Abstract
Mutations in the nicotinamide adenine dinucleotide phosphate+–dependent isocitrate dehydrogenase gene 2 (IDH2) have recently been found in patients with acute myeloid leukemia (AML) as well as in patients with leukemic transformation of myeloproliferative neoplasms. We analyzed 272 adult patients with cytogenetically normal AML (CN-AML) for the presence of IDH2 mutations in codons R140 and R172. IDH2 mutations of amino acid 140 or 172 could be identified in 12.1% of CN-AML patients, with the majority of mutations (90%) occurring at position R140. The incidence of IDH2 mutations in AML patients with aberrant karyotypes (n = 130) was significantly lower (3.8%, P = .006). IDH2 mutations were mutually exclusive with mutations in IDH1. IDH2 mutation status alone or in combination with IDH1 mutations had no impact on response to therapy, overall survival, and relapse-free survival in patients with CN-AML. In conclusion, IDH2 mutations are frequently found in CN-AML, but in our analysis these mutations did not influence treatment outcome. This study was registered at www.clinicaltrials.gov as #NCT00209833.
Introduction
In an attempt to discover unknown molecular alterations in patients with acute myeloid leukemia (AML), whole genome sequencing was performed on AML patients.1 With this approach, a novel mutation in nicotinamide adenine dinucleotide phosphate+–dependent isocitrate dehydrogenase gene (IDH1) at codon R132 has been identified2 previously described in gliomas.3 Since this first description of IDH1 mutations in AML, several reports have confirmed that IDH1 mutations occur in patients with cytogenetically normal AML (CN-AML), with a frequency of 5.5% to 11%.4-7 A strong association between IDH1 mutations and the intermediate-risk karyotypes and concurrent NPM1 mutations has been described.4-7 The prognostic impact of IDH1 mutations in AML is currently being investigated.4-7 A recent study also found mutations in IDH2 in codon R172 at a low frequency in AML patients.6 In addition, a novel mutation in IDH2 in codon R140 could be identified in 2 patients with leukemic transformation of myeloproliferative neoplasm as well as in patients with AML.8,9 IDH2 has the same enzymatic activity as IDH1 but is located in the mitochondrial matrix.
In the present study, we performed a comprehensive analysis of mutations occurring in exon 4 of IDH2 (including both codons R140 and R172) in 272 patients with CN-AML in the context of other known prognostic markers. These patients were intensively treated with a uniform protocol in 2 consecutive multicenter trials. Overall, our data indicate that IDH2 mutations in codons R140 and R172 are frequent but have no prognostic implications in patients with CN-AML when considered alone or in combination with IDH1 mutations and treated with these intensive protocols.
Methods
Patients
We examined 272 patients with CN-AML and 130 AML patients with aberrant karyotypes for mutations in IDH2. Of the latter, 45 had a core-binding factor leukemia, 15 had aberrations of chromosome band 11q23, 13 had a complex karyotype, 12 had an isolated trisomy 8, 7 had a monosomy 7, 5 had aberrations of chromosome 3q, 4 had a del(9q), 3 had a t(6;9), and 26 had various other aberrations. All patients were treated within the multicenter treatment trials AML SHG 0295 or AML SHG 0199. Details of the treatment protocols have been previously reported.10,11 Written informed consent was obtained in accordance with the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Hannover Medical School.
Mutational analysis of IDH2
Preparation of mononuclear cells and analysis of prognostic markers were performed as previously reported.5,12 The genomic region of the IDH2 gene (exon 4), containing the mutational hotspot codons R140 and R172, was amplified using polymerase chain reaction. Sequences of the primers were as follows: forward 5′-GGGGTTCAAATTCTGGTTGA-3′, and reverse 5′-CTAGGCGAGGAGCTCCAGT-3′. Purified polymerase chain reaction fragments were directly sequenced as previously described.12 Point mutations were confirmed in an independent second experiment.
Results and discussion
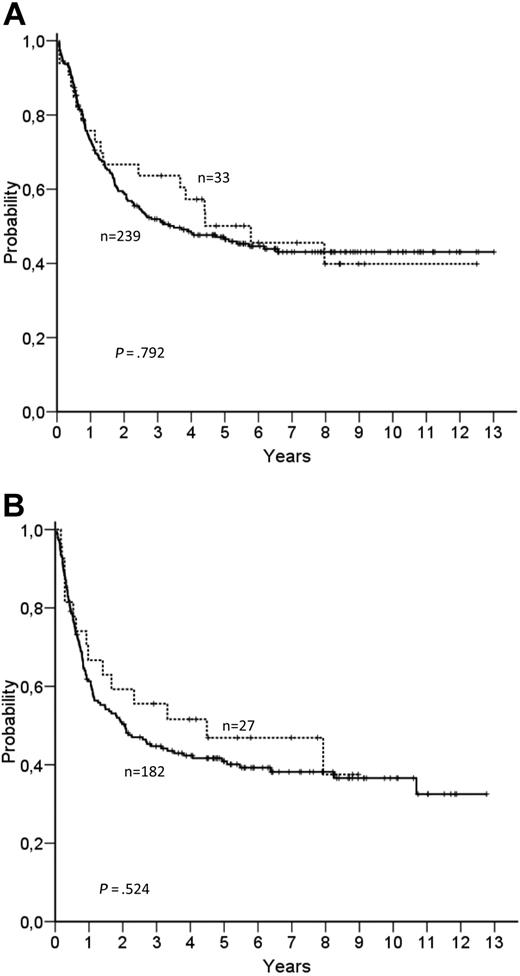
IDH2 mutations were found in 33 of 272 patients (12.1%) with CN-AML (30 patients [11%] in codon R140 and 3 patients [1.1%] in codon R172) and 3.8% of 130 AML patients with aberrant karyotypes. As already described for IDH1,7 IDH2 mutations were associated with a normal karyotype (P = .006), whereas no association with a single chromosomal aberration could be identified for the group of AML patients with aberrant karyotypes (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). IDH2 R140 mutations in CN-AML seem to be similarly common as IDH1 mutations,4,5 but IDH2 mutations in AML appear to be different from those in gliomas where IDH2 R140 mutations have not been described yet.3 The majority (31 of 33 patients, 93.9%) of IDH2 R140 mutations showed a heterozygous conversion of CGG → CAG leading to an arginine to glutamine substitution, whereas one patient had a homozygous CGG → CAG conversion and one patient showed a heterozygous conversion of CGG → CTG leading to an arginine to leucine substitution. Four of 5 patients with mutations in codon 172 had a heterozygous conversion of AGG → AAG leading to an arginine to lysine substitution, whereas one patient showed a heterozygous conversion of AGG → AGT leading to an arginine to serine substitution. By comparing the clinical and hematologic characteristics of IDH2-mutated versus unmutated CN-AML patients, apart from a higher platelet count in patients with IDH2 mutations, no significant difference in relevant markers was found between the 2 groups (supplemental Table 2). However, IDH2 mutations were mutually exclusive with mutations in IDH1. This has also been described in gliomas suggesting overlapping effects of both mutations.3,9 To determine whether patients lose their IDH2 mutation in remission, we examined 5 patients with an IDH2 mutation at the time of diagnosis during the postinduction phase. All 4 patients in continuous remission lost the IDH2 mutation during remission. However, 1 relapsing patient, who had lost the IDH2 mutation in remission, was again positive at relapse. An additional patient with mutant IDH2 at the time of diagnosis was again positive at the time of relapse. These data suggest that IDH2 mutations are stable during disease progression and might be a suitable marker for minimal residual disease monitoring. To analyze the prognostic impact of IDH2 mutations in CN-AML, IDH2 R140 and R172 mutations were combined because of the low incidence of R172 mutations. In univariate and multivariate analysis with adjustment for NPM1, CEBPA, WT1 SNP rs16754, and FLT3-ITD, IDH2 mutations had no influence on achieving complete remission. The complete remission rate was 81.8% versus 78.2% in the IDH2 mutated versus in the IDH2 WT group, respectively (P = .82). Of the 272 patients, 145 died, resulting in a median overall survival (OS) of 46 months. IDH2 mutations had no impact on OS in univariate or multivariate analysis (Figure 1A, Table 1, supplemental Table 3). In addition, no influence on OS was found for IDH2 mutations in an exploratory subgroup analysis for the NPM1mut/FLT3-ITD− patients (supplemental Figure 1A). Of the 214 patients who achieved complete remission, 98 relapsed and 30 died in complete remission, resulting in a median relapse-free survival (RFS) of 25 months and a 6-year RFS of 40% (95% confidence interval, 37%-43%). IDH2 also had no impact on RFS in univariate or multivariate analysis (Figure 1B, Table 1, supplemental Table 3). Again, no influence on RFS was found for IDH2 mutations in an exploratory subgroup analysis for the NPM1mut/FLT3-ITD− patients (supplemental Figure 1B). The lack of a prognostic impact of IDH2 mutations on treatment response and survival is in accordance with results for IDH1 R132 mutations for which we and others did not find any prognostic implications in CN-AML.4,5 When analyzing IDH1/IDH2 mutations together, IDH mutations remained without prognostic effect. The high frequency of IDH2 mutations in CN-AML but the missing prognostic implications for this marker proposes that the potential oncogenic effect of the mutation might be overcome by therapy. As already demonstrated for markers, such as RAS and WT1 mutations, the therapy administered can have a significant influence on prognostic markers.13,14
Kaplan-Meier curves for OS. (A) OS in CN-AML patients according to IDH2 mutations. Dotted line represents patients with mutated IDH2 (n = 33); and solid line, patients with unmutated IDH2 (n = 239; log-rank test, P = .792). (B) RFS in CN-AML patients according to IDH2 mutations. Dotted line represents patients with mutated IDH2 (n = 27); and solid line, patients with unmutated IDH2 (n = 182) (log-rank test, P = .524).
Kaplan-Meier curves for OS. (A) OS in CN-AML patients according to IDH2 mutations. Dotted line represents patients with mutated IDH2 (n = 33); and solid line, patients with unmutated IDH2 (n = 239; log-rank test, P = .792). (B) RFS in CN-AML patients according to IDH2 mutations. Dotted line represents patients with mutated IDH2 (n = 27); and solid line, patients with unmutated IDH2 (n = 182) (log-rank test, P = .524).
Significant variables in multivariate analysis for OS and RFS
| Endpoint/variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| OS | |||
| Age: above vs below median | 2.04 | 1.41-2.99 | < .001 |
| NPM1/FLT3 mutation status: low risk* vs high risk | 0.66 | 0.44-0.97 | .035 |
| Platelets: above vs below median | 0.61 | 0.42-0.88 | .008 |
| WT1 SNP rs16754 | 0.43 | 0.26-0.71 | .001 |
| RFS | |||
| WT1 SNP rs16754 | 0.60 | 0.39-0.92 | .019 |
| NPM1 mutation | 0.51 | 0.35-0.74 | < .001 |
| CEBPA mutation | 0.36 | 0.19-0.67 | .001 |
| Endpoint/variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| OS | |||
| Age: above vs below median | 2.04 | 1.41-2.99 | < .001 |
| NPM1/FLT3 mutation status: low risk* vs high risk | 0.66 | 0.44-0.97 | .035 |
| Platelets: above vs below median | 0.61 | 0.42-0.88 | .008 |
| WT1 SNP rs16754 | 0.43 | 0.26-0.71 | .001 |
| RFS | |||
| WT1 SNP rs16754 | 0.60 | 0.39-0.92 | .019 |
| NPM1 mutation | 0.51 | 0.35-0.74 | < .001 |
| CEBPA mutation | 0.36 | 0.19-0.67 | .001 |
Variables with P values of less than or equal to .1 in univariate analysis were included for the multivariate analysis. For OS, these included age, FLT3-ITD, WT1 expression, extramedullary disease, platelets, NPM1/FLT3 status low risk vs others, CEBPA mutation, and WT1 SNP rs16754. For RFS, these included CEBPA mutation, WT1 SNP rs16754, NPM1 mutation, and NPM1/FLT3 status low risk vs others. Variables evaluated in univariate analysis (see also supplemental Table 3) included FLT3-ITD, age, WT1 expression, extramedullary disease, platelets high vs low, NPM1/FLT3 status low risk vs others, CEBPA mutation, WT1 SNP rs16754, NPM1 mutation, WT1 mutation, sibling vs no sibling donor, white blood cells high vs low, peripheral blasts high vs low, hemoglobin high vs low, de novo vs secondary AML, sex, IDH2 mutation, and IDH1 or IDH2 mutation combined.
HR indicates hazard ratio; and CI, confidence interval.
NPM1/FLT3 low risk is defined as the presence of an NPM1 mutation in the absence of an FLT3-ITD.
In conclusion, we have identified IDH2 mutations of amino acids 140 and 172 in 12.1% of patients with CN-AML, with the majority of the mutations (90%) occurring in position R140. Although in our study these mutations had no prognostic impact, further analyses are necessary to define their role in leukemogenesis and their use as a marker for minimal residual disease monitoring.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and the members of the AML-Study Group as well as Kerstin Görlich, Martin Wichmann, Elvira Lux, Diana Dudacy, and Sylvia Horter for their excellent technical support.
This work was supported by the Deutsche-José-Carreras Leukämie-Stiftung e.V. (grant DJCLS H 06/04v), Kompetenznetz “Akute und chronische Leukämien” (grant 01GI0378), and the Bundesministerium für Bildung und Forschung and the Dieter-Schlag-Stiftung (grant 01KG0605).
Authorship
Contribution: F.T., M.H., J.K., and A.G. designed the research; F.T., F.D., K.W., and M.H. performed the research; D.H., M.L., W.H., L.K., G.S., A.R., W.F., H.K., and G.H. contributed patient samples and clinical data; G.G. and B.S. performed cytogenetic studies; F.T., J.K., and A.G analyzed the data and wrote the paper; and all authors read and agreed to the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Felicitas Thol, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl-Neuberg Str 1, 30625 Hannover, Germany; e-mail: thol.felicitas@mh-hannover.de.
References
Author notes
J.K. and A.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal