Abstract
Although allogeneic hematopoietic stem cell transplantation has recently been applied to patients with myelofibrosis with reproducible engraftment and resolution of marrow fibrosis, no data describe the outcomes of umbilical cord blood transplantation. We describe 14 patients with primary (n = 1) and secondary myelofibrosis (n = 13) who underwent reduced-intensity umbilical cord blood transplantation. Conditioning regimens included fludarabine and graft-versus-host disease prophylaxis composed cyclosporine/tacrolimus alone (n = 6) or a combination of tacrolimus and mycophenolate mofetil (n = 8). Thirteen patients achieved neutrophil engraftment at a median of 23 days. The cumulative incidence of neutrophil and platelet engraftment was 92.9% at day 60 and 42.9% at day 100, respectively. Posttransplantation chimerism analysis showed full donor type in all patients at a median of 14 days. The use of umbilical cord blood could be feasible even for patients with severe marrow fibrosis, from the viewpoint of donor cell engraftment.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered the only curative therapy for primary myelofibrosis (MF) and MF secondary to hematologic malignancies.1 Myeloablative conditioning regimens are associated with high rates of transplantation-related mortality (TRM), especially among elderly patients.2-4 Recent reports indicate that reduced-intensity conditioning (RIC) regimens can improve outcomes in such patients.5-8 These reports also confirm the safety and effectiveness of bone marrow (BM) and mobilized peripheral blood stem cells (PBSCs) from matched related or unrelated donors as stem cell sources. In contrast, the feasibility of umbilical cord blood transplantation (CBT) for MF is unknown.
CBT is a valuable alternative to allo-HSCT for treating patients with hematologic diseases who do not have matched related or unrelated donors and who need urgent transplantation.9-12 On the other hand, engraftment delay or failure is one of the most critical issues that can arise after CBT. The limited doses of total nucleated cells and CD34+ cells in umbilical cord blood and a human leukocyte antigen (HLA) disparity influence the kinetics of hematopoietic recovery.13-15 Considering these disadvantages of CBT, delayed engraftment or engraftment failure is a great concern for MF patients who undergo CBT.16 The goal of this study is to evaluate the feasibility of reduced-intensity CBT (RI-CBT) for MF.
Methods
The records of all patients who underwent RI-CBT at Toranomon Hospital from August 2003 and December 2008 were reviewed to identify patients who had histologically confirmed MF before starting the conditioning regimen. Marrow fibrosis was assessed on silver-stained BM trephine biopsies and classified into 4 grades according to the World Health Organization classification.17 All the patients were incurable using conventional approaches and lacked an HLA-identical sibling or a suitable unrelated donor from the Japan Marrow Donor Program. Cord blood units serologically matching more than or equal to 4 of 6 HLA antigens and containing at least 1.8 × 107 nucleated cells/kg of recipient body weight before freezing were obtained from the Japan Cord Blood Bank Network. Conditioning regimens were determined at the discretion of each physician according to the patients' disease, disease status, and history of prior therapy. Information about baseline demographics, clinical characteristics, transplantation, and its outcome were collected from medical records. Assessment of engraftment, chimerism (one or more times a week), pre-engraftment immune reactions, graft-versus-host disease (GVHD), and supportive care during transplantation were performed as previously reported.18-20 Cumulative incidences were estimated for neutrophil and platelet engraftment. Overall survival was estimated using the Kaplan-Meier method, taking the interval from date of transplantation to death or last contact.21 The Institutional Review Board of Toranomon Hospital approved the study, and written informed consent was provided by all patients to use their records in accordance with the Declaration of Helsinki.
Results and discussion
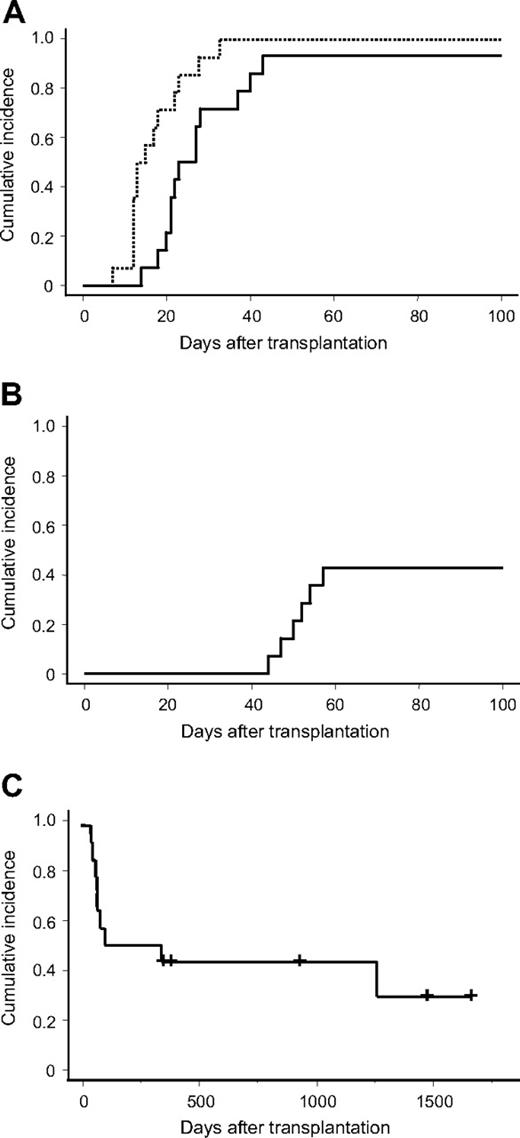
Fourteen MF patients (median age, 57.5 years; range, 46-72 years) were extracted. Table 1 shows the clinical characteristics of the patients. They had primary MF (n = 1), leukemic transformation from MF secondary to polycythemia vera or essential thrombocytosis (n = 2), or MF secondary to acute myeloid leukemia (AML; n = 11; AML with multilineage dysplasia in all patients except for one with de novo AML). All but one patient had the highest-grade MF. The median time from diagnosis to transplantation was 303 days (range, 92-1732 days). Table 2 shows the transplantation characteristics. All received purine analog–based conditioning regimens composing fludarabine phosphate (125-180 mg/m2), melphalan (80-140 mg/m2), or intravenous busulfan (12.8 mg/kg) and 0 to 8 Gy of total body irradiation. GVHD prophylaxis included tacrolimus and mycophenolate mofetil for 8 patients, tacrolimus, or cyclosporine A alone in 6. Neutrophil and platelet engraftment was achieved in 13 and 6 patients, respectively, of the 14 patients. The median time to engraftment was 23 days (range, 14-43 days) and 53 days (range, 44-102 days) for neutrophils and platelets, respectively. The cumulative incidence of neutrophil engraftment at day 60 and platelet engraftment at day 100 was 92.9% and 42.9%, respectively (Figure 1A-B). Chimerism analysis of the peripheral blood of 8 patients and the BM of 6 showed that donor chimerism was complete (donor > 90%) in all of them. The median length of time required to achieve complete donor chimerism was 14 days (range, 7-33 days; Figure 1A). Of the 14 patients, 9 (64%) developed pre-engraftment immune reactions. Five (36%) developed acute GVHD grades 2 to 4. No extensive chronic GVHD was observed in 6 evaluable patients (Table 3). Five patients remained alive at last contact, representing an estimated probability of overall survival of 28.6% at 4 years (Figure 1C). All the patients who could not achieve platelet engraftment died, whereas 4 of 7 patients (57%) who achieved platelet engraftment survived. In 9 patients who died after RI-CBT, 5 patients died of relapsed leukemia. Non–relapse-related causes of death composed infection (n = 2), GVHD (n = 1), and multiple organ failure (n = 1). Marrow fibrosis disappeared in 2 evaluated patients who survived beyond 100 days.
Patient characteristics
| Patient no. . | Age, y/sex . | Diagnosis . | Disease status . | Time from diagnosis to transplantation, d . | Pretransplantation MF grade . | Splenomegaly . | Cytogenetics . |
|---|---|---|---|---|---|---|---|
| 1 | 55/M | AML/MF/ET | PIF | 1732 | 3 | Yes | Normal |
| 2 | 53/M | PMF | Untreated | 307 | 3 | Yes | NA |
| 3 | 61/M | AML/MDS | PIF | 116 | 3 | Yes | Complex* |
| 4 | 51/F | AML/MDS | PIF | 740 | 3 | Yes | Normal |
| 5 | 61/F | AML/MDS | PIF | 227 | 3 | Yes | NA |
| 6 | 55/M | AML/MDS | Untreated | 299 | 3 | Yes | Complex |
| 7 | 46/M | AML/MDS | Untreated | 600 | 3 | Yes | NA |
| 8 | 58/M | AML/MDS | Untreated | 544 | 3 | Yes | Complex |
| 9 | 67/F | AML/MF/PV | Untreated | 150 | 3 | Yes | t(3;3)(q21;q26), −7 |
| 10 | 53/M | De novo AML | PIF | 111 | 3 | No | Complex |
| 11 | 57/F | AML/MDS | Untreated | 352 | 3 | Yes | Complex with t(9;22)(q34;q11) |
| 12 | 62/M | AML/MDS | Untreated | 147 | 3 | Yes | add(1)(p32), −7 |
| 13 | 72/F | AML/MDS | PIF | 329 | 2 | No | Complex with t(9;22)(q34;q11) |
| 14 | 66/M | AML/MDS | Untreated | 92 | 3 | No | Normal |
| Patient no. . | Age, y/sex . | Diagnosis . | Disease status . | Time from diagnosis to transplantation, d . | Pretransplantation MF grade . | Splenomegaly . | Cytogenetics . |
|---|---|---|---|---|---|---|---|
| 1 | 55/M | AML/MF/ET | PIF | 1732 | 3 | Yes | Normal |
| 2 | 53/M | PMF | Untreated | 307 | 3 | Yes | NA |
| 3 | 61/M | AML/MDS | PIF | 116 | 3 | Yes | Complex* |
| 4 | 51/F | AML/MDS | PIF | 740 | 3 | Yes | Normal |
| 5 | 61/F | AML/MDS | PIF | 227 | 3 | Yes | NA |
| 6 | 55/M | AML/MDS | Untreated | 299 | 3 | Yes | Complex |
| 7 | 46/M | AML/MDS | Untreated | 600 | 3 | Yes | NA |
| 8 | 58/M | AML/MDS | Untreated | 544 | 3 | Yes | Complex |
| 9 | 67/F | AML/MF/PV | Untreated | 150 | 3 | Yes | t(3;3)(q21;q26), −7 |
| 10 | 53/M | De novo AML | PIF | 111 | 3 | No | Complex |
| 11 | 57/F | AML/MDS | Untreated | 352 | 3 | Yes | Complex with t(9;22)(q34;q11) |
| 12 | 62/M | AML/MDS | Untreated | 147 | 3 | Yes | add(1)(p32), −7 |
| 13 | 72/F | AML/MDS | PIF | 329 | 2 | No | Complex with t(9;22)(q34;q11) |
| 14 | 66/M | AML/MDS | Untreated | 92 | 3 | No | Normal |
AML indicate acute myeloid leukemia; MF, myelofibrosis; ET, essential thrombocythemia; PIF, primary induction failure; PMF, primary myelofibrosis; AML/MDS, acute myeloid leukemia with multilineage dysplasia; NA, not available; and PV, polycythemia vera.
Complex karyotype was defined as 3 or more abnormalities at pretransplantation evaluation.
Transplantation characteristics
| Patient no. . | TNC, ×107/kg . | CD34+, ×105/kg . | Sex match . | HLA match . | Blood type match . | Conditioning regimen . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|
| 1 | 2.52 | 0.823 | MM | 4/6 | MM | F125/M80/TBI4 | CsA |
| 2 | 2.62 | 0.678 | MM | 4/6 | MM | F125/M80/TBI4 + SRT | TAC |
| 3 | 3.17 | 1.60 | Match | 4/6 | Match | F125/M80/TBI4 | TAC |
| 4 | 2.43 | NA | MM | 4/6 | Match | F125/M80/TBI4 | TAC |
| 5 | 3.94 | 2.26 | MM | 5/6 | Match | F180/M140 | TAC/MMF |
| 6 | 2.31 | 0.887 | MM | 4/6 | MM | F125/M80/TBI4 | TAC |
| 7 | 2.72 | 1.03 | Match | 4/6 | MM | F125/Mel140/TBI4 | TAC/MMF |
| 8 | 2.46 | 0.773 | MM | 4/6 | Match | F180/M140 | TAC |
| 9 | 1.99 | 1.24 | MM | 4/6 | MM | F125/M80/TBI4 + SRT | TAC/MMF |
| 10 | 3.25 | 0.547 | MM | 4/6 | Match | F125/M140/TBI4 | TAC/MMF |
| 11 | 3.31 | 1.31 | MM | 4/6 | Match | F125/M80/TBI8 | TAC/MMF |
| 12 | 2.37 | 0.873 | MM | 4/6 | MM | F125/M80/TBI8 | TAC/MMF |
| 13 | 2.51 | 0.993 | MM | 4/6 | Match | Flu180/B12.8/TBI2 | TAC/MMF |
| 14 | 2.50 | 0.554 | MM | 5/6 | Match | F125/M120 | TAC/MMF |
| Patient no. . | TNC, ×107/kg . | CD34+, ×105/kg . | Sex match . | HLA match . | Blood type match . | Conditioning regimen . | GVHD prophylaxis . |
|---|---|---|---|---|---|---|---|
| 1 | 2.52 | 0.823 | MM | 4/6 | MM | F125/M80/TBI4 | CsA |
| 2 | 2.62 | 0.678 | MM | 4/6 | MM | F125/M80/TBI4 + SRT | TAC |
| 3 | 3.17 | 1.60 | Match | 4/6 | Match | F125/M80/TBI4 | TAC |
| 4 | 2.43 | NA | MM | 4/6 | Match | F125/M80/TBI4 | TAC |
| 5 | 3.94 | 2.26 | MM | 5/6 | Match | F180/M140 | TAC/MMF |
| 6 | 2.31 | 0.887 | MM | 4/6 | MM | F125/M80/TBI4 | TAC |
| 7 | 2.72 | 1.03 | Match | 4/6 | MM | F125/Mel140/TBI4 | TAC/MMF |
| 8 | 2.46 | 0.773 | MM | 4/6 | Match | F180/M140 | TAC |
| 9 | 1.99 | 1.24 | MM | 4/6 | MM | F125/M80/TBI4 + SRT | TAC/MMF |
| 10 | 3.25 | 0.547 | MM | 4/6 | Match | F125/M140/TBI4 | TAC/MMF |
| 11 | 3.31 | 1.31 | MM | 4/6 | Match | F125/M80/TBI8 | TAC/MMF |
| 12 | 2.37 | 0.873 | MM | 4/6 | MM | F125/M80/TBI8 | TAC/MMF |
| 13 | 2.51 | 0.993 | MM | 4/6 | Match | Flu180/B12.8/TBI2 | TAC/MMF |
| 14 | 2.50 | 0.554 | MM | 5/6 | Match | F125/M120 | TAC/MMF |
TNC indicates total nucleated cell count; MM, mismatch; F, fludarabine (mg/m2); M, melphalan (mg/m2); TBI, total body irradiation; CsA, cyclosporine; SRT, splenic radiation; TAC, tacrolimus; MMF, mycophenolate mofetil; and B, intravenous busulfan (mg/kg).
Cumulative incidence of engraftment. (A) Solid and broken lines indicate cumulative incidence of neutrophil engraftment and complete donor chimerism, respectively. (B) Cumulative incidence of platelet engraftment. (C) Overall survival.
Cumulative incidence of engraftment. (A) Solid and broken lines indicate cumulative incidence of neutrophil engraftment and complete donor chimerism, respectively. (B) Cumulative incidence of platelet engraftment. (C) Overall survival.
Outcome of RI-CBT
| Patient no. . | Neutrophil engraftment, d . | Platelet engraftment, d . | Pre-engraftment immune reactions* . | aGVHD 2-4 . | aGVHD 3-4 . | cGVHD . | Survival . | Survival from transplantation, d . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 52 | No | Yes | No | No | Dead | 1264 | Relapse |
| 2 | 22 | 54 | Yes | No | No | NE | Alive | 1672 | NA |
| 3 | 23 | Not engrafted | Yes | Yes | Yes | NE | Dead | 68 | Infection |
| 4 | 40 | 102 | Yes | Yes | No | Limited | Alive | 1481 | NA |
| 5 | 18 | 44 | Yes | No | No | No | Dead | 344 | Relapse |
| 6 | 14 | Not engrafted | Yes | No | No | NE | Dead | 78 | Relapse |
| 7 | 21 | 57 | Yes | Yes | Yes | Limited | Alive | 937 | NA |
| 8 | Not engrafted | Not engrafted | No | No | No | NE | Dead | 42 | Infection |
| 9 | 37 | Not engrafted | Yes | No | No | NE | Dead | 45 | MOF |
| 10 | 28 | Not engrafted | Yes | Yes | Yes | NE | Dead | 64 | GVHD |
| 11 | 27 | Not engrafted | Yes | No | No | NE | Dead | 61 | Relapse |
| 12 | 43 | NA | No | No | No | Limited | Alive | 392 | NA |
| 13 | 21 | 47 | No | No | No | Limited | Alive | 355 | NA |
| 14 | 20 | 50 | No | No | No | NE | Dead | 100 | Relapse |
| Patient no. . | Neutrophil engraftment, d . | Platelet engraftment, d . | Pre-engraftment immune reactions* . | aGVHD 2-4 . | aGVHD 3-4 . | cGVHD . | Survival . | Survival from transplantation, d . | Cause of death . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | 52 | No | Yes | No | No | Dead | 1264 | Relapse |
| 2 | 22 | 54 | Yes | No | No | NE | Alive | 1672 | NA |
| 3 | 23 | Not engrafted | Yes | Yes | Yes | NE | Dead | 68 | Infection |
| 4 | 40 | 102 | Yes | Yes | No | Limited | Alive | 1481 | NA |
| 5 | 18 | 44 | Yes | No | No | No | Dead | 344 | Relapse |
| 6 | 14 | Not engrafted | Yes | No | No | NE | Dead | 78 | Relapse |
| 7 | 21 | 57 | Yes | Yes | Yes | Limited | Alive | 937 | NA |
| 8 | Not engrafted | Not engrafted | No | No | No | NE | Dead | 42 | Infection |
| 9 | 37 | Not engrafted | Yes | No | No | NE | Dead | 45 | MOF |
| 10 | 28 | Not engrafted | Yes | Yes | Yes | NE | Dead | 64 | GVHD |
| 11 | 27 | Not engrafted | Yes | No | No | NE | Dead | 61 | Relapse |
| 12 | 43 | NA | No | No | No | Limited | Alive | 392 | NA |
| 13 | 21 | 47 | No | No | No | Limited | Alive | 355 | NA |
| 14 | 20 | 50 | No | No | No | NE | Dead | 100 | Relapse |
aGVHD indicates acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; NE, not evaluable; NA, not applicable; and MOF, multiple organ failure.
Pre-engraftment immune reactions were diagnosed when febrile patients developed skin eruption, diarrhea, jaundice, or body weight gain of more than 10% of baseline, with no direct evidence of infection or adverse effects of medication, developing more than 6 days before engraftment.18
This study demonstrated that umbilical cord blood results in successful engraftment, even for patients with severe marrow fibrosis in the setting of the RIC regimen, which was similar to that of other stem cell sources, such as BM and PBSCs.2-8,22 Although marrow fibrosis has historically been considered as a relative contraindication to transplantation because of concerns over an insufficient and/or dysfunctional niche in which allogeneic hematopoietic stem cell engraftment may proceed, recent outcomes of allo-HSCT for MF support the concept that marrow fibrosis is not an absolute barrier to allogeneic hematopoietic stem cell engraftment.1 However, data from these reports are limited to transplantations with BM and PBSCs, and no information is available about umbilical cord blood. Delayed hematopoietic recovery and low engraftment rate, perhaps because of limited infused cell doses and HLA disparities, might limit the use of umbilical cord blood in these cases.13-15,19 However, the present study demonstrated an equivalent or superior engraftment rate after CBT compared with allo-HSCT using other stem cell sources.1-8 We also confirmed an early chimerism switching in the present study. All 14 patients achieved complete donor chimerism at a median of 14 days, which was much earlier than that with neutrophil engraftment. Moreover, we histologically confirmed that RI-CBT had the potential to cure marrow fibrosis in 2 evaluated patients. These data suggest that RI-CBT is an encouraging strategy for treating MF.
Despite successful engraftment, overall survival was poor in the present study compared with previous reports. However, this result does not eliminate the feasibility of RI-CBT for MF patients. Our patient series included only one primary MF. In 13 of 14 patients, MF coexisted with AML simultaneously. High prevalence of concurrent AML with MF in the present study probably made overall survival poorer. However, MF with AML is also challenging issues in real clinical settings. Physicians occasionally face rapidly growing AML cases with concurrent marrow fibrosis, especially in the elderly, for whom urgent allo-HSCT is the only curative therapy. For those patients, CBT is attractive because of its accessibility. In this viewpoint, we think that the feasibility of RI-CBT suggested in the present study is encouraging.
In conclusion, our data suggest that RI-CBT is feasible, even for patients with severe marrow fibrosis, from the viewpoint of donor cell engraftment. Especially for MF with AML, further improvements are required in the next place to overcome poor survival resulting from relapse.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all physicians (Dr Akiko Yoneyama, Dr Shigeyoshi Makino, Dr Hideki Araoka), nurses, pharmacists (Mr Tadaaki Ito, Ms Yumiko Uchida), transplantation coordinator (Ms Madoka Narita), data managers (Ms Naomi Yamada, Ms Kaoru Kobayashi, and Ms Rumiko Tsuchihashi), and support personnel for their care of the patients involved in this study.
This work was supported in part by the Japanese Ministry of Health, Labor, and Welfare (Research Grant for Tissue Engineering H17-014 and Research Grant for Allergic Disease and Immunology H20-015).
National Institutes of Health
Authorship
Contribution: S. Takagi performed transplantation, analyzed extracted data, and contributed to writing the paper; Y.O. analyzed histologic sections; N.U., K.T., K.I., M.T., H.Y., Y.A.-M., K.M., A.W., and S.M. performed transplantation and contributed to writing the paper; N.M. performed transplantation and supported statistical analysis; K.O. reviewed histologic sections and contributed to writing the paper; and S. Taniguchi reviewed the study method and organized this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shinsuke Takagi, Department of Hematology, Toranomon Hospital, 2-2-2 Toranomon, Minato-Ku, Tokyo 105-0001, Japan; e-mail: shinsuke-takagi@umin.net.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal