Abstract
Tissue factor (TF) is the cellular receptor for plasma protease factor VIIa (FVIIa), and the TF-FVIIa complex initiates coagulation in both hemostasis and thrombosis. Cell surface-exposed TF is mainly cryptic and requires activation to fully exhibit the procoagulant potential. Recently, the protein disulfide isomerase (PDI) has been hypothesized to regulate TF decryption through the redox switch of an exposed disulfide in TF extracellular domain. In this study, we analyzed PDI contribution to coagulation using an in vitro endothelial cell model. In this model, extracellular PDI is detected by imaging and flow cytometry. Inhibition of cell surface PDI induces a marked increase in TF procoagulant function, whereas exogenous addition of PDI inhibits TF decryption. The coagulant effects of PDI inhibition were sensitive to annexin V treatment, suggesting exposure of phosphatidylserine (PS), which was confirmed by prothrombinase assays and direct labeling. In contrast, exogenous PDI addition enhanced PS internalization. Analysis of fluorescent PS revealed that PDI affects both the apparent flippase and floppase activities on endothelial cells. In conclusion, we identified a new mechanism for PDI contribution to coagulation on endothelial cells, namely, the regulation of PS exposure, where PDI acts as a negative regulator of coagulation.
Introduction
Tissue factor (TF) is a transmembrane glycoprotein that binds with high affinity to the plasma protease factor VII in either zymogen (FVII) or activated form (FVIIa). The formation of the TF-FVIIa complex is crucial for initiation of coagulation, leading to thrombin generation and fibrin formation. Although the primary role of TF-FVIIa is to maintain hemostasis after vascular injury, aberrant activation of coagulation underlies thrombosis, the major cause of mortality in most industrialized countries.1
In physiologic conditions, initiation of coagulation is maintained silent by restricting the exposure of TF to the plasma factors.2,3 In pathologic conditions, however, TF may become exposed on the endothelium4,5 and on circulating monocytes.6 At these sites, TF can initiate thrombotic events associated with sepsis,4,7 cancer,8,9 or atherosclerosis.10,11 Multiple in vitro studies have indicated that most of the cell-exposed TF is “cryptic,” thus not fully active toward coagulation,12,13 and TF “decryption” has been proposed as the initial step in the activation of coagulation.14 Although the molecular mechanisms of TF decryption are not completely understood, many of the stimuli that decrypt TF also increase the exposure of phosphatidylserine (PS),15-18 which is known to enhance coagulation. PS-independent mechanisms of TF decryption have also been postulated, such as TF self-association,19 association with lipid rafts,20-22 and the redox switch of an exposed disulfide in the membrane proximal domain of TF.23
Protein disulfide isomerase (PDI) is an oxidoreductase24 localized mainly in the endoplasmic reticulum (ER), but also reported on the cell surface of vascular cells, such as platelets, monocytes, and endothelial cells (ECs).24,25 On platelets, PDI influences coagulation by enhancing integrin-mediated platelet activation.26 On monocytes, PDI apparently modulates TF decryption and coagulation, by regulating the redox state of the Cys186-Cys209 pair in the extracellular domain of TF.14,23 Although the molecular mechanism for PDI modulation of TF function has been highly debated,27,28 in vivo inhibition of PDI with monoclonal antibodies reduced fibrin deposition in 2 mouse models of vascular injury.14,29 Recently, both PDI and TF have been identified on ECs and leukocytes in a deep vein thrombosis model, suggesting their involvement in yet another thrombotic manifestation.30
PS exposure is a strong signal for multiple physiologic processes, such as coagulation and clearance of apoptotic cells. In resting cells, most of the PS is localized on the cytosolic leaflet of the lipid bilayers, an asymmetry maintained by the activity of phospholipid translocases.31 Both efflux and influx of PS are sensitive to sulfhydryl modification. Sulfhydryl oxidizing or cross-linking reagents activate PS efflux and inhibit the flippase activity, whereas sulfhydryl reduction activates the flippase and inhibits PS efflux.32 This reciprocal modulation suggests that PS transporters may be regulated by a common redox element. Our study identifies PDI, a known modulator of sulfhydryl modifications, as also being a pivotal regulator of PS transport.
The present study analyzes PDI contribution to coagulation, using an in vitro EC model in which TF was either expressed as yellow fluorescence protein TF (YFP-TF) or induced by inflammatory mediators. Our data show that extracellular PDI could modulate coagulation, but surprisingly it acts as a negative regulator. Surface PDI inhibition increased the PS exposure, whereas exogenous addition of PDI enhanced the aminophospholipid translocase activity. For the first time, we show that PDI modulates the membrane phospholipid environment, which may have multiple implications, both for coagulation and clearance of apoptotic cells.
Methods
Reagents and cells
The antibodies and reagents used in this study are detailed in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For this study, we used the immortalized hybrid EC line EA.hy926 (gift from Dr Cora-Jean Edgell, University of North Carolina). We have previously reported the use of this cell model for analysis of TF pathway inhibitor-dependent regulation of TF procoagulant function.33 Characterization of TF expression and activity in this cellular model is detailed in supplemental data and supplemental Figure 1. Cells were washed twice with HEPES-buffered saline (HBS; 10mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 137mM NaCl, 40mM KCl, 20mM glucose, and 0.5% bovine serum albumin, pH 7.4) before use. Unless otherwise noted, the assays were performed in HBS containing 2mM CaCl2 (HBS/Ca).
Generation of EC cell lines stably expressing YFP-TF and/or PDI shRNA
Generation and characterization of the YFP-TF chimera containing the monomeric yellow fluorescent protein34 (mYFP; gift from Dr Roger Tsien, University of California, San Diego) as N-terminal tag is detailed under Supplemental data. Functional characterization of the YFP-TF chimera, transiently or stably expressed in ECs, is shown in supplemental Figure 2. ECs expressing low levels of YFP-TF, renamed TF-ECs, were used in this study.
For PDI silencing, we used PDI shRNA vectors specific for human P4HB/PDIA1 (Origene). For transfection, we used Effectene (QIAGEN). Parallel assays were run on nontransfected or control-transfected cells. Geneticin was used to select ECs stably expressing low levels of YFP-TF, whereas puromycin was used for selection of PDI shRNA-transfected ECs.
Immunofluorescence
The analysis was carried out on ECs fixed with 3% paraformaldehyde in the presence or absence of permeabilization with 0.1% Triton X-100.33,35 Images were collected with a Nikon C1 confocal system on a Nikon TE2000U microscope (Nikon Instruments) as described,33 using a PlanApo 60× NA 1.2 water immersion objective lens and/or a PlanApo 20× NA 0.7 dry objective lens. The acquisition software was EZ-C1 Version 3.6 (Nikon). Mean fluorescence intensity (MFI), expressed in arbitrary units (AU), was measured on a minimum of 50 cells per group randomly chosen from at least 5 pictures for each experiment.
Flow cytometry
After detachment with cell stripper (Cellgro), ECs were washed with phosphate-buffered saline (PBS), fixed with 3% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in PBS. Approximately 106 intact and/or permeabilized ECs were blocked with 5% donkey serum in PBS and then stained for surface or total PDI with a rabbit polyclonal (Sigma-Aldrich, 10 μg/mL) followed by donkey anti–rabbit immunoglobulin G–fluorescein isothiocyanate (IgG-FITC). Data were collected on a FACScan flow cytometer using CellQuest software (BD Biosciences).
Western blot
Western blot was performed essentially as described,33 using chemiluminescent detection (GE Healthcare). Densitometric analysis was performed with ImageJ software 1.42b (NIH).
TF and prothrombinase activity assays
TF procoagulant activity was measured on confluent monolayers by the 2-stage Xa generation assay in the presence of inhibitory rabbit anti–TF pathway inhibitor IgG (30 μg/mL).33 ECs were treated for 30 minutes with anti-PDI, control IgG (10 μg/mL), or human recombinant PDI (hPDI; 100nM) and/or ionomycin (5μM) for 10 minutes, before the addition of FVIIa (10nM) and excess substrate FX (100nM). Aliquots were taken during a 30-minute incubation at 37°C, FXa generation was quenched with ethylenediaminetetraacetic acid, and the amount of FXa was measured in a chromogenic assay.33 For prothrombinase assays, monolayers were treated and then incubated with FVa (50nM), FXa (5nM), and substrate prothrombin (0.5μM) in HBS/Ca. The reaction was stopped after 10 minutes with ethylenediaminetetraacetic acid, and the amount of thrombin was measured with Spectrozyme TH.
Insulin reduction assay
Antibody inhibition of the enzymatic activity of bovine PDI was assessed using an in vitro insulin reduction assay.36
Calcium influx
ECs were loaded with 3μM of either Fluo-3 or Fluo-4-AM calcium probes in culture media for 1 hour and then washed twice with PBS and detached with cell stripper. Residual ethylenediaminetetraacetic acid was washed, and cells were incubated in HBS/Ca with BD34 anti-PDI, isotype control, or no monoclonal antibody (mAb) for 30 minutes at room temperature. Changes in MFI of the calcium probe were detected by flow cytometry in the FITC channel. After collection of 10 000 events, ionomycin (5μM) was added for 10 minutes and samples reanalyzed by flow.
Annexin V detection of PS exposure
EC monolayers on glass coverslips were incubated in HBS/Ca with 10 μg/mL BD34 anti-PDI, isotype control, or no antibody for 30 minutes, or 5μM ionomycin for 10 minutes at 37°C. Cells were incubated with annexin V–FITC for 15 minutes at room temperature in the dark, washed, fixed with 3% paraformaldehyde, and processed for imaging as for immunofluorescence analysis.
For analysis of flippase activity, ECs were treated with 1μM thapsigargin (TG+) or vehicle (TG−) for 1 hour at 37°C in culture media supplemented with 2mM CaCl2. After washing, cells were incubated for 30 minutes at 37°C with glutathione (GSH; 10μM) or PDI (800nM) preincubated for 30 minutes with either GSH or equimolar concentration of BD34 anti-PDI in the presence of GSH. Cells were washed and labeled with annexin V–FITC.
Analysis of PS dynamics using fluorescent NBD-PS
Flippase activity.
For live cell imaging, ECs cultured in glass-bottom dishes were preincubated with BD34 anti-PDI or isotype control for 30 minutes at 37°C in HBS/Ca. The fluorescence was recorded in time-lapse microscopy (4 frames/minute for 30 minutes) with 1-oleoyl-2-{6-[(7-nitro-2-1,3-benzooxadiazol-4-yl)amino]hexamoyl}-sn-glycero-3-phosphoserine (NBD-PS; 2μM final concentration) added after the fifth frame. The NBD-PS uptake is represented as the average MFI from approximately 20 cells as a function of time. For endpoint analysis, fluorescence intensity measurements were performed on at least 80 cells randomly chosen from 10 pictures from each experiment.
For flow cytometric analysis of flippase activity, we used the impermeant reducing agent sodium dithionite to quench the NBD-PS exposed on the cell surface.37,38 Approximately 106 cells, preincubated with either BD34 anti-PDI or isotype control, were incubated on ice with 2μM NBD-PS for 2 to 5 minutes. PS internalization was initiated by warming the cells to room temperature. During a 1-hour incubation, aliquots were transferred to test tubes either containing or not containing 25mM sodium dithionite in HBS/Ca. Residual MFI of NBD-PS in the presence (FD) or absence (Ftotal) of dithionite was measured by flow cytometry, and the percentage of internalized NBD-PS was represented as the normalized FD/Ftotal ratio. In control experiments, Triton X-100 (0.1%) was added to the dithionite group to allow quenching of internalized NBD-PS. Residual FD/Ftotal ratio in permeabilized samples was less than 5%. Analysis of NBD-PC internalization was performed similarly.
Floppase activity.
For live-cell imaging, ECs treated with BD34 anti-PDI or isotype control IgG were loaded for 20 minutes with NBD-PS, washed, and the initial MFI measurements performed in the absence of dithionite. Time-lapse (4 frames/minute for 12 minutes) changes in MFI for 20 different cells were measured, with dithionite (25mM) added after the ninth frame. For bleaching correction, NBD-PS loaded ECs were fixed with 3% paraformaldehyde, and the laser-induced bleaching was measured in the absence of dithionite under an identical collection protocol. For individual cells, the corrected MFI at each time point was normalized to the average MFI measured in the absence of dithionite (first 9 frames). The changes in normalized residual NBD-PS fluorescence over time, which inversely correlate with the PS “flop,” are represented. Thirty minutes from the start of the time-lapse protocol, 10 different fields per experiment were collected for endpoint analysis. The PS flop is represented as the normalized (Ftotal − FD)/Ftotal ratio.
Data collection
All results are reported as mean plus or minus SD for activity assays and mean plus or minus SEM for image analysis. Differences between groups were analyzed by the unpaired t test or 1-way analysis of variance using Prism (GraphPad) and were considered significant when P less than .05. The experiments were repeated at least 3 times.
Results
PDI distribution in ECs
We analyzed the distribution of PDI in ECs using fluorescence microscopy and flow cytometry. PDI was immunodetected on nonpermeabilized or Triton X-100–treated cells with a rabbit polyclonal anti-PDI. On nonpermeabilized cells, the anti-PDI staining was significantly higher (P < .001) than either an irrelevant rabbit IgG or secondary-alone controls (Figure 1A). The surface PDI staining was punctuated, and coalescent patches were observed on many cells, although the significance of this distribution pattern is yet unclear. PDI staining in permeabilized cells, also significantly higher than controls (Figure 1B), exhibited a perinuclear accumulation consistent with an ER/secretory pathway distribution. The PDI staining was higher under permeabilized conditions than surface only (1237 ± 42.68, n = 55, vs 234.3 ± 11.10, n = 61, fluorescence arbitrary units, P < .001).
Low levels of PDI are expressed on the surface of ECs. Confocal analysis of PDI immunostaining was performed on nonpermeabilized (A) or Triton X-100-permeabilized (B) EA.hy926 cells and compared with an irrelevant rabbit IgG or secondary antibody alone. Scatter plot representation and statistical analysis (one-way analysis of variance [ANOVA]) of MFIs of more than 50 individual cells per experimental condition are shown on the right. (C) Flow cytometric analysis of PDI immunostaining on surface or Triton-permeabilized ECs, shown as MFI ± SEM. ***P < .001, unpaired t test.
Low levels of PDI are expressed on the surface of ECs. Confocal analysis of PDI immunostaining was performed on nonpermeabilized (A) or Triton X-100-permeabilized (B) EA.hy926 cells and compared with an irrelevant rabbit IgG or secondary antibody alone. Scatter plot representation and statistical analysis (one-way analysis of variance [ANOVA]) of MFIs of more than 50 individual cells per experimental condition are shown on the right. (C) Flow cytometric analysis of PDI immunostaining on surface or Triton-permeabilized ECs, shown as MFI ± SEM. ***P < .001, unpaired t test.
We used flow cytometry (Figure 1C) to confirm the microscopy results in the EC population. The intensity of PDI staining was significantly higher (P < .001) than controls under both nonpermeabilized and permeabilized conditions (10.094 ± 0.199 vs 3.840 ± 0.073 AU, and 51.436 ± 3.382 vs 22.840 ± 1.151 AU, respectively). PDI staining was higher under permeabilized conditions compared with surface staining. These results confirm the microscopy data and show that low levels of PDI are present on the surface of ECs.
Extracellular PDI inhibition enhances coagulation
In the absence of specific PDI inhibitors,39 we tested PDI contribution to coagulation by TF-dependent Xa generation in the presence of anti-PDI antibodies and compared their effects on coagulation with proper controls. As shown in Figure 2A, the 2 mAbs used at 10 μg/mL significantly enhanced the Xa generation on TF-EC, whereas the 2 polyclonal antibodies did not. In addition to RL-90 mAb, a reported inhibitor of PDI enzymatic activity in vitro40,41 and in vivo,14,29 we found that BD34 mAb was the most potent inhibitor in our screening. We used an insulin reduction assay to test the functional properties of BD34 mAb against purified bovine PDI (Figure 2B). In the presence of GSH, PDI reduces insulin disulfides, leading to aggregation of denatured insulin and changes in turbidity. GSH alone is not able to reduce insulin. Preincubation of PDI with equimolar concentrations of BD34 mAb inhibits PDI reductase function and decreases insulin aggregation. In contrast, PDI pretreatment with either one of the polyclonal antibodies did not affect insulin aggregation and thus PDI reductase activity. We observed some variability between different batches of antibody; however, PDI inhibition by equimolar concentrations of BD34 mAb was never less than 60%. In comparison, RL-90 inhibition of PDI activity was reportedly approximately 25% to 30%.41
PDI is a negative regulator of coagulation on ECs. (A) TF-ECs were treated with anti-PDI antibodies (10 μg/mL) for 30 minutes or ionomycin (Iono, 5μM) for 10 minutes before TF procoagulant activity was measured by a 2-stage Xa generation assay. TF activity is normalized against control samples and represented as fold increase over control IgG-treated ECs. *P < .05, ***P < .001 vs control. (B) PDI enzymatic activity was determined by an insulin turbidity assay in the presence of antibody-treated PDI (□, rabbit polyclonal; ▴, BD34 mAb) or PDI alone (■) and compared with no PDI controls (○). Not shown: IgG1k isotype or irrelevant rabbit IgG treated PDI was indistinguishable from PDI alone (■), whereas insulin reduction in the presence of antibody without PDI was indistinguishable from no PDI samples (○). (C) Antibody inhibition of surface PDI enhanced Xa generation in EA.hy926 cells stimulated with TNF-α (TNF+, **P < .01) but not in vehicle-treated cells (TNF−). (D) Procoagulant effects of surface PDI inhibition are sensitive to anti-TF antibodies (**P < .01) similar to ionomycin-induced decryption (***P < .001). (E) The procoagulant effects of extracellular PDI inhibition are observed on both EC surface and in the supernatant released from the cells (P < .01). (F) PDI inhibition increases TF procoagulant function, which can be further enhanced by ionomycin treatment. In contrast, preincubation with recombinant human PDI limits the amplitude of ionomycin-induced TF decryption. **P < .01, ***P < .001, 1-way ANOVA. (A-F) Data are mean ± SD of triplicates.
PDI is a negative regulator of coagulation on ECs. (A) TF-ECs were treated with anti-PDI antibodies (10 μg/mL) for 30 minutes or ionomycin (Iono, 5μM) for 10 minutes before TF procoagulant activity was measured by a 2-stage Xa generation assay. TF activity is normalized against control samples and represented as fold increase over control IgG-treated ECs. *P < .05, ***P < .001 vs control. (B) PDI enzymatic activity was determined by an insulin turbidity assay in the presence of antibody-treated PDI (□, rabbit polyclonal; ▴, BD34 mAb) or PDI alone (■) and compared with no PDI controls (○). Not shown: IgG1k isotype or irrelevant rabbit IgG treated PDI was indistinguishable from PDI alone (■), whereas insulin reduction in the presence of antibody without PDI was indistinguishable from no PDI samples (○). (C) Antibody inhibition of surface PDI enhanced Xa generation in EA.hy926 cells stimulated with TNF-α (TNF+, **P < .01) but not in vehicle-treated cells (TNF−). (D) Procoagulant effects of surface PDI inhibition are sensitive to anti-TF antibodies (**P < .01) similar to ionomycin-induced decryption (***P < .001). (E) The procoagulant effects of extracellular PDI inhibition are observed on both EC surface and in the supernatant released from the cells (P < .01). (F) PDI inhibition increases TF procoagulant function, which can be further enhanced by ionomycin treatment. In contrast, preincubation with recombinant human PDI limits the amplitude of ionomycin-induced TF decryption. **P < .01, ***P < .001, 1-way ANOVA. (A-F) Data are mean ± SD of triplicates.
Next, we sought to confirm these findings in ECs expressing endogenous TF in response to tumor necrosis factor (TNF). As shown in Figure 2C, PDI inhibition with BD34 mAb significantly increased coagulation only on cells treated with TNF-α (P = .003), suggesting that enhanced Xa generation required TF. Furthermore, PDI-enhanced Xa generation on TF-EC was sensitive to inhibitory TF antibodies (Figure 2D), confirming TF requirements for PDI effects in this assay.
Inhibition of extracellular PDI significantly enhanced TF procoagulant activity on both EC surface and in the supernatant collected from these cells (Figure 2E), which suggests that cells treated with the inhibitory anti-PDI mAb release TF-bearing microparticles. Furthermore, anti-PDI treatment enhanced TF procoagulant function similar to, but to a lesser extent than, ionomycin, which could further increase TF activity after PDI inhibition (Figure 2F). In contrast, preincubation of ECs with 100nM hPDI reduced by 56% the effects of ionomycin-induced TF decryption (P < .001). Altogether, these data indicate that extracellular PDI is a negative regulator of TF-dependent initiation of coagulation.
Extracellular PDI inhibition does not affect calcium influx
Because both calcium and sulfhydryl modifying agents affect the distribution of phospholipids, we tested whether PDI inhibition enhances coagulation through a mechanism similar to ionomycin: calcium-induced PS exposure.42 We used permeable fluorescent calcium indicators (Fluo-3 or Fluo-4) and flow cytometry to analyze the effects of PDI inhibition on calcium influx. Treatment of ECs with BD34 mAb did not change the calcium fluorescence compared with control cells (Figure 3A). A total of 5μM ionomycin increased calcium fluorescence in both control (Figure 3B,D) and anti-PDI treated cells (Figure 3C-D), indicating proper loading of cells with the calcium probes as well as feasibility of the method. These results show that, in contrast to ionomycin treatment, extracellular PDI inhibition does not induce a detectable calcium influx.
Extracellular PDI inhibition does not induce a detectable calcium influx in ECs. Flow cytometric analysis of calcium influx in EA.hy926 cells using Fluo-3 calcium probes. The fluorescent profiles are indistinguishable between BD34 and isotype IgG-treated cells (A) but shifted by ionomycin in both control EC (B) and anti-PDI treated ECs (C). (D) MFI ± SEM representation of panels A through C.
Extracellular PDI inhibition does not induce a detectable calcium influx in ECs. Flow cytometric analysis of calcium influx in EA.hy926 cells using Fluo-3 calcium probes. The fluorescent profiles are indistinguishable between BD34 and isotype IgG-treated cells (A) but shifted by ionomycin in both control EC (B) and anti-PDI treated ECs (C). (D) MFI ± SEM representation of panels A through C.
Surface PDI inhibition induces PS exposure
We first tested whether PDI inhibition affects PS exposure in ECs using prothrombinase assays. In control experiments, ionomycin induced a 7.132 plus or minus 0.320-fold increase in prothrombinase activity (Figure 4A). Similarly, PDI inhibition enhanced the prothrombinase activity 5.303 plus or minus 1.432-fold compared with control cells, suggesting that PDI inhibition translates into PS exposure. Furthermore, EC preincubation with hPDI reduced by half the ionomycin-dependent enhancement of thrombin generation (P < .01), indicating that exogenous PDI addition limits the exposure of anionic phospholipids.
Surface PDI inhibition induces PS exposure on ECs. (A) Ea.hy926 cells were treated with BD34 anti-PDI or isotype control (10 μg/mL), or 100nM hPDI for 30 minutes and/or ionomycin (Iono, 5μM) for 10 minutes before addition of FXa, FVa, and prothrombin substrate. Normalized thrombin generation after 10 minutes, measured in a chromogenic assay, is represented as fold increase over control samples. Data are mean ± SD of triplicates. ***P < .001, 1-way ANOVA. (B) PDI inhibition-induced enhancement of TF procoagulant activity is sensitive to annexin V treatment (P = .033, unpaired t test). (C) Representative micrographs of annexin V–FITC labeling of PS exposure on EA.hy926 cells treated with IgG1k isotype control, BD34 anti-PDI, or ionomycin. (D) Scatter plot representation of MFI and statistical analysis (1-way ANOVA) of annexin V–FITC labeling of PS exposure (n ≥ 50 per group).
Surface PDI inhibition induces PS exposure on ECs. (A) Ea.hy926 cells were treated with BD34 anti-PDI or isotype control (10 μg/mL), or 100nM hPDI for 30 minutes and/or ionomycin (Iono, 5μM) for 10 minutes before addition of FXa, FVa, and prothrombin substrate. Normalized thrombin generation after 10 minutes, measured in a chromogenic assay, is represented as fold increase over control samples. Data are mean ± SD of triplicates. ***P < .001, 1-way ANOVA. (B) PDI inhibition-induced enhancement of TF procoagulant activity is sensitive to annexin V treatment (P = .033, unpaired t test). (C) Representative micrographs of annexin V–FITC labeling of PS exposure on EA.hy926 cells treated with IgG1k isotype control, BD34 anti-PDI, or ionomycin. (D) Scatter plot representation of MFI and statistical analysis (1-way ANOVA) of annexin V–FITC labeling of PS exposure (n ≥ 50 per group).
In contrast to TF procoagulant assays, subsequent ionomycin treatment did not significantly enhance the prothrombinase activity on PDI-inhibited cells. This apparent discrepancy could be the result of the anti–PDI-induced release of membrane microparticles (Figure 2E). The association of the prothrombinase complex with the cell surface occurs exclusively through interactions with anionic phospholipid-binding sites, whereas the tenase complex is kept on the cell surface by the TF-FVIIa interaction. As such, the prothrombinase activity is more sensitive to the release of microparticles than is the TF procoagulant activity. Furthermore, normalization of TF procoagulant activity to TF antigen could account for the antigen loss in microparticles, whereas normalization of prothrombinase activity to total protein, as a measure of cell number, may not properly account for the loss of membrane-binding sites.
To confirm that PS exposure was also involved in the PDI-dependent enhancement of TF activity, we performed annexin V sensitivity assays of PDI-induced TF activity. Annexin V (100nM) treatment for 15 minutes before substrate FX addition decreased the PDI inhibition effect on TF activity in TF-ECs by 50% (Figure 4B). Similar to previous reports,18,43 100nM annexin V decreased the ionophore-induced TF decryption by approximately 70% (supplemental Figure 3).
PDI contribution to PS exposure was confirmed by direct detection of surface PS using annexin V–FITC (Figure 4C). Annexin V–FITC did not significantly bind to isotype-treated ECs, suggesting minimal PS exposure. PDI inhibition induced a significant surface binding of annexin V–FITC, similar to, but not to the extent of, ionomycin treatment used as a positive control (Figure 4D).
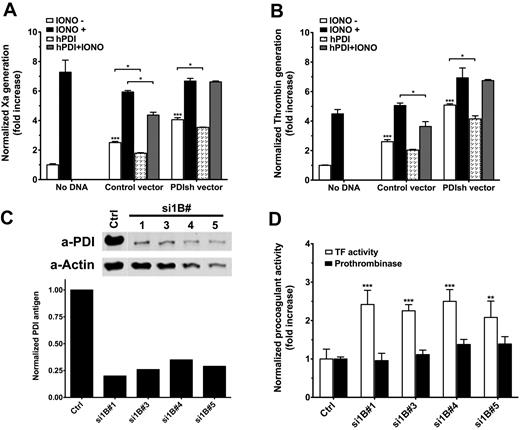
PDI silencing enhances TF procoagulant function
Complementary to antibody studies, we decreased PDI expression in TF-ECs using shRNA transfection. Transient transfection with PDI shRNA vectors significantly increased both TF procoagulant function (Figure 5A) and prothrombinase activity (Figure 5B) measured 48 hours after transfection. Under each condition, ionomycin could further enhance both procoagulant activities, suggesting that PDI silencing does not maximally enhance coagulation. Incubation with exogenous hPDI decreased by approximately 20% the basal TF procoagulant function in both ECs treated with a control vector (P = .014) or PDI shRNA vectors (P = .019). hPDI treatment had a more pronounced effect on ionomycin-induced enhancement of TF procoagulant activity on control cells; however, it did not affect ionomycin treatment on PDI shRNA-treated cells. The reasons behind this apparent discrepancy are not clear at the moment. Similar effects were observed for prothrombinase activity measured on parallel plates (Figure 5B).
PDI silencing affects procoagulant activities on ECs. (A) Analysis of basal and ionomycin-decrypted TF procoagulant activity in response to transient PDI silencing and/or exogenous hPDI addition. TF activity is normalized to TF antigen and represented as fold increase over nontransfected ECs. (B) Analysis of basal and ionomycin-enhanced prothrombinase activity in response to transient PDI silencing and/or exogenous hPDI addition. The prothrombinase activity is normalized to the total protein content and represented as fold increase over nontransfected ECs. (C) Representative Western blot and densitometric analysis of PDI in puromycin-selected PDI shRNA-tranfected TF-EC lines. (D) Comparative analysis of basal TF procoagulant activity (open bars) and prothrombinase activity (solid bars) of PDI shRNA-transfected TF-EC cell lines shown in panel C. Procoagulant activities were normalized to nontransfected TF-ECs. (A-B,D) Data are mean ± SD of triplicates. Statistical analysis was performed by 1-way ANOVA: *P < .05, **P < .01, ***P < .001.
PDI silencing affects procoagulant activities on ECs. (A) Analysis of basal and ionomycin-decrypted TF procoagulant activity in response to transient PDI silencing and/or exogenous hPDI addition. TF activity is normalized to TF antigen and represented as fold increase over nontransfected ECs. (B) Analysis of basal and ionomycin-enhanced prothrombinase activity in response to transient PDI silencing and/or exogenous hPDI addition. The prothrombinase activity is normalized to the total protein content and represented as fold increase over nontransfected ECs. (C) Representative Western blot and densitometric analysis of PDI in puromycin-selected PDI shRNA-tranfected TF-EC lines. (D) Comparative analysis of basal TF procoagulant activity (open bars) and prothrombinase activity (solid bars) of PDI shRNA-transfected TF-EC cell lines shown in panel C. Procoagulant activities were normalized to nontransfected TF-ECs. (A-B,D) Data are mean ± SD of triplicates. Statistical analysis was performed by 1-way ANOVA: *P < .05, **P < .01, ***P < .001.
Several cell lines with low PDI expression (25%-30%) were selected (Figure 5C). Basal TF activity, assessed by Xa generation (Figure 5D open bars), doubled in response to approximately 70% decrease of the endogenous PDI (P < .01 vs control). However, basal prothrombinase activity was not changed on these stable transfected cells (Figure 5D solid bars), suggesting that a new steady-state equilibrium of PS asymmetry is maintained in the absence of PDI. Furthermore, the differences between TF procoagulant function and the prothrombinase assays on these cells support the annexin sensitivity assays (Figure 4B) and indicate that control of PS exposure is one, but probably not the only, mechanism by which extracellular PDI modulates TF-dependent initiation of coagulation.
Extracellular PDI enhances PS internalization
Although hPDI preincubation limits the ionomycin-dependent effects on both TF procoagulant and prothrombinase activities, it is not clear whether this is the result of inhibition of PS efflux or enhanced internalization of exposed PS. To test whether extracellular PDI can modulate PS internalization, we treated ECs with thapsigargin to expose PS, followed by exogenous addition of hPDI. Residual PS was detected by annexin V–FITC labeling.
Annexin V staining is minimal in ECs not treated with thapsigargin, regardless of the subsequent treatment with GSH or PDI (Figure 6A, TG−). In contrast, thapsigargin treatment induced significant PS exposure (Figure 6A, TG+, and Figure 6B). GSH treatment at 10μM, which represents the median reported plasma GSH concentration, induced a small but statistically significant decrease of the thapsigargin-induced PS exposure (Figure 6B). Furthermore, exogenous addition of hPDI reduced annexin V staining to control levels and was statistically different (P < .001) compared with either thapsigargin or thapsigargin plus GSH-treated cells. These results indicate that PDI enhances the internalization of PS exposed in response to thapsigargin. Furthermore, PDI pretreatment with the inhibitory antibody diminished the effects of the oxidoreductase on the apparent flippase activity, suggesting the specificity of the response to PDI. Similar results were obtained by flow cytometry (supplemental Figure 4).
Surface PDI enhances PS internalization in ECs. A small PS efflux was induced in ECs in response to TG stimulation for 1 hour, and subsequent PDI effect on PS internalization was assessed by annexin V–FITC labeling of residual exposed PS. (A) Representative micrographs. (B) Scatter plot representation of MFI ± SEM of 180 to 240 cells per group. Statistical analysis was performed by 1-way ANOVA: ***P < .001.
Surface PDI enhances PS internalization in ECs. A small PS efflux was induced in ECs in response to TG stimulation for 1 hour, and subsequent PDI effect on PS internalization was assessed by annexin V–FITC labeling of residual exposed PS. (A) Representative micrographs. (B) Scatter plot representation of MFI ± SEM of 180 to 240 cells per group. Statistical analysis was performed by 1-way ANOVA: ***P < .001.
Surface PDI modulates PS dynamics
We used the fluorescent analog NBD-PS to study the dynamics of transmembrane transport of PS.37,42 Live cell imaging indicates that the NBD-PS upload is specifically affected by pretreatment with the PDI inhibitory mAb BD34 compared with control ECs (Figure 7A). Although the maximal fluorescence is similar after 15 minutes, NBD-PS uptake in PDI-inhibited ECs is delayed and the slope is milder than in controls. The total lipid upload is dependent on the adsorption of NBD-PS in the exoplasmic leaflet of the plasma membrane and the flippase activity that transports it across the bilayers; and, as such, this is an indirect measure of the flippase activity. To confirm that PDI affects the transmembrane PS transport, we measured, by flow cytometry, the NBD-PS fluorescence during uptake in the presence or absence of sodium dithionite as impermeant quencher of NBD. Figure 7B shows that the percentile PS “flip” was significantly lower in PDI-inhibited ECs compared with control cells (58.28% ± 0.44% vs 69.35% ± 0.60%) after 2 minutes of incubation with NBD-PS (considered T0), and this difference decreased with time (81.98% ± 0.43% vs 87.45% ± 0.40%, at T10). At T20, the NBD-PS quenching was similar between the 2 experimental conditions and plateaued at approximately 95% flip. Once again, the total fluorescent uptake was similar between anti-PDI–treated and isotype-treated ECs. We concluded that PDI inhibition slows the apparent flippase activity responsible for the outside-in PS transport. Similar to previous reports,38 the dynamics of NBD-PC internalization were different from that of NBD-PS, and no significant difference was observed between anti-PDI– and isotype IgG–treated ECs (supplemental Figure 5).
Surface PDI inhibition affects PS dynamics in ECs. (A) Time-lapse imaging analysis of NBD-PS uptake in ECs. Pretreatment with BD34 anti-PDI (■) delayed the NBD-PS uptake compared with isotype control IgG (◇). The arrow indicates the addition of NBD-PS. Data are mean ± SEM of approximately 20 cells. (B) Flow cytometric analysis of the apparent flippase activity on EA.hy926 cells pretreated with the inhibitory BD34 anti-PDI (■) or isotype control (◇), using NBD-PS and the impermeant quencher sodium dithionite. The internalized PS is represented as the percentile dithionite-resistant NBD-PS fluorescence from total NBD-PS fluorescence. Data are mean ± SEM of more than or equal to 10 000 gated events. (C) Time-lapse imaging analysis of NBD-PS efflux in ECs. Pretreatment of ECs with inhibitory BD34 anti-PDI mAb (■) resulted in accelerated PS efflux and faster dithionite quenching compared with isotype-treated ECs (◇). Data are mean ± SEM of 20 cells corrected for bleaching and normalized against the total NBD-PS fluorescence in the absence of dithionite. (D) Scatter plot representation of NBD-PS efflux in ECs. PDI inhibited (■) or control (◇) ECs were exposed to the impermeant sodium dithionite quencher for 30 minutes, and the PS flop is represented by the difference between the total NBD-PS fluorescence in the absence of dithionite and the residual, dithionite-resistant NBD-PS fluorescence. Statistical analysis was performed by the unpaired t test.
Surface PDI inhibition affects PS dynamics in ECs. (A) Time-lapse imaging analysis of NBD-PS uptake in ECs. Pretreatment with BD34 anti-PDI (■) delayed the NBD-PS uptake compared with isotype control IgG (◇). The arrow indicates the addition of NBD-PS. Data are mean ± SEM of approximately 20 cells. (B) Flow cytometric analysis of the apparent flippase activity on EA.hy926 cells pretreated with the inhibitory BD34 anti-PDI (■) or isotype control (◇), using NBD-PS and the impermeant quencher sodium dithionite. The internalized PS is represented as the percentile dithionite-resistant NBD-PS fluorescence from total NBD-PS fluorescence. Data are mean ± SEM of more than or equal to 10 000 gated events. (C) Time-lapse imaging analysis of NBD-PS efflux in ECs. Pretreatment of ECs with inhibitory BD34 anti-PDI mAb (■) resulted in accelerated PS efflux and faster dithionite quenching compared with isotype-treated ECs (◇). Data are mean ± SEM of 20 cells corrected for bleaching and normalized against the total NBD-PS fluorescence in the absence of dithionite. (D) Scatter plot representation of NBD-PS efflux in ECs. PDI inhibited (■) or control (◇) ECs were exposed to the impermeant sodium dithionite quencher for 30 minutes, and the PS flop is represented by the difference between the total NBD-PS fluorescence in the absence of dithionite and the residual, dithionite-resistant NBD-PS fluorescence. Statistical analysis was performed by the unpaired t test.
We used time-lapse microscopy and dithionite quenching of exposed NBD-PS to analyze PDI contribution to the apparent floppase activity. The kinetics of PS efflux, shown as normalized residual PS fluorescence versus time (Figure 7C), indicate that the anti-PDI treatment accelerates the rate of exofacial exposure of NBD-PS compared with control. Endpoint analysis of the normalized residual NBD-PS fluorescence (Figure 7D) shows that the percentile PS “flop” is significantly higher in PDI-inhibited cells than in control cells (37.69% ± 1.83%, n = 96, vs 22.94% ± 2.09%, n = 100). Because NBD-PS that reaches the endomembrane system is not subject to dithionite quenching regardless of being the substrate for bilayer transport or not, the observed difference in PS efflux is expected to be lower than the actual PS flop occurring at plasma membrane. Overall, these results indicate that the net PS exposure observed in response to inhibition of extracellular PDI on EC is the result of accelerated PS efflux and inhibited PS influx.
Discussion
This study provides new insights into the mechanisms of PDI contribution to coagulation in ECs. To our knowledge, this is the first experimental evidence showing that modulation of extracellular PDI regulates PS exposure. In this model, PDI is a negative regulator of coagulation and PS exposure by affecting both the apparent flippase and floppase activities.
Under basal conditions, cellular TF is “cryptic” with little procoagulant activity. Activation of TF procoagulant function (“decryption”) is thought to be one of the initial steps in the coagulation process.14 Although evidence of TF decryption in vivo is still elusive, the procoagulant potential of TF in vitro is enhanced by cell lysis, calcium ionophores, or apoptotic signals.13 The most widely accepted mechanism for TF decryption is the exposure of anionic phospholipids, such as PS, on the cell surface, although PS-independent mechanisms have also been proposed.19-23 In this study, we show that PDI can directly modulate the PS exposure and that its effects on coagulation on ECs are at least in part the result of PS exposure.
In agreement with previous reports,44 we detected low levels of surface PDI on nonpermeabilized ECs. It is not clear how PDI escapes the ER because it contains a KDEL retention signal, or how it is retained on the cell surface.25 One possibility is that PDI reaches the surface in association with a secreted protein. Although TF-PDI complexes have been documented,14,45 TF may not be the primary carrier as we have detected PDI on the cell surface of nonstimulated ECs with minimal TF expression. The punctated staining of surface PDI suggests the association of PDI with specialized membrane rafts. Interestingly, PDI is one of the highest hits identified in the proteome of EC shed microparticles,46 and lipid rafts seem to be involved in the generation of microparticles.22 Although further studies are needed to understand the mechanisms of PDI release and its distribution on the cell surface, the extracellular localization of the enzyme allows for the redox modulation of cell surface reactions, such as the initiation of coagulation.
Contradictory reports from in vitro cellular models have debated the redox switch model of PDI-dependent TF decryption.47 In monocytic cell lines, TF decryption correlates with oxidized TF monomers.23 In keratinocytes, PDI was proposed to regulate the equilibrium between the coagulation and signaling pools of TF,45 and, similar to our results, siRNA-mediated PDI knockdown enhanced coagulation, suggesting a negative correlation between PDI and coagulation. These observations, however, were not recapitulated in a breast cancer cell line27 with increased TF expression. The group proposed that PS contaminants in the PDI reagent may be responsible for the increased TF procoagulant activity.43 None of these studies, however, addresses the exposure of anionic phospholipids, which is known to influence coagulation at multiple levels.
In the present study, we show that PDI is a negative regulator of coagulation in ECs because both antibody-mediated inhibition of cell surface PDI and PDI silencing enhance TF procoagulant activity, whereas exogenous hPDI limits ionomycin-induced TF decryption. Specifically, inhibitory antibodies to PDI had a procoagulant effect, suggesting that PDI oxidoreductase activity is important for the regulation of coagulation. The enhanced prothrombinase activity observed in response to PDI inhibition suggests a net PS exposure that may also contribute to TF procoagulant function. Accordingly, PDI-dependent enhancement of Xa generation was sensitive to annexin V. However, the annexin V treatment, which inhibits 70% of ionomycin-induced TF decryption, reduced by only half the PDI-dependent increase in TF activity, suggesting that other mechanisms, such as the proposed TF redox switch, may also be involved. In agreement, PDI-silenced cell lines exhibited enhanced TF procoagulant function in the absence of significant changes in prothrombinase activities. Further studies are needed to understand the relative propensity of PS exposure and TF redox switch in TF decryption.
Although it is known that sulfhydryl-modifying agents can affect both PS efflux and influx,32 there is no evidence that modulation of an extracellular oxidoreductase could regulate the activity of PS translocases. Using annexin V–FITC labeling, we show in this study that surface PDI modulation affects PS exposure directly and the PS dynamics are influenced by the availability of the oxidoreductase and the redox conditions. In light of our data, the sulfhydryl-modifying reagents could affect PDI, the substrate transporters, or both. Because the dynamic PS equilibrium at the plasma membrane is maintained by the concerted action of 3 classes of aminophospholipid transporters (flippases, floppases, and scramblases), we further investigated PDI effects on the apparent flippase and floppase activities. Our data suggest that scramblases (PLSCR) may not be directly involved in PDI regulation of PS exposure because in silico analysis of PLSCR members did not reveal any cysteine residues in their extracellular domain, and PDI inhibition did not induce a detectable calcium influx. Consequently, the net exofacial exposure of PS in response to surface PDI inhibition on ECs could be achieved by an increased floppase activity, a decreased flippase activity combined with a small constitutive efflux of PS, or a combination of the two.
The dynamics of NBD-PS uptake indicate that the apparent flippase activity decreases in response to surface PDI inhibition, although, given enough time, all NBD-PS is internalized. In contrast, the apparent flippase activity on ECs is sensitive to PDI concentration because exogenous addition of PDI accelerated PS internalization after thapsigargin treatment. These results indicate that surface PDI modulation can either inhibit or enhance the flippase activity.
Because the nonmediated flop of plasma membrane aminophospholipids is very slow, the rapid net exposure of PS in response to PDI inhibition (30 minutes) cannot be explained by flippase inhibition alone. In agreement with this, we show that the efflux rate of PS is accelerated in response to PDI inhibition compared with controls. If floppase activity were not affected by PDI, one would expect a similar rate of quenching of NBD-PS in response to spontaneous inside-out flop of the lipid. Interestingly, PDI has been shown to associate with the transporter associated with antigen processing (TAP1),48 a member of the MDR/TAP subfamily of ABC-type transporters that also includes putative floppases. Furthermore, the putative floppases from the MRP/ABCC subfamily of ABC transporters translocate glutathione and/or glutathione derivates,49,50 and their function may be modulated by reversible S-glutathionylation reactions. Identification of transporters and reconstitution studies will be required to analyze the mechanisms of PDI contribution to PS transport.
In conclusion, our work has established that PDI oxidoreductase function on cell surface is important for the maintenance of membrane asymmetry, acting as a negative regulator of PS externalization by affecting both the apparent flippase and floppase activities. Thus, PDI may significantly contribute to the regulation of coagulation on the cell surface, as well as other physiopathologic processes involving PS exposure, such as the clearance of apoptotic and tumoral cells. This study reveals a new pathway that could ultimately provide novel targets for therapeutic interventions in thrombotic and inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Cora-Jean Edgell (University of North Carolina, Chapel Hill) for the EA.hy926 cells, Dr James H. Morrissey (University of Illinois at Urbana-Champagne) for the anti-TF antibodies, Dr Roger Tsien (University of California, San Diego) for mYFP, and Dr Charles T. Esmon (Oklahoma Medical Research Foundation) for prothrombinase reagents.
N.I.P. is a PhD candidate at Oklahoma University Health Sciences Center, and this work was submitted in partial fulfillment of the requirement for the PhD.
This work was supported in part by the American Heart Association (grant 0615595Z, N.I.P.; grant 0755765Z, C.L.) and the National Institutes of Health (RO1 GM037704, F.L.).
National Institutes of Health
Authorship
Contribution: N.I.P. performed experiments; and N.I.P., C.L., and F.L. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Florea Lupu, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: florea-lupu@omrf.org.

![Figure 1. Low levels of PDI are expressed on the surface of ECs. Confocal analysis of PDI immunostaining was performed on nonpermeabilized (A) or Triton X-100-permeabilized (B) EA.hy926 cells and compared with an irrelevant rabbit IgG or secondary antibody alone. Scatter plot representation and statistical analysis (one-way analysis of variance [ANOVA]) of MFIs of more than 50 individual cells per experimental condition are shown on the right. (C) Flow cytometric analysis of PDI immunostaining on surface or Triton-permeabilized ECs, shown as MFI ± SEM. ***P < .001, unpaired t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/6/10.1182_blood-2009-10-249607/4/m_zh89991055410001.jpeg?Expires=1767715008&Signature=r-eUyN7R-bRweZlEk-GDGhUMcF~JsrXb5wh1SeT6jtCFw3I3ivMU2Apx3BA9g0yteEp5ehVHU7-AongHDSD3s4diMZ4IgocHf9pm7uRIp~aN2aPaaM1Ptb0xRR~J-KDZJFeH17gY9aBAgJLmiZwamq264u8pvi7q7-Xe7fSl0dkCJBBsTIjMiUtebPYgx63qmMH9ACqIkzsRB8daoYQdNu4~hxVoYwJG4sEiEFjxXsX0~DVg7d1c4nK-uz9HgIRDMn6xZzmwgF49q~jjs-PJlzoPUb7ZkzM6Hg1VKeV9ZAd4JbqzEQ0rcBy7C98di1iB0iBg5YLTcdnOCrGZt-~wPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal