Abstract
Despite the clear importance of Hedgehog (Hh) signaling in blood vascular development as shown by genetic analysis, its mechanism of action is still uncertain. To better understand the role of Hh in vascular development, we further characterized its roles in vascular development in mouse embryos and examined its interaction with vascular endothelial growth factor (VEGF), a well-known signaling pathway essential to blood vascular development. We found that VEGF expression in the mouse embryo depended on Hh signaling, and by using genetic rescue approaches, we demonstrated that the role of Hh both in endothelial tube formation and Notch-dependent arterial identity was solely dependent on its regulation of VEGF. In contrast, overactivation of the Hh pathway through deletion of Patched1 (Ptch1), a negative regulator of Hh signaling, resulted in reduced vascular density and increased Delta-like ligand 4 expression. The Ptch1 phenotype was independent of VEGF pathway dysregulation and was not rescued when Delta-like ligand 4 levels were restored to normal. These findings establish that Hh uses both VEGF- and Notch-dependent and -independent mechanisms to pattern specific events in early blood vascular development.
Introduction
The blood vascular system is the first organ to develop in the mammalian embryo, establishing a basic circulatory system between embryonic (E) day 7.5 and E8.5. The vessels that initially comprise the blood vascular system form by the process of vasculogenesis: the assembly of endothelial precursors (angioblasts) into simple endothelial tubes in the absence of preexisting vessels. Failure to establish these initial vessels results in growth arrest and embryonic lethality by E9.5.1 Subsequent remodeling and expansion of these and other vessels requires the intricate and coordinated process of angiogenesis: the branching, splitting, pruning, and proliferation of preexisting vessels. The Hedgehog (Hh) signaling pathway is known to play an important role in blood vessel development,2 but its mechanism of action remains incompletely defined. To better understand this role, we have used the mouse embryo as a model to examine the interplay between Hh and vascular endothelial growth factor (VEGF) signaling, another pathway essential for blood vascular development.3
The interplay between Hh and VEGF in vascular development is exemplified in the establishment of artery/vein identity in zebrafish. In this pathway, notochordal Hh induces expression of VEGF in neighboring somites, which in turn induces Notch-dependent arterial identity in endothelial cells.4 In mice, Notch signaling is essential for artery specification,5 and numerous receptors and ligands for this pathway become restricted to arteries,6 key among them are Notch1,7 Notch4,8 and Delta-like ligand 4 (Dll4).9 Although the role for Notch in assignment of arterial identity in mice is unequivocal, the requirement for either Hh or VEGF upstream of Notch in vivo during mouse development has not been determined.
Hh is also essential for endothelial tube formation. In mouse embryos, this requirement is limited to the anterior (but not posterior) region of the dorsal aorta and to the vessels of the yolk sac.10 Endothelial cells express the Hh-binding receptor Patched1 (Ptch1),10,11 and Hh ligand can induce endothelial cord and tube formation in vitro.10,12 However, mice lacking the common Hh signal transducer smoothened (Smo), specifically in endothelial cells, develop normally into adulthood,13 arguing the role of Hh in endothelial tube formation is nonautonomous. Hh is known to induce expression of proangiogenic cytokines, including VEGF in nonendothelial tissues,11 and mice lacking VEGF experience severe defects in blood vessel development, resulting in embryonic lethality around the same time as embryos lacking Smo,14,15 making it a likely candidate for a downstream mediator of Hh in endothelial tube formation.
In this study, we investigated the interaction between Hh and VEGF signaling pathways in vasculogenesis in the mouse embryo. We found that Hh is required for VEGF expression during mouse vasculogenesis and that both were required for artery/vein identity in addition to their role in endothelial tube formation. Restoration of VEGF pathway activation to endothelial cells of Hh mutants was sufficient to rescue both the arterial identity and endothelial tube formation and defects in Hh mutants. In contrast, we found that hyperactivation of the Hh signaling pathway through deletion of Ptch1 caused increased aorta diameter and increased expression of the arterial Notch ligand Dll4; however, levels of VEGF and its major receptors were not affected in Ptch1 mutants. Together, these results demonstrate that Hh signaling modulates distinct vascular patterning events in mammalian embryos through both VEGF-dependent and -independent mechanisms.
Methods
Mice
Experiments were approved by the Animal Care Committee of the Hospital for Sick Children and were conducted in accord with guidelines established by the Canadian Council on Animal Care. The following mouse lines were used in this study: Smo (generated by crossing Smoflox/flox16 with a ubiquitous cre deleter strain, resulting in Smodel/+ followed by outcrossing to wild-type CD1 strain mice to remove cre) and Ptch1 (generated by crossing Ptch1flox/flox17 with a ubiquitous cre deleter strain, resulting in Ptch1del/+ followed by outcrossing to wild-type CD1 strain mice to remove cre). Flt1lacZ/+,18 Dll4lacZ/+,9 and VEGFhypo/+14 were all maintained on a CD1 background. Embryos from timed pregnancy were collected either at E8.5 (6- to 8-somite stage), or at E9.5 (approximately 20-somite stage). Noon on the day the plug was observed was considered to be E0.5.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed by the use of a modified version of Lickert et al19 but with the following modifications: proteinase K treatment was replaced with a 20-minute treatment with 3% hydrogen peroxide, levamisole was excluded from all solutions, and (with the exception of VEGF assessment in Smo mutant and control embryos), methanol fixation was replaced with a 20-minute treatment with 50mM sodium azide before hydrogen peroxide treatment. Antisense DIG-labeled RNA probes were transcribed from sequence verified clones encoding mouse Dll4 (a generous gift from Prof Antonio Duarte, Instituto Gulbenkian de Ciência, Oeiras, Portugal) or mouse VEGF. Stained embryos were imaged with a Leica MZ16F stereoscope equipped with a Micropublisher 5.0 RTV (QImaging) camera and Volocity software (Improvision). All postacquisition image analysis and modification were conducted with Photoshop (Adobe).
Whole-mount immunofluorescence and optical projection tomography imaging
Whole-mount immunohistochemistry was performed by the use of a modified version of Walls et al,20 but methanol fixation was replaced with a 20-minute treatment with 50mM sodium azide in phosphate-buffered saline containing 0.1% Tween 20. Stained embryos were cleared in a graded series of glycerol in phosphate-buffered saline (up to 80%) before imaging. Epifluorescence imaging was performed by the use of a Zeiss Axiovert 200M inverted microscope with a Ph1 Plan-Neofluar 5×/0.15 NA objective. Images were captured with an Axiocam HRm camera (Zeiss) with Axiovision 4.6 software (Zeiss). All postacquisition image analysis and modification were conducted by the use of Photoshop (Adobe). Confocal imaging was performed with a Zeiss LSM510 META confocal microscope, Plan-Neofluar 25×/0.8 NA immersion correction objective with pinhole set to 1 Airy Unit. YZ and XZ stacks were compiled from the original data with Volocity software (Improvision). Optical projection tomography (OPT) was performed as previously described.20
Quantitative reverse transcription polymerase chain reaction
In all cases, the extraembryonic structures of yolk sac, allantois, and amnion were removed from the embryo before RNA was extracted. Where indicated in Figures 1 and 2, embryos were further subdissected into specific embryonic regions. Total RNA was extracted from embryos by the use of Trizol Reagent (Invitrogen) and reverse transcribed (RT) with the QuantiTect RT kit (QIAGEN) according to the manufacturer's instructions. Quantitation of gene expression was performed by real-time polymerase chain reaction (PCR) with a LightCycler 480 (Roche). Each reaction was performed in technical triplicate with 2X SYBR mix (Roche) containing 100 ng of cDNA and 300 nmol/L each of gene-specific forward and reverse oligonucleotides (ie, Vegf sense caggctgctgtaacgatgaa, antisense tatgtgctggctttggtgag; Flt1 sense caagcaggccagactctctt, antisense gggagtgatgctcagccttt; Dll4 sense tgcctgggaagtatcctcac, antisense tagagtccctgggagagcaa; Ptch1 sense gcgctaatgttctgaccaca, antisense agcacaaatgttccaacttcc; Gli1 sense cttcaaggcccaatacatgc antisense taggacttccgacagccttc; Flk1/Kdr sense tttggcaaatacaacccttcaga, antisense gcagaagatactgtcaccaccg; Nrp1 sense aacccacatttcgatttgga, antisense aaggtgcaatcttcccacag; and Polr2a sense cccaaacttgggggactaat, antisense aggaagcccacatgaaacac) in a 50-cycle reaction (95°C 10 seconds, 60°C 10 seconds, 72°C 10 seconds). Specificity of oligonucleotides was confirmed by melt curve analysis and gene expression determined by relative quantification with correction for amplification efficiency for each oligonucleotide pair (determined by standard curve analysis). All data were analyzed by the ΔCT method and expressed as gene expression relative to the internal loading control, Polr2a.
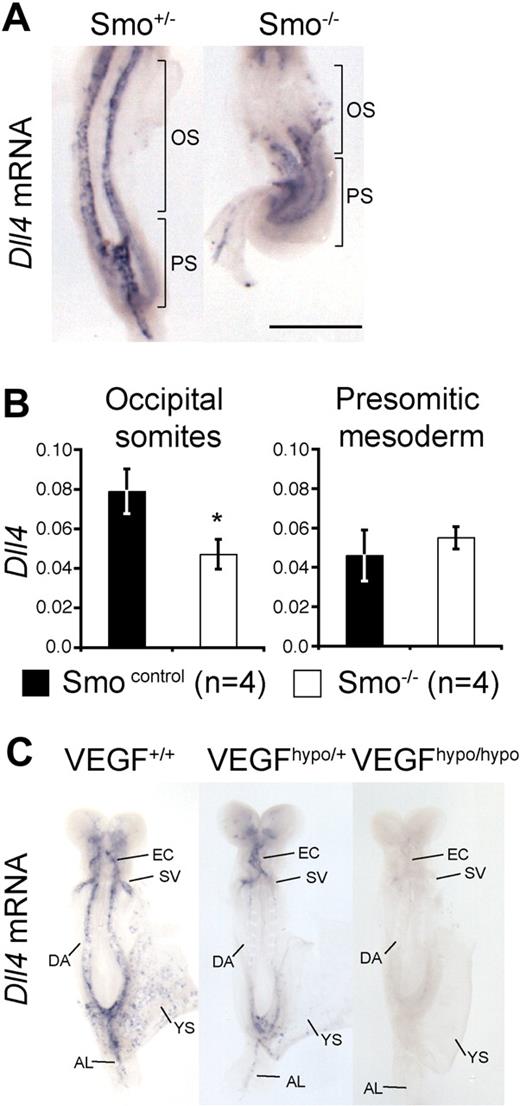
Hh and VEGF are required for arterial identity in mice. (A) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (approximately 8-somite stage) Smo+/− and Smo−/− embryos. Brackets indicate the occipital somite (OS) and presomitic/primitive streak (PS) regions of the embryo microdissected for Q-RT-PCR in panel B. Scale bar equals 500 μm. (B) Q-RT-PCR for Dll4 mRNA in Smo control (Smo+/+, n = 2 and Smo+/−, n = 2) and Smo−/− (n = 4) littermates. Q-RT-PCR was performed on distinct regions of 8-somite stage embryos corresponding to the occipital somites region (labeled OS in panel A) and presomitic region (labeled PS in panel A) in control or mutant embryos. Data are presented as Dll4 expression relative to the loading control, Polr2a. Asterisk indicates statistical significance between control and mutant sample groups, P < .05. (C) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (7- to 8-somite stage) VEGF hypomorphic embryos. Representative embryos from VEGF+/+ (n = 4), VEGFhypo/+ (n = 3), and VEGFhypo/hypo (n = 2) littermates are shown. EC indicates endocardium; SV, sinus venosus; DA, dorsal aorta; YS, yolk sac plexus; and AL, allantoic artery.
Hh and VEGF are required for arterial identity in mice. (A) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (approximately 8-somite stage) Smo+/− and Smo−/− embryos. Brackets indicate the occipital somite (OS) and presomitic/primitive streak (PS) regions of the embryo microdissected for Q-RT-PCR in panel B. Scale bar equals 500 μm. (B) Q-RT-PCR for Dll4 mRNA in Smo control (Smo+/+, n = 2 and Smo+/−, n = 2) and Smo−/− (n = 4) littermates. Q-RT-PCR was performed on distinct regions of 8-somite stage embryos corresponding to the occipital somites region (labeled OS in panel A) and presomitic region (labeled PS in panel A) in control or mutant embryos. Data are presented as Dll4 expression relative to the loading control, Polr2a. Asterisk indicates statistical significance between control and mutant sample groups, P < .05. (C) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (7- to 8-somite stage) VEGF hypomorphic embryos. Representative embryos from VEGF+/+ (n = 4), VEGFhypo/+ (n = 3), and VEGFhypo/hypo (n = 2) littermates are shown. EC indicates endocardium; SV, sinus venosus; DA, dorsal aorta; YS, yolk sac plexus; and AL, allantoic artery.
Hh requires VEGF for arterial identity and endothelial tube formation. (A) Whole-mount in situ hybridization for Vegf mRNA in E8.5 Smo mutant embryos. Representative embryos and all yolk sacs from 8 somite stage Smo+/+ (n = 2) and Smo−/− (n = 3) littermates are shown. Brackets indicate the occipital somite (OS) and presomitic/primitive streak (PS) regions of the embryo microdissected for Q-RT-PCR in panel B. (B) Q-RT-PCR for Vegf mRNA in Smo control (Smo+/+, n = 2 and Smo+/−, n = 2) and Smo−/− (n = 4) embryos. Q-RT-PCR was performed on distinct regions of 8-somite stage littermates corresponding to the occipital somites region (labeled OS in panel A) and presomitic region (labeled PS in panel A) in control or mutant embryos. Data are presented as Vegf expression relative to the loading control, Polr2a. Asterisk indicates statistical significance (t test) between control and mutant sample groups, P < .05. (C) Whole-mount PECAM1 staining of E8.5 (approximately 8-somite stage) Smo;Flt1 compound mutant embryos. Representative examples of littermate Smo+/+;Flt1+/+ (n = 2), Smo+/+;Flt1+/− (n = 2), Smo−/−;Flt1+/+ (n = 1), and Smo−/−;Flt1+/− (n = 4) embryos are shown. Brackets indicate the region of the dorsal aorta affected in Smo−/−;Flt1+/+ embryos and rescued in Smo−/−;Flt1+/− embryos. The affected region corresponds exactly to the location of the somites in each embryo. Scale bar represent 500 μm. (D) Whole-mount PECAM1 staining of E8.5 (approximately 8-somite stage) Smo;Flt1 compound mutant yolk sacs. Representative examples of littermate Smo+/+;Flt1+/+ (n = 2), Smo+/+;Flt1+/− (n = 2), Smo−/−;Flt1+/+ (n = 1), Smo−/−;Flt1+/− (n = 4) embryos are shown. Brackets indicate the position of the blood islands. Scale bar represents 500 μm. (E) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (approximately 8-somite stage) Smo+/−;Flt1+/− (n = 1), Smo−/−;Flt1+/+ (n = 3), and Smo−/−;Flt1+/− (n = 2) littermate embryos. Bracket indicates occipital somite region. Scale bar represents 500 μm.
Hh requires VEGF for arterial identity and endothelial tube formation. (A) Whole-mount in situ hybridization for Vegf mRNA in E8.5 Smo mutant embryos. Representative embryos and all yolk sacs from 8 somite stage Smo+/+ (n = 2) and Smo−/− (n = 3) littermates are shown. Brackets indicate the occipital somite (OS) and presomitic/primitive streak (PS) regions of the embryo microdissected for Q-RT-PCR in panel B. (B) Q-RT-PCR for Vegf mRNA in Smo control (Smo+/+, n = 2 and Smo+/−, n = 2) and Smo−/− (n = 4) embryos. Q-RT-PCR was performed on distinct regions of 8-somite stage littermates corresponding to the occipital somites region (labeled OS in panel A) and presomitic region (labeled PS in panel A) in control or mutant embryos. Data are presented as Vegf expression relative to the loading control, Polr2a. Asterisk indicates statistical significance (t test) between control and mutant sample groups, P < .05. (C) Whole-mount PECAM1 staining of E8.5 (approximately 8-somite stage) Smo;Flt1 compound mutant embryos. Representative examples of littermate Smo+/+;Flt1+/+ (n = 2), Smo+/+;Flt1+/− (n = 2), Smo−/−;Flt1+/+ (n = 1), and Smo−/−;Flt1+/− (n = 4) embryos are shown. Brackets indicate the region of the dorsal aorta affected in Smo−/−;Flt1+/+ embryos and rescued in Smo−/−;Flt1+/− embryos. The affected region corresponds exactly to the location of the somites in each embryo. Scale bar represent 500 μm. (D) Whole-mount PECAM1 staining of E8.5 (approximately 8-somite stage) Smo;Flt1 compound mutant yolk sacs. Representative examples of littermate Smo+/+;Flt1+/+ (n = 2), Smo+/+;Flt1+/− (n = 2), Smo−/−;Flt1+/+ (n = 1), Smo−/−;Flt1+/− (n = 4) embryos are shown. Brackets indicate the position of the blood islands. Scale bar represents 500 μm. (E) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (approximately 8-somite stage) Smo+/−;Flt1+/− (n = 1), Smo−/−;Flt1+/+ (n = 3), and Smo−/−;Flt1+/− (n = 2) littermate embryos. Bracket indicates occipital somite region. Scale bar represents 500 μm.
Results
Hh and VEGF are required upstream of Notch for arterial identity in mice
Because the Notch ligand Dll4 is specific to arterial endothelial cells and is essential for arterial identity in mouse,9 we chose to investigate its expression as a readout for arterial fate in mice in which Hh signaling was blocked (Smo−/−). Dll4 mRNA expression was readily detected in the dorsal aorta of 8 somite (E8.5) Smo+/− embryos (Figure 1A). In contrast, Dll4 mRNA was extremely weak or absent from the anterior portion of the aorta underlying the occipital somites of Smo−/− embryos but normal in the portion underlying the presomitic tail bud region (Figure 1A). By using quantitative RT-PCR (Q-RT-PCR), we confirmed that Dll4 mRNA was significantly reduced in the occipital somite region but expressed at normal levels in the presomitic region of Smo−/− embryos (Figure 1B).
We next examined Dll4 expression in mice carrying a hypomorphic allele for VEGF. Embryos homozygous for this allele phenocopy VEGF heterozygote-null embryos.14 Dll4 mRNA was lost from all arterial endothelial populations in VEGFhypo/hypo embryos (Figure 1C). Interestingly, we observed a dose-dependent reduction of Dll4 mRNA levels in VEGFhypo/+ and VEGFhypo/hypo embryos compared with littermate controls (Figure 1C). This result is consistent with observations made during angiogenesis that suggest arterial identity is defined by a gradient of VEGF activity.21,22 These results demonstrate that both Hh and VEGF are required upstream of Notch for arterial identity in mammalian embryos, but that although the role of Hh is region specific, VEGF is required for all arterial populations.
Hh and VEGF are required for tube formation in mouse embryos
Embryos lacking Hh signaling experience region-specific defects in endothelial tube formation.10 Because VEGF mutants also have severe defects in vessel formation,14,15,23 we sought to determine the similarity between the Hh and VEGF phenotypes. Consistent with previous reports,10 we found tube formation defects in the anterior dorsal aorta and yolk sac vessels of 8-somite stage (E8.5) Smo−/− embryos but not in the posterior dorsal aorta when staining with the pan-endothelial marker platelet/endothelial cell adhesion molecule-1 (PECAM1; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Whole-mount PECAM1 staining revealed the dorsal aortas of 8-somite stage VEGFhypo/hypo embryos to be severely constricted compared with their control littermates (supplemental Figure 1B). Tube formation was affected along the entire length of the VEGFhypo/hypo aorta, demonstrating that VEGF is required for tube formation in all endothelial cells of the aorta. Yolk sac vessels were essentially absent from VEGFhypo/hypo embryos, preventing assessment of tube formation in this population.
In addition to reduced vessel diameter, whole-mount PECAM1 staining revealed an additional vascular patterning defect in Smo mutants. Whereas the region of the embryo underlying the lateral plate mesoderm of control embryos was avascular, in Smo mutants, this region was occupied by a vascular plexus, which appeared to connect the dorsal aorta to the yolk sac vascular plexus (arrowheads in supplemental Figure 1A). This phenotype was not evident in VEGFhypo/hypo embryos (arrowheads in supplemental Figure 1B), suggesting the requirement for Hh in this process is independent of VEGF.
Hh is required for VEGF expression in a regionally restricted fashion in mouse embryos
Given that VEGF loss-of-function mutants experienced similar arterial identity and tube formation defects as Smo mutants, we next sought to assess whether VEGF expression was reduced in Smo mutants. Whole-mount in situ hybridization for Vegf mRNA was used to assess Vegf levels in Smo−/− and littermate controls. Vegf mRNA levels in 6- and 8-somite stage Smo−/− embryos were reduced specifically in the occipital somites compared with their wild-type littermates but appeared normal in the presomitic mesoderm of the tail bud (Figure 2A; supplemental Figure 2). Q-RT-PCR showed Vegf mRNA levels to be significantly reduced in the cephalic somite region but not the tail bud of Smo−/− embryos compared with their littermate controls (Figure 2B). Reduced Vegf mRNA also was evident in the yolk sacs of Smo mutants at the 6- and 8-somite stages by whole-mount in situ hybridization (Figure 2A; supplemental Figure 2). Collectively, these results indicate that in mouse embryos, Hh is necessary for normal VEGF expression in those regions of the Smo−/− embryo where Hh is required for tube formation and arterial endothelial identity.
VEGF is sufficient for endothelial tube formation and arterial identity in the absence of Hh signaling
We next sought to address whether the vascular patterning defect of Smo−/− embryos could be rescued by restoration of VEGF signaling. We excluded using VEGFhyper mice (which have elevated VEGF expression from the endogenous Vegf locus as the result of increased mRNA stability24 ) for this analysis because their elevated levels of VEGF require the endogenous Vegf promoter and hence likely require an intact Hh signaling pathway. Furthermore, VEGF expression levels vary significantly between tissues and developmental stages in the VEGFhyper embryos, and VEGF levels at the stage in which we were interested (E8.5) had not been assessed.24 Therefore, we could not be certain this mutant would generate sufficient levels of VEGF to test the epistatic relationship between VEGF and Smo. Instead, because Smo mutants retained some residual levels of VEGF expression (∼ 60% of normal) in areas of defective vascular development, we reasoned that increasing the sensitivity of endothelial cells to the VEGF still present might rescue the Smo phenotype. We addressed this by breeding Smo mutants to mice lacking the high-affinity VEGF receptor Flt1. During vasculogenesis, Flt1 is inhibitory for VEGF signaling and is generated both as membrane-bound and soluble isoforms.25,26
Consistent with previous observations, Flt1−/− embryos lacked a defined cranial plexus at E8.5 (approximately 8 somites) and developed what appeared to be severe endothelial and blood island overgrowth in the yolk sac (supplemental Figure 3A).18 Flt1+/− embryos also showed subtle abnormalities in yolk sac and dorsal aorta vascular development, with wider vessel channels, suggesting VEGF perception is heightened in the absence of one copy of Flt1 (Figure 2C). When we used Q-RT-PCR, we determined Dll4 mRNA expression to be 2.1-fold greater in Flt1 mutant embryos at E8.5 (8 somites) compared with stage-matched littermates (supplemental Figure 3B), which is consistent with VEGF-promoting arterial identity.
Because Flt1+/− mice presented a phenotype consistent with elevated VEGF signaling, we assessed whether removal of one copy of Flt1 could rescue the Smo phenotype. Vascular morphology was assessed in Smo−/−;Flt1+/− embryos by whole-mount PECAM1 staining of embryos at E8.5. Smo−/−;Flt1+/+ embryos displayed the expected constriction of the anterior dorsal aorta and yolk sac vessels at E8.5 (Figure 2C). In contrast, the dorsal aorta of Smo−/−;Flt1+/− embryos at E8.5 were of comparable diameter to those of their wild-type littermates (4 of 4 embryos; Figure 2C). In contrast, the ectopic vessels present adjacent to the dorsal aorta in the Smo−/− embryos were not rescued by removal of one copy of Flt1 (Figure 2C). In the yolk sac, the majority (3 of 4) of Smo−/−;Flt1+/− embryos showed restoration of vessel diameter (Figure 2D).
In situ hybridization for Dll4 mRNA showed only partial restoration of Dll4 expression to the anterior dorsal aorta in Smo−/−;Flt1+/− embryos (Figure 2E). Because VEGF regulates Dll4 expression in a dose-dependent fashion (see “Hh and VEGF are required upstream of Notch for arterial identity in mice”) the removal of 1 copy of Flt1 may not have restored VEGF signaling to high enough levels to promote complete arterialization of the dorsal aorta. It remains to be addressed whether removal of both copies of Flt1 could fully restore Dll4 expression and arterial identity in Smo−/− aortas. These results demonstrate that Hh does not act cell autonomously to regulate endothelial tube formation and arterial identity but instead acts to induce VEGF in nonendothelial tissues, which then regulates tube formation and arterial identity.
Vascular patterning in Ptch1 mutants is phenotypically similar to Flt1 mutants
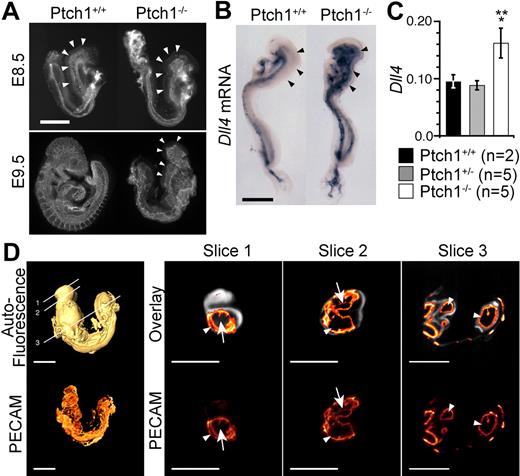
Because Hh ligand is sufficient to promote an angiogenic response when added exogenously to organ cultures or to mice and chicks,10,11,13,27 we sought to determine what effect overactivation of the Hh pathway would have on early stages of vascular development and its relationship to VEGF by assessing vascular development in embryos lacking the inhibitory Hh receptor Ptch1. PECAM1 staining revealed that the normally branched network of small-caliber capillaries that comprised the cephalic plexus of E8.5 (8 somite) Ptch1+/+ embryos was absent in stage matched Ptch1−/− littermates (Figure 3A,D). Instead, these embryos presented a dilated dorsal aorta with few side branches and no anterior cardinal vein, similar to Flt1 mutants. Whole-mount in situ hybridization at the 8-somite stage showed that the cephalic vasculature in Ptch1−/− embryos consisted exclusively of Dll4-expressing endothelium (Figure 3B). Increased Dll4 expression was confirmed by Q-RT-PCR, which found Dll4 levels to be 1.8-fold greater in Ptch1−/− mutants compared with their littermates (Figure 3C), similar to Flt1 mutants. By E9.5, all Ptch1 mutants examined showed the expected developmental arrest and had failed to turn.17
Defective vascular patterning in Ptch1 mutant embryos. (A) Lateral view of whole-mount PECAM1 staining of E8.5 (8-somite stage) and E9.5 Ptch1 mutant and littermate control embryos. Representative images from 8-somite stage Ptch1+/+ (n = 3) and Ptch1−/− (n = 3) embryos are shown. Arrowheads indicate cranial plexus region. Scale bar represents 500 μm. (B) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (8-somite stage) Ptch1 mutant embryos. Representative embryos from Ptch1+/+ (n = 2) and Ptch1−/− (n = 2) littermate embryos are shown. Arrowheads indicate the region in which Dll4 is ectopically expressed in Ptch1 mutants versus controls. Scale bar represents 500 μm. (C) Q-RT-PCR for Dll4 mRNA in Ptch1+/+ (n = 2), Ptch1+/− (n = 5), and Ptch1−/− (n = 5) embryos. Q-RT-PCR was performed on 8-somite stage embryos from which the yolk sac, allantois, and amnion had been removed. Data are presented as Dll4 expression relative to the loading control, Polr2a. Single asterisk indicates statistical significance (t test) between Ptch1+/+ and Ptch1−/− sample groups (P < .05). Double asterisk indicates statistical significance (t test) between Ptch1+/− and Ptch1−/− sample groups, (P < .005). (D) FDR-OPT images of an E9.5 Ptch1−/− embryo. Top left side is a surface-rendered image of the 3D reconstructed autofluoresence signal with locations of digital slices shown in right side panels indicated. Bottom left side is a 3D reconstruction of PECAM1-Cy3 signal alone. Right side images are digital slices from 3D reconstructed FDR-OPT data, top are PECAM1-Cy3 signal overlaid on autofluoresence signal, bottom are PECAM1-Cy3 signal from the same slice alone. Arrows indicate PECAM1+ structures surrounding other tissue (autofluoresence signal present), arrowheads indicate PECAM1+ signal encompassing hollow space (autofluoresence signal not present).
Defective vascular patterning in Ptch1 mutant embryos. (A) Lateral view of whole-mount PECAM1 staining of E8.5 (8-somite stage) and E9.5 Ptch1 mutant and littermate control embryos. Representative images from 8-somite stage Ptch1+/+ (n = 3) and Ptch1−/− (n = 3) embryos are shown. Arrowheads indicate cranial plexus region. Scale bar represents 500 μm. (B) Whole-mount in situ hybridization for Dll4 mRNA in E8.5 (8-somite stage) Ptch1 mutant embryos. Representative embryos from Ptch1+/+ (n = 2) and Ptch1−/− (n = 2) littermate embryos are shown. Arrowheads indicate the region in which Dll4 is ectopically expressed in Ptch1 mutants versus controls. Scale bar represents 500 μm. (C) Q-RT-PCR for Dll4 mRNA in Ptch1+/+ (n = 2), Ptch1+/− (n = 5), and Ptch1−/− (n = 5) embryos. Q-RT-PCR was performed on 8-somite stage embryos from which the yolk sac, allantois, and amnion had been removed. Data are presented as Dll4 expression relative to the loading control, Polr2a. Single asterisk indicates statistical significance (t test) between Ptch1+/+ and Ptch1−/− sample groups (P < .05). Double asterisk indicates statistical significance (t test) between Ptch1+/− and Ptch1−/− sample groups, (P < .005). (D) FDR-OPT images of an E9.5 Ptch1−/− embryo. Top left side is a surface-rendered image of the 3D reconstructed autofluoresence signal with locations of digital slices shown in right side panels indicated. Bottom left side is a 3D reconstruction of PECAM1-Cy3 signal alone. Right side images are digital slices from 3D reconstructed FDR-OPT data, top are PECAM1-Cy3 signal overlaid on autofluoresence signal, bottom are PECAM1-Cy3 signal from the same slice alone. Arrows indicate PECAM1+ structures surrounding other tissue (autofluoresence signal present), arrowheads indicate PECAM1+ signal encompassing hollow space (autofluoresence signal not present).
By this stage, the cranial plexus had still failed to develop in Ptch1 mutants, and the dorsal aorta was dilated relative to their control littermates (Figure 3A). Because of the complexity of the E9.5 Ptch1−/− phenotype, vascular development in Ptch1−/− embryos was assessed by the use of high-resolution 3-dimensional frequency distance relationship (FDR)-based OPT.20 The severe developmental arrest of Ptch1 mutants before turning (approximately 10 somites) prevented meaningful comparison with control littermates at E9.5 (Figure 3A). However, the E9.5 Ptch1 mutant vasculature can be compared with equivalently staged 8- to 9- and 10- and 11-somite stage embryos, which have been visualized by FDR-OPT previously, the data for which are freely available for analysis online.20
Overlaying the PECAM1 signal on the autofluoresence background in digital slices generated from reconstructed 3D FDR-deconvolved OPT data of Ptch1−/− embryos showed that the dorsal aorta and branchial arch were hollow and dilated in Ptch1−/− embryos, displacing much of the surrounding mesenchyme (enclosed PECAM1+ structures lacking internal autofluoresence signal, arrowheads in slices 2 and 3; Figure 3D), whereas the cranial vessels formed a sheet surrounding the cranial mesenchyme (PECAM1+ structure surrounding a region of autofluoresence signal, arrows in slice 1 and 2; Figure 3D). This phenotype contrasts that of Flt1, in which the cranial mesenchyme is completely displaced by a dilated, hollow vascular sinus.18 Occasional hollow lumens could be distinguished in the Ptch1−/− cranial endothelial sheet, suggesting it was composed of an endothelial bi-layer lacking the organized, branched structure that normally occurs in wild-type animals (arrowhead, slice 1, Figure 3D). This analysis demonstrates that despite strong phenotypic similarity, Ptch1−/− mutants do not recapitulate all aspects of the Flt1−/− vascular phenotype.
VEGF expression in Ptch1 mutants is normal
The striking similarity of the Ptch1 vascular phenotype to that of Flt1 mutants prompted us to test whether an up-regulation of VEGF expression could account for the Ptch1 vasculogenesis phenotype. Somewhat surprisingly, we could find no evidence for elevated Vegf mRNA in Ptch1 mutants at E8.5 (8 somites) either by whole-mount in situ hybridization or Q-RT-PCR (Figure 4A,B). We then tested the possibility that Ptch1−/− endothelial cells had heightened sensitivity to VEGF because of altered VEGF receptor expression. Levels of the major VEGF receptors Flk1 (Kdr, MGI:96 683) and its coreceptor Nrp1 and Flt1 were not altered in Ptch1 mutants (supplemental Figure 4). Although not statistically significant, levels of Flk1 were if anything reduced in Ptch1 mutants, possibly because of inhibition by elevated Dll4 levels.28-30 No difference was found in the expression of the Hh-responsive genes Gli1 or Ptch1 between Flt1−/− and control embryos, suggesting that Hh signaling was not enhanced in Flt1 mutants (Figure 4C). Collectively, these results suggest the vascular phenotype observed in Ptch1 mutants is not attributable to dysregulated VEGF expression or perception. We next investigated expression of Foxc1 and Foxc2, direct transcriptional activators of Dll4 whose expression in endothelial cells is not regulated by VEGF,31-34 in Ptch1 mutants. Expression of both these transcription factors was normal in Ptch1 mutant embryos, suggesting that increased Dll4 expression was not caused by misexpression of Foxc1 or Foxc2.
Ptch1 vascular phenotype is independent of VEGF and Notch. (A) Whole-mount in situ hybridization for Vegf mRNA in E8.5 Ptch1 mutants. Representative embryos from 8-somite stage Ptch1+/+ (n = 4), Ptch1+/− (n = 5), and Ptch1−/− (n = 3) littermate embryos are shown. (B) Q-RT-PCR for Vegf mRNA in Ptch1+/+ (n = 2), Ptch1+/− (n = 5), and Ptch1−/− (n = 5) embryos. Q-RT-PCR was performed on 8-somite stage littermates from which the yolk sac, allantois, and amnion had been removed. Data are presented as gene expression relative to the loading control, Polr2a. (C) Q-RT-PCR for Ptch1 and Gli1 mRNA in Flt1+/− (n = 4) and Flt1−/− (n = 4) embryos. Q-RT-PCR was performed on 7- to 8-somite stage littermates from which the yolk sac, allantois, and amnion had been removed. Data are presented as gene expression relative to the loading control, Polr2a. (D) Confocal images for whole-mount PECAM1-stained Ptch;Dll4 compound mutants. Representative embryos from 8-somite stage Ptch+/−;Dll4+/+ (n = 2), Ptch+/+;Dll4+/− (n = 2), Ptch−/−;Dll4+/+ (n = 2), Ptch−/−;Dll4+/− (n = 3) littermates are shown. Top panel for each embryo represents projected PECAM1 stain (red) with DRAQ5 nuclear stain (gray). Bottom panels represent single confocal section (xy plane) from each embryo with yz (left) and xz (top) stacks shown (PECAM1, red; DRAQ5 nuclear, gray). Arrows indicate the abnormal, hollow vascular structures in Ptch1−/−;Dll4+/+, and Ptch1−/−;Dll4+/− embryos. Scale bars represent 100 μm.
Ptch1 vascular phenotype is independent of VEGF and Notch. (A) Whole-mount in situ hybridization for Vegf mRNA in E8.5 Ptch1 mutants. Representative embryos from 8-somite stage Ptch1+/+ (n = 4), Ptch1+/− (n = 5), and Ptch1−/− (n = 3) littermate embryos are shown. (B) Q-RT-PCR for Vegf mRNA in Ptch1+/+ (n = 2), Ptch1+/− (n = 5), and Ptch1−/− (n = 5) embryos. Q-RT-PCR was performed on 8-somite stage littermates from which the yolk sac, allantois, and amnion had been removed. Data are presented as gene expression relative to the loading control, Polr2a. (C) Q-RT-PCR for Ptch1 and Gli1 mRNA in Flt1+/− (n = 4) and Flt1−/− (n = 4) embryos. Q-RT-PCR was performed on 7- to 8-somite stage littermates from which the yolk sac, allantois, and amnion had been removed. Data are presented as gene expression relative to the loading control, Polr2a. (D) Confocal images for whole-mount PECAM1-stained Ptch;Dll4 compound mutants. Representative embryos from 8-somite stage Ptch+/−;Dll4+/+ (n = 2), Ptch+/+;Dll4+/− (n = 2), Ptch−/−;Dll4+/+ (n = 2), Ptch−/−;Dll4+/− (n = 3) littermates are shown. Top panel for each embryo represents projected PECAM1 stain (red) with DRAQ5 nuclear stain (gray). Bottom panels represent single confocal section (xy plane) from each embryo with yz (left) and xz (top) stacks shown (PECAM1, red; DRAQ5 nuclear, gray). Arrows indicate the abnormal, hollow vascular structures in Ptch1−/−;Dll4+/+, and Ptch1−/−;Dll4+/− embryos. Scale bars represent 100 μm.
The Ptch1 vascular phenotype is not caused by elevated Dll4
We then tested whether the increased Dll4 expression we observed in Ptch1 mutants was responsible for the Ptch1 vascular phenotype. Because Ptch1 mutants displayed 1.8-fold increase in Dll4 expression over controls (see “Vascular patterning in Ptch1 mutants is phenotypically similar to Flt1 mutants”) and Dll4 is haploinsufficient,9,35,36 we reasoned that the removal of a single copy of Dll4 would rescue the Ptch1 phenotype if it were caused by increased Dll4. At E8.5, PECAM1-stained Ptch1−/−;Dll4+/− embryos showed the same hollow, sinus-like structure and lack of a cranial plexus network observed in Ptch1−/− littermates (Figure 4D). These results show that reducing Dll4 levels in Ptch1 mutants is not sufficient to rescue the Ptch1 mutant vascular phenotype.
Discussion
Hh signaling is a key regulator of vascular development, essential to the assignment of arterial identity to endothelial cells in zebrafish4 and mice (this study), and the process of endothelial tubulogenesis.10 Hh ligands are also known to induce expression of proangiogenic cytokines, including VEGF during neoangiogenesis in the adult11 and during development of the coronary37 and pulmonary13 vascular systems. Interestingly, coronary vascular development appears to require both angiogenesis and vasculogenesis.38 We have addressed whether during mouse vasculogenesis, Hh acts autonomously on endothelial cells, or indirectly via the proangiogenic cytokine VEGF to pattern blood vascular development and found Hh uses both VEGF-dependent and -independent mechanisms (Figure 5).
Interplay between Hh, VEGF, and Notch signaling during mouse vasculogenesis. Summary diagram illustrating the interaction between Hh and VEGF signaling during mouse vascular development. Bold arrows indicate new pathways identified by this study. See “Discussion” for details.
Interplay between Hh, VEGF, and Notch signaling during mouse vasculogenesis. Summary diagram illustrating the interaction between Hh and VEGF signaling during mouse vascular development. Bold arrows indicate new pathways identified by this study. See “Discussion” for details.
We found that Hh was required for the expression of VEGF specifically in those regions of the embryo in which Hh was necessary for artery/vein identity and endothelial tube formation. Notably, although Hh was necessary for VEGF expression, hyperactivation of the Hh pathway through deletion of Ptch1 did not result in elevated VEGF levels. This result would suggest that during vasculogenesis, Hh may simply be required to derepress the VEGF locus, possibly by reducing levels of Gli repressors (the Gli isoform present in Smo mutants), whereas Gli activators (the Gli isoform present in Ptch1 mutants) are not required for transcriptional activation of the VEGF locus, similar to how Hh regulates Gremlin expression during limb development.39-41
VEGF was sufficient in the absence of Hh signaling to promote Notch-dependent arterial identity and endothelial tube formation. These results confirm, we believe for the first time, that the Hh-VEGF-Notch signaling axis required for arterial specification during vasculogenesis in zebrafish is also active in mammalian development. Second, these results show that Hh is entirely dispensable for endothelial tube formation provided sufficient VEGF pathway activation occurs. The results presented here plus the report that endothelial specific deletion of Smo did not cause a vascular phenotype13 strongly argue that Hh regulates endothelial tube formation and arterial identity during mouse vasculogenesis through regulation of VEGF expression rather than through an autonomous action on endothelial cells.
Our findings also demonstrate that Hh uses VEGF-independent means to pattern vessel development. Ptch1 mutants displayed a vascular phenotype remarkably similar to that of the negative VEGF receptor Flt1,18 including elevated expression of Dll4. Despite this, the Ptch1 phenotype was not accompanied by dysregulated expression of VEGF or its major receptors. Forced activation of the Notch signaling pathway in endothelial cells (including by the overexpression of Dll4) results in an expansion of aortic vessel diameter at the expense of veins, and reduced branching morphogenesis.28,42 Restoration of Dll4 to normal levels however, was not sufficient to overcome the Ptch1 vascular phenotype, indicating the Ptch1 phenotype was independent of Dll4 and Notch in general because Dll4 accounts for most if not all activation of Notch in the vasculature between E8.5 and E9.5.9,35,36 Notably, mice engineered to overexpress Dll4 in endothelial cells (4-fold greater than normal) displayed a vascular phenotype that was less severe and occurred later than what we observed in Ptch1 mutants, further arguing that the Ptch1 phenotype could not be explained by dysregulated Dll4 expression.28 Exactly why Dll4 is overexpressed in Ptch1 mutants is unknown; however, we have excluded dysregulated VEGF signaling or misexpression of the VEGF-independent regulators of Dll4 transcription, Foxc1 and Foxc2.
In addition to their tube formation defect, Smo dorsal aortas had aberrant connections to an ectopic vascular plexus under the lateral plate mesoderm. Although this arrangement is similar to veins, which have increased vascular density and connections compared with arteries,43 it is unlikely this aspect of the Smo mutant phenotype occurs by the same mechanism that creates the difference between arteries and veins. Increased vascular density around veins is due to increased VEGF levels in venous territory,43 whereas the increased vascular density around the dorsal aorta in Smo mutants occurs despite reduced VEGF expression. Instead we prefer a model in which an unidentified endothelial repellent normally expressed in the lateral plate mesoderm creates an avascular region adjacent to the dorsal aorta and this repellent is silenced in Smo mutant embryos. We do not believe the ectopic vessels in Smo mutants are responsible for the aorta tube formation defect (either because of diverted blood flow or because they are representative of failed aortic endothelial migration) for the following reasons: Ectopic vessel connections occurred in the posterior region of the Smo mutant embryo (where aorta formation was otherwise normal), failure of aorta tube formation in VEGFhypo mutant was not accompanied by ectopic vessel connections, and finally aorta tube formation was restored in Smo−/−;Flt1+/− embryos despite persistence of these ectopic vessels.
We summarize the effects of Hh on vascular development as follows (Figure 5): On the basis of the Smo mutant phenotype, Hh is required for endothelial tube formation through its regulation of VEGF, which we speculate is through regulation of lumen expansion (not initiation). In contrast sufficient activation of the Hh pathway, as occurs in Ptch1 mutants, regulates tube diameter not by affecting lumen formation or expansion, but instead by reducing vascular density either through the fusion of many small vessel segments into one large vessel, or reduced endothelial sprouting or branching from a precursor vessel resulting in its increased size. Ptch1 mutants underwent growth arrest before the onset of intersomitic vessel sprouting, the best characterized example of dorsal aorta branching morphogenesis, so we were unable to assess whether their dilated aorta diameter was caused by failed endothelial sprouting and migration such as occurs in Notch mutants28,42 or another mechanism.
Because Ptch1 mutants undergo growth arrest before the onset of well-characterized examples of branching morphogenesis or plexus bed remodeling (key angiogenic events), we were unable to assess whether Ptch1 affects aspects of angiogenesis in the same way it affects vasculogenesis. In addition, whether the VEGF-independent Ptch1 phenotype acts autonomously, thereby directly affecting endothelial cell behavior, or nonautonomously via other proangiogenic factors known to be induced by Hh such as the angiopoietins,11 remains to be established. These will be important questions to resolve because Hh signaling is required in general for angiogenesis, and the growth of certain tumors overexpressing Hh ligand was recently found to depend on activation of the Hh pathway in neighboring stroma by tumor-derived ligand.44 Because this is likely to induce a proangiogenic response by the stroma45 a more comprehensive understanding of which cell types respond to the Hh ligand and how they respond during a proangiogenic response may help in devising strategies to treat such tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Rong Mo for expert technical assistance and all members of the Rossant and Hui laboratories for insightful discussions.
This work was supported by grants from the National Cancer Institute of Canada Terry Fox Foundation, Genome Canada, and the Canadian Cancer Society. R.M.H. holds a Canada Research Chair in Imaging, J.R. is a Distinguished Investigator of the Canadian Institutes of Health Research, and L.C. is a C. J. Martin postdoctoral fellow of the National Health and Medical Research Council (Canberra, Australia).
National Institutes of Health
Authorship
Contribution: L.C., C.-C.H., and J.R. conceived and designed the experiments and wrote the manuscript; L.C., E.N., G.A.A., J.C., and R.M.H. performed all experiments; and A.N. provided reagents.
Conflict-of-interest disclosure: R.M.H. is named as an inventor and coapplicant with the Hospital for Sick Children on a patent application “Resolution Improvement in Emission Optical Projection Tomography,” PCT/CA2007/001637. The remaining authors declare no competing financial interests.
The current affiliation for Dr Coultas is The Walter and Eliza Hall Institute of Medical Research, Parkville, Australia.
Correspondence: Leigh Coultas, PhD, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Vic, 3050, Australia; e-mail: lcoultas@wehi.edu.au; or Janet Rossant, PhD, The Hospital for Sick Children, 555 University Ave, Toronto, ON M5G 1X8 Canada; e-mail: janet.rossant@sickkids.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal