Abstract
Induced pluripotent stem (iPS) cells offer a unique potential for understanding the molecular basis of disease and development. Here we have generated several human iPS cell lines, and we describe their pluripotent phenotype and ability to differentiate into erythroid cells, monocytes, and endothelial cells. More significantly, however, when these iPS cells were differentiated under conditions that promote lympho-hematopoiesis from human embryonic stem cells, we observed the formation of pre-B cells. These cells were CD45+CD19+CD10+ and were positive for transcripts Pax5, IL7αR, λ-like, and VpreB receptor. Although they were negative for surface IgM and CD5 expression, iPS-derived CD45+CD19+ cells also exhibited multiple genomic D-JH rearrangements, which supports a pre–B-cell identity. We therefore have been able to demonstrate, for the first time, that human iPS cells are able to undergo hematopoiesis that contributes to the B-cell lymphoid lineage.
Introduction
Reprogramming of adult human somatic cells toward induced pluripotent stem cells1-3 is a groundbreaking technology that opens the way for the development of novel diagnostics and therapeutics. Although traditionally human embryonic stem (hES) cells have been used as a convenient tool with which to understand hematopoietic and lymphoid development in health and disease, the usefulness of human induced pluripotent stem (iPS) cells for this purpose has recently been questioned.4 For hES cells, a limited number of studies have derived B cells5 and T cells,6,7 with some reports suggesting why this might be difficult to achieve,8 and which has not been described thus far for B cells from human iPS cells. Development of lymphoid progenitors from such pluripotent sources is of particular interest because it will help to understand better the early stages of B-cell development, which is currently not well understood.9 This includes which B cells are formed and when and how these lineages relate to the formation of both normal and leukemic progenitors during development.10,11
Methods
Derivation, identification, and maintenance of human iPS cell lines
iPS cells were generated essentially as decided by Takahashi et al,1 with changes highlighted herein. pMX vectors hOct4, hSox2, and hKlf4 were purchased from Addgene and viral supernantants for each transgene produced in pantropic PLATGP packaging cells (Cell Biolabs). Transduced Normal Human Dermal Fibroblasts (NHDFs; Lonza Biologics) were cocultured with mitomycin C-treated mouse embryonic fibroblasts in modified embryonic stem cell media (Dulbecco modified Eagle medium/F12, 20% [v/v] Knock Out Serum Replacement, 10 ng/mL basic fibroblast growth factor, 2mM sodium pyruvate, 1× nonessential amino acids [Invitrogen], and 1μM mercaptoethanol [Sigma-Aldrich]). Cultures were routinely maintained in 5% CO2 and 5% oxygen with half-media changes every 2 days, for up to 34 days. iPS colonies were picked and expanded before adaptation to feeder independent culture as described by Ludwig et al,12 using mTeSR (StemCell Technologies) and additional supplementation with Y27632 ROCK inhibitor (10μM final; Sigma-Aldrich) and further maintained in 5% CO2 and 5% oxygen. Three subclones (c15, c18, and c19) isolated from the same dermal fibroblast population were further characterized and used within this study. Immunofluorescent staining of iPS cultures was performed using anti–TRA-1-81 and SSEA4 (Millipore), anti-Nanog (R&D Systems), and appropriate Alexa-conjugated secondaries (Invitrogen).
Erythromyeloid and endothelial differentiation
Initial erythromyeloid differentiation studies were performed as embryoid bodies in suspension cultures in RPMI with B27 medium (Invitrogen; catalog no. 17504044) using a 3-stage protocol described by Yang et al.13 Differentiated cells were seeded into complete methocult H4434 (StemCell Technologies) with up to 40 000 cells/well, where colony-forming units (CFUs) were enumerated at day 14 or assessed by fluorescence-activated cell sorter (FACS) after staining using conjugated antibodies, anti–CD235-allophycocyanin (APC; clone HIR2, BD Biosciences), and anti–CD13-phycoerythrin (clone WM-47, Dako United Kingdom). To demonstrate endothelial potential of iPS-derived CD34 populations, CD34+ cells were isolated by magnetic-activated cell sorting (MACS), and cells were then expanded in endothelial growth media-2 (EGM-2; Lonza Biologics) up to passage 4, before seeding into the vasculogenesis tubule assay, as described previously.14
Characterizing B-cell potential of human iPS cells
To demonstrate B-cell lymphoid differentiation, iPS were differentiated on OP9 stroma (from Dr. I. Stubrin, University of Wisconsin, Madison, WI) on gelatinized dishes for 10 days as described previously.5 CD34+ cells were harvested by MACS and cocultured on MS-5 stroma (DSMZ) for a further 21 days, with appropriate cytokines, also described by Vodyanik et al.5 CD45+ cells were then isolated by MACS with subsequent staining of cells with relevant conjugated antibodies (CD19-APC [HIB19] and fluorescein isothiocyanate, CD45-APC [HI30], and CD10-phycoerythrin [HI10a]; BD Biosciences) performed before analysis by FACS (LSR II from BD Biosciences). This was repeated with 3 separate cell lines (subclones) and with c18 in triplicate.
Analysis of B cell–specific mRNA transcripts and genomic VDJ rearrangements
cDNA from CD45+ cells was prepared and subsequent polymerase chain reaction (PCR) reactions toward Pax5, IL7R, λ-like, and VpreB was performed with oligonucleotide primers and reaction conditions described elsewhere.5,15 PCR amplicons were visualized by 2% (w/v) agarose gel electrophoresis and image acquisition software (UVITEC; Essential Version 12.6). To analyze DJH rearrangement, genomic DNA was assessed by PCR amplification, using primers and conditions described by Reynaud et al.16 Amplicons were visualized as before with DJH fragments excised, cloned into TA-TOPO (Invitrogen), and confirmed by sequencing (ABI 3730 DNA Analyser, Weatherall Institute for Molecular Medicine, University of Oxford). Umbilical cord blood (UCB)-derived mononuclear cells (obtained with written informed consent) were used as positive control for rearrangements representative of DN, DXP, DG, and DA rearrangements with a further positive clonal control for DNJH rearrangement also included (In Vivo Scribe). NHDF and undifferentiated iPS genomic DNA were also assessed and shown to be negative for these rearrangements.
Results and discussion
Assessing reprogramming and pluripotency of iPS cell lines
We have generated iPS colonies from NHDFs with similar efficiencies to those previously described1 and shown their ES-like phenotype by demonstrating surface expression of TRA-1-81 and SSEA4 (Figure 1A) and SSEA3 with Nanog (Figure 1B). We also show typical hES morphology, additional surface staining for TRA-1-60, transgene silencing, and ES specific gene expression and demonstrate pluripotency in vitro and as teratoma formation in vivo (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We then showed that our iPS cells were competent to undergo erythromyeloid hematopoiesis and endothelial differentiation, as recently described by others17 using a 3-stage directed differentiation protocol. Here we found cells that were positive for the endothelial and hematopoietic marker CD34; and when these cells were assayed for their erythromyeloid CFU content (Figure 1C), a range of myeloid CFUs was evident, including CFU-granulocyte-erythrocyte-macrophage-megakaryocyte, burst-forming units-erythroid, CFU-erythroid, CFU-granulocyte macrophage, CFU-granulocyte, and CFU-macrophage. These were further assessed by FACS profiling after staining for CD235 (a marker for the erthromegakaryocytic progenitors in hES cells) and CD13 (Figure 1D). These results were highly reproducible, across 3 separately derived iPS cell lines. CD34+ selected cells, after growth in EGM-2 medium, were also cocultured with dermal fibroblasts in a vasculogenesis assay14 (Figure 1E) to demonstrate that our human iPS cells contribute to both the myeloid and endothelial lineage that is consistent with previous reports.17
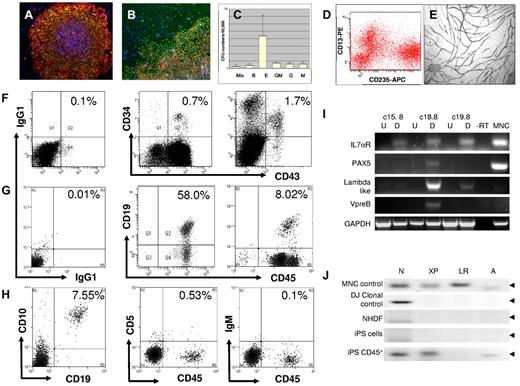
Analysis of iPS phenotype and hematopoietic potential. iPS cells were induced from adult human dermal fibroblasts as described, and ES-like phenotype was confirmed using markers specific to pluripotent stem cells. (A) Staining for TRA-1-81 (green) and SSEA4 (red) (original magnification ×10). (B) iPS colonies are stained for TRA-1-81 (red) and Nanog (green); original magnification, ×20. In all images, nuclear staining is with 4,6-diamidino-2-phenylindole (blue). After a 3-stage differentiation protocol, cell populations were further differentiated toward the hematopoietic lineage using complete methocult H4434; and after 14 days, we see the appearance of CFUs representative of the myeloid lineages. (C) CFU hematopoietic colonies formed from 3 separate iPS cell lines: c15, c18, and c19 (mean ± SEM). These cells were harvested and subsequently stained for CD13 (phycoerythrin) and CD235 (APC) before analysis by FACS (D) where we see distinct populations representing erythroid (CD13−CD235+) and monocytic lineages (CD13+CD235−) similar to that observed in MNC (data not shown). iPS-derived CD34+ cells isolated by MACS after differentiation were expanded in EGM-2 media and assessed for their endothelial function. (E) We demonstrate the ability of these endothelial cells to form tubules when cocultured with human dermal fibroblasts in EGM-2 media for 14 days, which are subsequently stained for CD31 (original magnification, ×10). All images were captured with a Nikon TE300 inverted fluorescent microscope using Hamamatsu Photonics CCD camera and processed with SimplePCI software (Hamamatsu Version 6.6) (Digital Pixel). Demonstration of B-cell lymphopoiesis from human iPS cells. To address whether human iPS cells are able to contribute to the lymphoid compartment, we cultured iPS cells with OP9 stroma for 10 days and cells were stained for CD34 and CD43 and assessed this by FACS. (F) We show (left) isotype and (middle) MNC controls for CD34+ and CD43+ staining with (right) representing populations observed from iPS cells differentiated on OP9. Next, we isolated these CD34+ cells by MACS and cultured on mouse MS-5 stroma for a further 21 days. We then isolated CD45+ cells, again by MACS separation, and stained these for CD45 and CD19. (G) We show (left) isotype control, (middle) staining for UCB-derived CD34+ cells cultured on MS-5 stroma for 21 days, and (right) staining for iPS-derived CD34+ cells also cultured under conditions to promote B-cell lymphopoiesis. (H) We show that a subpopulation of the CD45+ cells from human iPS sources stains double-positive for (left) CD45+ and CD10+ but negative for (middle) CD5 or (right) IgM. This experiment was repeated with 3 cell lines (c15, c18, and c19), thus representing 3 biologic replicates where CD19+CD45+ cells represent 4.67% ± 2.07% (mean ± SEM). B-cell formation was also performed in triplicate with the c18 line, which performed best previously, and here CD19+CD45+ cells represented 7.38% ± 0.86% (mean ± SEM; n = 3). With mRNA isolated from CD45+ cells from 3 separate iPS cell lines (c15, c18, and c19), we show (I) that these differentiated cells (D) are positive for B-cell specific transcripts, such as Pax5, IL7αR, λ-like, and VpreB receptor compared with their undifferentiated (U) counterparts. CD45+ cells from iPS cell line c18.8, which yielded the highest number of B cells by FACS, also had the highest levels B-cell specific transcripts when assessed by reverse-transcribed PCR. (J) We analyzed VDJ rearrangement by PCR amplification of genomic DNA, using primers specific for D-JH recombination. In UCB-derived MNCs (control), we observe clonal rearrangements representative of DN, DXP, DLR, and DA to JH recombinations; also shown is a positive clonal control for DNJ rearrangement (DJ clonal control). Whereas NHDF and iPS cell genomic DNA are shown to be negative for these rearrangements, DNA from CD45+ cells from OP9/MS5-differentiated iPS cells (c18.8 CD45+) are positive for DN, DXP, and DA rearrangements, although not DLR. Amplicons were sequenced and confirmed to map to the IgH locus of the human genome. Full VHJH rearrangement was not observed in any of the iPS-derived CD45+ populations assessed.
Analysis of iPS phenotype and hematopoietic potential. iPS cells were induced from adult human dermal fibroblasts as described, and ES-like phenotype was confirmed using markers specific to pluripotent stem cells. (A) Staining for TRA-1-81 (green) and SSEA4 (red) (original magnification ×10). (B) iPS colonies are stained for TRA-1-81 (red) and Nanog (green); original magnification, ×20. In all images, nuclear staining is with 4,6-diamidino-2-phenylindole (blue). After a 3-stage differentiation protocol, cell populations were further differentiated toward the hematopoietic lineage using complete methocult H4434; and after 14 days, we see the appearance of CFUs representative of the myeloid lineages. (C) CFU hematopoietic colonies formed from 3 separate iPS cell lines: c15, c18, and c19 (mean ± SEM). These cells were harvested and subsequently stained for CD13 (phycoerythrin) and CD235 (APC) before analysis by FACS (D) where we see distinct populations representing erythroid (CD13−CD235+) and monocytic lineages (CD13+CD235−) similar to that observed in MNC (data not shown). iPS-derived CD34+ cells isolated by MACS after differentiation were expanded in EGM-2 media and assessed for their endothelial function. (E) We demonstrate the ability of these endothelial cells to form tubules when cocultured with human dermal fibroblasts in EGM-2 media for 14 days, which are subsequently stained for CD31 (original magnification, ×10). All images were captured with a Nikon TE300 inverted fluorescent microscope using Hamamatsu Photonics CCD camera and processed with SimplePCI software (Hamamatsu Version 6.6) (Digital Pixel). Demonstration of B-cell lymphopoiesis from human iPS cells. To address whether human iPS cells are able to contribute to the lymphoid compartment, we cultured iPS cells with OP9 stroma for 10 days and cells were stained for CD34 and CD43 and assessed this by FACS. (F) We show (left) isotype and (middle) MNC controls for CD34+ and CD43+ staining with (right) representing populations observed from iPS cells differentiated on OP9. Next, we isolated these CD34+ cells by MACS and cultured on mouse MS-5 stroma for a further 21 days. We then isolated CD45+ cells, again by MACS separation, and stained these for CD45 and CD19. (G) We show (left) isotype control, (middle) staining for UCB-derived CD34+ cells cultured on MS-5 stroma for 21 days, and (right) staining for iPS-derived CD34+ cells also cultured under conditions to promote B-cell lymphopoiesis. (H) We show that a subpopulation of the CD45+ cells from human iPS sources stains double-positive for (left) CD45+ and CD10+ but negative for (middle) CD5 or (right) IgM. This experiment was repeated with 3 cell lines (c15, c18, and c19), thus representing 3 biologic replicates where CD19+CD45+ cells represent 4.67% ± 2.07% (mean ± SEM). B-cell formation was also performed in triplicate with the c18 line, which performed best previously, and here CD19+CD45+ cells represented 7.38% ± 0.86% (mean ± SEM; n = 3). With mRNA isolated from CD45+ cells from 3 separate iPS cell lines (c15, c18, and c19), we show (I) that these differentiated cells (D) are positive for B-cell specific transcripts, such as Pax5, IL7αR, λ-like, and VpreB receptor compared with their undifferentiated (U) counterparts. CD45+ cells from iPS cell line c18.8, which yielded the highest number of B cells by FACS, also had the highest levels B-cell specific transcripts when assessed by reverse-transcribed PCR. (J) We analyzed VDJ rearrangement by PCR amplification of genomic DNA, using primers specific for D-JH recombination. In UCB-derived MNCs (control), we observe clonal rearrangements representative of DN, DXP, DLR, and DA to JH recombinations; also shown is a positive clonal control for DNJ rearrangement (DJ clonal control). Whereas NHDF and iPS cell genomic DNA are shown to be negative for these rearrangements, DNA from CD45+ cells from OP9/MS5-differentiated iPS cells (c18.8 CD45+) are positive for DN, DXP, and DA rearrangements, although not DLR. Amplicons were sequenced and confirmed to map to the IgH locus of the human genome. Full VHJH rearrangement was not observed in any of the iPS-derived CD45+ populations assessed.
Definitive hematopoiesis and B-cell lymphopoiesis
To demonstrate whether our human iPS cells are also able to undergo B-cell lymphopoiesis, we differentiated iPS colonies on OP9 stoma and MS5 cells as previously described for human H1 ES cells.5 Here we showed that human iPS cells, cocultured with OP9 stroma for 10 days, gave rise to a dual CD34+ and CD43+ positive population (Figure 1F) similar to that observed for H1 hES cells (supplemental Figure 1) and with comparable staining to that observed with UCB-derived mononuclear cells. When CD34+ cells derived from iPS, hES, and UCB mononuclear cell (MNC) sources were cocultured on MS5 cells for a further 21 days and stained for CD45/CD19 (Figure 1G), we observed that iPS cells consistently produced double-positive cells for CD45 and CD19 (7.38% ± 0.86%, mean ± SEM, n = 3). Compared with the B-cell phenotype from UCB CD34+ cells, they had a similar profile of staining, where the intensity of CD19 staining for iPS-derived cells was comparable with those from UCB. Unfortunately, we were not able to demonstrate B-cell differentiation from H1 ES cells (supplemental Figure 1), which may be a consequence of extended passage. We also show that human iPS-derived CD19+ cells costain for CD10, further indicating a B lymphoid identity,18,19 but do not appear positive for the more mature B-cell markers CD5 and IgM (Figure 1H), suggesting a pre-B-cell phenotype.
To further characterize human iPS-derived B cells at a molecular level, we assessed expression of B-cell specific transcripts and the presence of VDJ recombination at the IgH locus. We showed that each differentiated iPS line, but particularly that of c18, expressed pre-B-cell specific transcripts (Figure 1I), namely Pax5, a regulator of B-cell maturation,20 IL7αR critical for B-cell proliferation, and components of the surrogate Ig receptor complex λ-like and VpreB receptor, which indicates a pre-B-cell identity.21,22 c15 and c19 lines expressed these transcripts at lower levels possibly because of a lower percentage of CD19+ cells in the whole population. When genomic DNA was analyzed for VH and DJH rearrangements, although VH-DJH rearrangement was not observed, PCR amplicons that represented several DJH rearrangements were observed specifically in iPS-derived B cells (Figure 1J) and, when sequenced, were confirmed to align with the IgH locus and further supports a pre-B-cell identity.21
In conclusion, we have shown that human iPS cells can efficiently contribute to the B-lymphoid lineage. In doing so, we have demonstrated that these cells can be considered as an alternative in an in vitro model with which to address developmental issues, such as ontogeny in the B-cell lineage and the relationship between “fetal” B1 and “adult” B2 cells,10,23 disease modeling for B-cell leukemias, which to date has not been described using human iPS cells, and to help provide for an informed discussion on issues relating to the development of hematopoietic stem cells in early development.24,25
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Maxim Vodyanik and Igor Slukvin (University of Wisconsin, Madison, WI) for the provision of OP9 cells (originally from Dr Nakano, Kyoto University, Yoshida, Japan) and Professor James Thomson (University of Wisconsin, Madison, WI) for the supply of H1 human ES cells.
L.C., R.J.F., and S.M.W. were supported by the National Institute for Health Research (program grant), the Oxford Stem Cell Institute (seed-funded grant), and National Health Service Blood and Transplant.
The sponsors of this study are public nonprofit organizations that support science in general and have no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: L.C. conceived and performed experiments and wrote the manuscript; R.M., C.-T.Y., K.J.P., R.J.F., and A.F. contributed toward experiments; J.S.-S. maintained an invaluable supply of OP9 and MS5 stroma; K.M.S., T.J.D., and P.J.F. conducted the teratoma studies; and S.M.W. and T.E. reviewed the experimental design, experiments, and manuscript and obtained funding for the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lee Carpenter, Stem Cell Research Laboratory, National Health Service Blood and Transplant, John Radcliffe Hospital, Headington, Oxford, OX3 9BQ, United Kingdom; e-mail: lee.carpenter@nhsbt.nhs.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal