the hemorrhagic complications of APL.
Plasmin plays an essential role in fibrinolysis. The first step in cellular plasmin production involves the binding of the zymogen, plasminogen to the cell surface which is mediated by a heterogeneous group of specific receptors. The plasminogen binding capacity of neutrophils, monocytes, and lymphocytes is high, with 104-106 molecules bound per cell, whereas that of certain transformed monocytoid and lymphoblastoid cell lines can exceed 107 molecules per cell. Several plasminogen receptors have been identified on the surface of leukocytes26 including α-enolase,27 TIP49a,28 integrin αMβ229 , histone H2B,30 annexin A2,31 and S100A10.32,33 Of these plasminogen receptors, annexin A2 was reported to be present at abnormally high levels on APL cells, and it was proposed that the elevated levels of annexin A2 were responsible for increased production of plasmin and the hemorrhagic complications of APL. Furthermore, in vitro treatment of t(15;17)–positive APL cells with ATRA was shown to significantly reduce both the cellular expression of annexin A2 and plasmin generation over a similar time course. Recently, we showed that S100A10 played a key role in macrophage plasminogen binding and plasmin generation despite the presence of significant levels of annexin A2 at the cell surface. In the current study we have examined the role of S100A10 in regulating the generation of plasmin at the surface of APL cells. We used the human APL cell line, NB4, for these studies. The NB4 cell has been shown to possess the t(15;17) translocation and to constitutively express the PML-RAR-α oncoprotein. Furthermore, these cells are induced to terminally differentiate to neutrophils with ATRA. We observed that depletion of S100A10 by RNA interference resulted in a 70% loss in plasminogen binding and a 64% loss in plasmin generation by the NB4 cells. In addition to measuring plasmin generation, we also examined the ability of NB4 cells to migrate through a fibrin barrier as an independent measure of fibrinolytic activity of the cells. We observed that depletion of S100A10 resulted in 60% fewer NB4 cells migrating through the fibrin barrier. These results establish the importance of S100A10 in plasmin generation in PML cells in vitro. We also observed that treatment of the NB4 cells with ATRA resulted in a rapid reduction in the PML-RAR-α oncoprotein concomitant with a loss in S100A10. ATRA treatment also resulted in a 60% loss in plasminogen binding, a 40% loss in plasmin activity, and a 60% loss in migration of the ATRA-treated NB4 cell through the fibrin barrier. The plasminogen receptor, α-enolase, was unaffected by ATRA treatment, and histone H2B was not detected on the surface of the NB4 cells. The complete loss of cellular levels of S100A10 after ATRA treatment presents the possibility that the remission of hemorrhagic complications observed during treatment of patients with APL with ATRA could be because of the ATRA-mediated loss S100A10 from the surface of the leukemic promyelocytes.
Because the treatment of NB4 cells with ATRA is known to result in the up-regulation of ∼ 119 genes as well as the down-regulation of 17 genes,34 it was unclear if the regulation of S100A10 by ATRA was directly through the PML-RAR-α oncoprotein or by other ATRA-regulated genes. This issue was addressed by examining S100A10 levels in the U937/PR9 cells, which express PML-RAR-α oncoprotein under the control of a zinc-inducible promoter. We observed that expression of PML-RAR-α oncoprotein resulted in a rapid and dramatic up-regulation of S100A10 protein but not mRNA and a subsequent increase in plasminogen binding and fibrinolytic activity. We also observed that annexin A2 was up-regulated by the induced expression of the PML-RAR-α oncoprotein. However, when the PML-RAR-α oncoprotein expressing PR-9 cells were depleted of S100A10 by RNA interference, we observed a dramatic loss of plasminogen binding and plasmin generation. These data suggest that PML-RAR-α oncoprotein regulates the S100A10 protein levels and also implicates S100A10 as a main component of the fibrinolytic system on development of APL. Collectively, the data suggest that up-regulation of S100A10 at the cell surface of PML cells may contribute to the bleeding complications that are associated with APL.
Previous studies have suggested that annexin A2 was the fibrinolytic regulatory protein whose overexpression was responsible for the hemorrhagic complications of APL.31 Unlike the well-characterized plasminogen receptors of myeloid cells, annexin A2 does not possess a C-terminal lysine. For example, S100A10 binds tPA and plasminogen through its carboxyl-terminal lysine residues which interact with the lysine-binding, kringle domains of tPA and plasminogen (reviewed in Kwon et al35 ). Although intact annexin A2 does not bind plasminogen,30,36 the proteolytic cleavage of annexin A2 at lysine 307 has been reported to be required for the interaction of cell surface annexin A2 with plasminogen.36-38 However, to date this truncated form of annexin A2 or the protease responsible for the cleavage event has not been shown on the cell surface. We have shown that HT1080 cells and macrophages that are actively engaged in plasmin generation do not express truncated annexin A2 at the cell surface. Similarly, we have been unable to detect the truncated form of annexin A2 on the surface of NB4 or PR9 cells that are actively engaged in fibrinolysis (supplemental Figure 2). These findings suggest that annexin A2 is not responsible for the hemorrhagic complications in APL.
In summary, we have shown that the induced expression of the PML-RAR-α oncoprotein, the hallmark of APL, increases both cell surface levels of S100A10 and cellular fibrinolytic activity. The enhanced fibrinolytic activity resulting from expression of the PML-RAR-α oncoprotein is inhibited by blocking the expression of S100A10. The enhanced fibrinolytic activity of NB4 cells, which constitutively express the PML-RAR-α oncoprotein, is reversed by treatment with ATRA which also causes a loss in cell surface S100A10 levels. Overexpression of S100A10 may therefore be responsible for the hemorrhagic complications of APL. In addition, S100A10 represents a new protein that could be therapeutically targeted to block the fibrinolytic activity of promyelocytes to prevent APL-associated hemorrhage. Given the persistence of this complication as the main cause of morbidity and mortality in this disease, this strategy could greatly affect overall outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Canadian Cancer Society Research Institute (CCSRI) and the Canadian Institutes of Health Research (CIHR). P.A.O. and P.A.M. were supported by a trainee award from The Beatrice Hunter Cancer Research Institute with funds provided by The Terry Fox Foundation Strategic Health Research Training Program in Cancer Research at CIHR.
Authorship
Contribution: P.A.O. designed and performed research, analyzed data, and wrote the manuscript; P.A.M. performed research; J.N.B. and R.S.L. analyzed data; and D.M.W. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David M. Waisman, Department of Biochemistry and Molecular Biology, Dalhousie University, Halifax, Nova Scotia, B3H 1X5 Canada; e-mail: david.waisman@dal.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal