Abstract
Treatment with oral melphalan and dexamethasone (M-Dex) was reported to be effective and feasible in patients with systemic light chain amyloidosis (AL) not eligible for high-dose melphalan. We report on 61 patients with advanced AL who were treated with intravenous M-Dex as first-line therapy. Estimated median overall survival (OS) was 17.5 months. Seventeen patients (28%) died within 3 months, mostly of disease-related complications. In addition, nonhematologic toxicity of Common Terminology Criteria grade 3 or 4 was observed in 20 patients, whereas hematologic toxicity was low. Twenty-seven patients (44%) had hematologic response, including complete in 7 patients (11%) and partial remission in 20 patients (33%). Organ response was observed in 15 patients (25%). The amount of the involved free light chains in serum and Karnofsky Index at diagnosis significantly influenced OS. Plasma levels of the cardiac biomarkers before start of treatment and their increase after the third M-Dex cycle also were strong negative predictors of OS. These parameters might help to identify patients who will not benefit from M-Dex chemotherapy.
Introduction
Immunoglobulin light chain amyloidosis (AL) is a monoclonal plasma cell disorder characterized by the accumulation of monoclonal light chain fragments that have undergone a conformational transformation and deposit as amyloid fibrils in different tissues. Heart, kidney, peripheral nerves, liver, and gut are the organs that are affected most often. Although the burden of plasma cells in the bone marrow is generally low, the accumulation of amyloid protein leads to progressive and end-stage organ failure and thereby death. Untreated patients with AL had a median overall survival (OS) of approximately 6 to 12 months.1-3 This short life expectancy is reduced even further to 5 months if patients have severe cardiac involvement.1,2 For approximately 20 years the combination of oral melphalan and prednisone has been the standard treatment, but median OS can only be prolonged to 12 or 18 months on this regimen.4,5
Successful treatment of multiple myeloma with high-dose melphalan (HDM) and autologous stem cell transplantation (auto-SCT) provided the rationale to establish this approach for selected AL patients. Skinner et al6 reported 2004 in a large retrospective study a median OS of 55 months after HDM and auto-SCT. Patients who were not eligible for HDM for any reason remained with a poor prognosis. Palladini et al7,8 suggested that this group of patients can be treated with oral melphalan and high-dose dexamethasone (M-Dex), resulting in a median OS of 61 months. Recently, the authors of a French prospective randomized multicenter trial compared oral M-Dex with HDM and subsequent auto-SCT.9 They could confirm the Italian results obtained with M-Dex, that is, a median OS of 60 months. Moreover, this study failed to show any survival benefit for patients in the HDM arm, probably because of the high treatment-related mortality in 19 (38%) of 50 patients. Currently, oral M-Dex is considered as a first-line standard treatment in AL amyloidosis, at least for patients not eligible for HDM.10
The intravenous application of melphalan might have some advantages in patients with AL, where intestinal resorption, already known to be highly variable in patients with multiple myeloma,11,12 is further complicated by gastrointestinal amyloid involvement. Intravenous administration also was used in other clinical myeloma trials without apparently increased toxicity.13
Measuring the amount of the involved free light chains (iFLCs) is a very useful tool for the initial assessment of the plasma cell dyscrasia in patients with AL. The iFLC concentration reflects the expansion of the aberrant clone and to some extent the severity of organ involvement.14
Among various prognostic factors the extent of cardiac involvement is the most important determinant of clinical outcome.15-17 However, echocardiography and the grading of New York Heart Association (NYHA) stages are not investigator independent. Sensitive and reliable tools to diagnose cardiac involvement are cardiac biomarkers such as cardiac troponins T (cTNT) and N-terminal prohormone of brain natriuretic peptide (NT-proBNP). cTNT is a highly specific marker of myocardial injury,18 and NT-proBNP is a sensitive indicator of myocardial dysfunction.19 In the context of AL, these 2 cardiac biomarkers provide potent prognostic information and led to the Mayo Clinic staging system.20-23 Here we report our detailed experience in treating patients with intravenous M-Dex who were not eligible for HDM by focusing on the impact of baseline iFLC and cardiac biomarkers before and after 3 months of treatment on survival.
Methods
Patients
From September 2004 to September 2007, 176 patients with newly diagnosed systemic AL and an indication for chemotherapy were referred to our outpatient amyloidosis clinic, and 61 patients were treated with M-Dex as first-line therapy and were retrospectively analyzed in this study. They had not been eligible for HDM and auto-SCT for the following reasons: NYHA class greater than stage II, older than 70 years of age, performance status (World Health Organization) greater than 2, systolic blood pressure less than 90 mmHg, symptomatic pleural effusion, or factor X deficiency less than 10% or refusal of HDM. The distribution of the remaining 115 patients was as follows: 60 patients received high-dose chemotherapy; in 32 patients treatment with melphalan/prednisone was recommended mostly because of advanced age (or severely reduced performance status), 10 patients were too sick or too old to be treated with any chemotherapy (n = 5 received heart transplants), and 13 patients died of advanced AL before chemotherapy could be started. Before initiation of M-Dex, patients underwent a complete physical examination, electrocardiogram, and echocardiography. Renal and liver function tests, cardiac biomarkers, urine and serum immunofixation electrophoresis, and an FLC assay were performed. The study was approved by the Ethics Committee of the University of Heidelberg, and patients gave written informed consent in accordance with the Declaration of Helsinki.
Treatment
Patients were treated with 16 mg/m2 of melphalan intravenously on day 1 and 40 mg of Dex orally on days 1 to 4 in 28-day cycles. The melphalan dose was adjusted for kidney function (creatinine clearance 20-40 mL/min: 12 mg/m2 of melphalan; creatinine clearance < 20 mL/min: 8 mg/m2 melphalan). In case of hematologic toxicity (ie, National Cancer Institute Common Terminology Criteria [CTC] greater than grade 2), the dose of melphalan was reduced during the cycles that followed. If patients did not reach a hematologic nadir (white blood cell count < 4/nL and platelet count < 150/nL) the melphalan dose was increased by 25%. Thirty-six patients with symptomatic heart or kidney involvement received a reduced dose of 20 mg of Dex for 4 days. The next cycle was started after 28 days if bone marrow function was recovered (white blood cell count > 2.5/nL and platelets > 100/nL). To reduce the rate of infection we recommended treating patients with ciprofloxacin twice daily from day 1 to 21. Chemotherapy was given at the University of Heidelberg in 22 patients (36%). A total of 48 patients (79%) were admitted as inpatients for the first cycle of M-Dex. Evaluation of hematologic as well as organ response (OR) was performed during a consultation at our outpatient AL clinic after each 3 cycles of M-Dex. Second-line chemotherapy was administered in case of hematologic relapse/progression, if no hematologic remission (HR) could be achieved after 3 to 6 cycles of M-Dex or if organ function deteriorated although the monoclonal gammopathy was in partial remission (PR).
Outcome measures
Primary outcome was assessed by HR and OS.24 Additional outcome variables were event-free survival (EFS), OR, and toxicity. All time-to-event end points were measured beginning from the start of M-Dex therapy. OS was defined as time until death of any cause (failure time) or date of last follow-up (censored time). An event to calculate EFS was considered to be relapse or progression of the monoclonal gammopathy, organ progression, and death as the result of any cause or second-line chemotherapy for patients without HR. Patients who received at least 3 cycles of M-Dex were evaluated for HR and OR. HR and OR were determined according to the international consensus criteria.24 Patients who had an iFLC less than 100 mg/L at diagnosis (n = 11) were evaluated by the use of M protein greater than 5 g/L (n = 8, at least 50% reduction for PR) or FLC difference kappa-lambda greater than 50 mg/L (n = 3, 50 mg/L reduction for PR). OR of soft tissues, lung, polyneuropathy, and gut (which are not included in the consensus criteria) was judged by clinical evidence. Hematologic toxicity was evaluated only by the measurement of white blood cell and platelet counts (anemia was excluded because of the high rate of renal insufficiency). Organ toxicity was classified according to National Cancer Institute CTC for Adverse Events.25 All analyses involving cTNT, NT-proBNP, creatinine clearance, and FLC levels were restricted to patients who had not been dialyzed at that time to avoid a strong bias by renal function. In our study population there was only a limited number of patients with Mayo Clinic I staging (Table 1). Therefore, we focused our analyses on cTNT and NT-proBNP as continuous variables.
Patient characteristics
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 65 (44-76) |
| Sex | |
| Female | 27 |
| Male | 34 |
| Presence of heavy chain | |
| Yes | 24 |
| No | 37 |
| Monoclonal light chain, no. of patients | |
| Kappa | 15 |
| Lambda | 46 |
| Median absolute involved free light chain concentration, mg/L (range)* | 237 (27-1840) |
| Kappa | 467 |
| Lambda | 202 |
| Plasma cell content of the bone marrow, median % (range) | 10 (1-90) |
| Number of organs involved, no. of patients | |
| 1 organ | 5 |
| 2 organs | 25 |
| 3 or more organs | 31 |
| Dominant organ, no. of patients | |
| Heart | 28 |
| Liver | 5 |
| Gut | 4 |
| Kidney | 21 |
| Peripheral nerves | 1 |
| Soft-tissue involvement | 2 |
| NYHA stage, no. of patients | |
| 0 | 4 |
| 1 | 2 |
| 2 | 14 |
| 3 | 36 |
| n.a. | 5 |
| NT-proBNP, ng/L, median (range)* | 4420 (157-27 624) |
| cTNT, μg/L, median (range)* | 0.04 (< 0.005-0.6) |
| Mayo stage, no. of patients* | |
| 1 | 5 |
| 2 | 20 |
| 3 | 28 |
| n.a. | 8 |
| Median Karnofsky Index (range) | 80% (50-90) |
| Median creatinine clearance, mL/min (range)* | 51 (17-134) |
| Median albumin in serum, g/L (range) | 35 (21-49) |
| Reason not eligible for ASCT, no. of patients | |
| Age | 17 |
| Patient refusal | 2 |
| NYHA stage > 2 | 33 |
| Performance status > 2 or Karnofsky Index < 80% | 6 |
| Severe factor X deficiency | 1 |
| Symptomatic pleural effusion | 2 |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 65 (44-76) |
| Sex | |
| Female | 27 |
| Male | 34 |
| Presence of heavy chain | |
| Yes | 24 |
| No | 37 |
| Monoclonal light chain, no. of patients | |
| Kappa | 15 |
| Lambda | 46 |
| Median absolute involved free light chain concentration, mg/L (range)* | 237 (27-1840) |
| Kappa | 467 |
| Lambda | 202 |
| Plasma cell content of the bone marrow, median % (range) | 10 (1-90) |
| Number of organs involved, no. of patients | |
| 1 organ | 5 |
| 2 organs | 25 |
| 3 or more organs | 31 |
| Dominant organ, no. of patients | |
| Heart | 28 |
| Liver | 5 |
| Gut | 4 |
| Kidney | 21 |
| Peripheral nerves | 1 |
| Soft-tissue involvement | 2 |
| NYHA stage, no. of patients | |
| 0 | 4 |
| 1 | 2 |
| 2 | 14 |
| 3 | 36 |
| n.a. | 5 |
| NT-proBNP, ng/L, median (range)* | 4420 (157-27 624) |
| cTNT, μg/L, median (range)* | 0.04 (< 0.005-0.6) |
| Mayo stage, no. of patients* | |
| 1 | 5 |
| 2 | 20 |
| 3 | 28 |
| n.a. | 8 |
| Median Karnofsky Index (range) | 80% (50-90) |
| Median creatinine clearance, mL/min (range)* | 51 (17-134) |
| Median albumin in serum, g/L (range) | 35 (21-49) |
| Reason not eligible for ASCT, no. of patients | |
| Age | 17 |
| Patient refusal | 2 |
| NYHA stage > 2 | 33 |
| Performance status > 2 or Karnofsky Index < 80% | 6 |
| Severe factor X deficiency | 1 |
| Symptomatic pleural effusion | 2 |
ASCT indicates autologous stem cell transplantation; cTNT, cardiac troponin T; n.a., not applicable; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; and NYHA, New York Heart Association.
Patients on dialysis (n = 6) were excluded.
Laboratory methods
NT-proBNP and cTNT were measured with enhanced chemiluminescence immunoassay on the COBAS E 411 (reagents and analyzer from Roche Diagnostics). The FLC were analyzed with nephelometry on the BNII (reagent from The Binding Site and analyzer from Siemens Healthcare Diagnostics). For the calculation of the creatinine clearance, the serum creatinine was analyzed with an enzymatic assay, whereas the urine creatinine was measured with the Jaffe method (both reagents from Siemens Healthcare Diagnostics measured on ADVIA 2400, also Siemens Healthcare Diagnostics).
Reference ranges were as follows: NT-proBNP, age less than 70 years, less than 125 ng/L, age greater than 70 years, less than 450 ng/L; cTNT less than .03 μg/L; creatinine clearance 90 to 120 mL/min; free lambda light chain 5.7 to 26.3 mg/L; free kappa light chain 3.3 to 19.4 mg/L.
Statistical analysis
Data were analyzed as of March 2009. Median duration of follow-up was calculated according to Korn.26 Percentages were calculated on an intention-to-treat basis; all 61 patients were set 100%. Survival curve estimation was performed by the method of Kaplan and Meier. Corresponding 95% confidence intervals (95% CIs) were determined on the basis of the log-log survival scale with the use of the Greenwood formula for the variance. Multivariate analysis of HR was performed by logistic regression. The statistical analysis of OS was performed by the use of Cox proportional hazards regression. Distributions of categorical variables were compared with the Fisher exact or χ2 test. To compare the change of minimal white blood and platelet counts during the first 3 cycles of M-Dex with the corresponding values during the following 3 cycles of treatment, we applied the Wilcoxon signed rank test.
For statistical evaluation, HR was defined as observing complete remission (CR) or PR; stable disease (SD) or progression was considered as no HR. The Karnofsky index (KI) was dichotomized at 80% to compare KI smaller than 80% with values being 80% or larger. For the statistical analyses iFLC, NT-proBNP, and cTNT levels were log-transformed. Odds and hazard ratios were reported according to a 2-fold increase of the original values. Covariates in logistic regression models for HR were iFLC levels as well as age, bone marrow plasma cell count, presence of heavy chain, and type of light chain. For Cox proportional hazards regression of OS iFLC, NT-proBNP, and cTNT levels as well as age, sex, KI, type of light chain, number of involved organs, creatinine clearance, and presence of heavy chain were considered as additional predictors. In addition univariable Cox regression was performed for the change of NT-proBNP and cTNT plasma levels after 3 cycles of M-Dex compared with baseline levels. Testing for a possible cutoff value for the NT-proBNP plasma levels with respect to OS was performed by the use of maximally selected log-rank statistics.27 All statistical tests were 2-sided, and results with P values less than .05 were considered to be statistically significant. All statistical analyses were performed by the use of R, version 2.7.2.28
Results
Patients
Patient characteristics are summarized in Table 1. Median age at start of M-Dex treatment was 65 years (range, 44-76 years) and the median Karnofsky Index was 80% (range, 50%-90%). Thirty-six patients (59%) had a cardiac failure NYHA stage III. Five patients could not be evaluated for NYHA stage because they were confined to bed as the result of poor performance status caused by polyneuropathy or other noncardiac involvement. The median NT-proBNP level was 4420 ng/L (range, 157-27 624 ng/L). According to the NT-proBNP– and cTNT-based Mayo Clinic staging system, 5 patients (8%) were stage I, 20 patients (33%) were stage II, and 28 patients (46%) were stage III.20 The second most affected organ was the kidney (21 patients, 34%). Proteinuria was 2.8 g/day in median (range, 0-16 g/day). Six patients (10%) were dependent on dialysis when chemotherapy was started. More than 50% of the patients had 3 or more organs involved. Underlying plasma cell disease has mostly been monoclonal gammopathy as previously defined (n = 56).14 Three patients had stage I multiple myeloma, and 2 patients had stage III multiple myeloma (Durie-Salmon staging system). The amyloidogenic monoclonal light chain was predominantly lambda in 46 patients (75%). Median iFLC concentration was 237 mg/L (range, 27-1840 mg/L).
Treatment and response
Median time from diagnosis to start of treatment was 50 days (95% CI 37-71 days). Patients received a median of 4 cycles of M-Dex (range, 1-12 cycles). Forty-one of 61 patients were evaluable for response (17 patients died before the third cycle of M-Dex was completed; 3 further patients did not continue with M-Dex after the first or second cycle because of deterioration of their performance status, and therefore, response was not assessed). OR was observed in 15 patients (25% of all study patients). Median time to OR was 6 months (95% CI 5-16 months). The distribution of OR was as follows: kidney, 7 patients; heart, 4 patients; liver, 3 patients; gut, 3 patients; polyneuropathy, 2 patients; soft tissue and lung, 1 patient each. Twenty-seven patients achieved a HR with CR in 7 and PR in 20 patients (11% and 33% of all study patients, respectively). Six of 7 patients with CR and 9 of 20 patients with PR achieved an OR; vice versa, all patients who did not respond hematologically did not achieve an OR (Table 2; P < .001). Time-to-first HR was 3.2 months in median (95% CI 2.6-5.4 months). Twenty-one patients showed HR already after the third cycle, and 6 patients further improved their remission status later in the treatment (5 from SD to PR, 1 from PR to CR).
Number of patients with or without hematologic and organ response (n = 41 evaluable)
| Response . | Organ response . | Total . | |
|---|---|---|---|
| No . | Yes . | ||
| Complete remission | 1 | 6 | 7 |
| Partial remission | 11 | 9 | 20 |
| Stable disease | 14 | 0 | 14 |
| Total | 26 | 15 | 41 |
| Response . | Organ response . | Total . | |
|---|---|---|---|
| No . | Yes . | ||
| Complete remission | 1 | 6 | 7 |
| Partial remission | 11 | 9 | 20 |
| Stable disease | 14 | 0 | 14 |
| Total | 26 | 15 | 41 |
Significant association of hematologic remission and organ response (P < .001).
In the univariate logistic regression analysis, iFLC level was a significant predictor for best HR (P = .04) with an estimated odds ratio of .33 (95% CI .11-.97) for a 2-fold increase of iFLC level, whereas age, plasma cell content of the bone marrow, presence of heavy chain, or type of light chain failed to predict HR. In multivariate analysis FLC was still marginally significant to predict for HR (P = .08; odds ratio, .56 for a 2-fold increase of iFLC level; Table 3).
Multivariate logistic regression analysis for hematologic response after chemotherapy (n = 36)
| . | Effect . | Odds ratio . | Lower .95 . | Upper .95 . | P . |
|---|---|---|---|---|---|
| Plasma cell content | 10% increase | 1.16 | 0.62 | 2.15 | .65 |
| abs. iFLC | 2-fold increase | 0.56 | 0.30 | 1.08 | .08 |
| Kappa/lambda | Kappa: lambda | 1.12 | 0.15 | 8.66 | .92 |
| Heavy chain | Yes: no | 2.38 | 0.49 | 11.44 | .28 |
| . | Effect . | Odds ratio . | Lower .95 . | Upper .95 . | P . |
|---|---|---|---|---|---|
| Plasma cell content | 10% increase | 1.16 | 0.62 | 2.15 | .65 |
| abs. iFLC | 2-fold increase | 0.56 | 0.30 | 1.08 | .08 |
| Kappa/lambda | Kappa: lambda | 1.12 | 0.15 | 8.66 | .92 |
| Heavy chain | Yes: no | 2.38 | 0.49 | 11.44 | .28 |
abs.iFLC is of borderline significance.
abs.iFLC indicates absolute involved free light chain concentration.
Six patients relapsed or progressed hematologically within a time frame of 4 to 23 months (median, 18 months) after M-Dex was started. Nineteen patients received second-line therapy because they did not respond to M-Dex or because they developed hematologic progression or organ progression while still in PR. Median time to second-line therapy was 9 months (range, 4-30 months). In 4 patients thalidomide/Dex, in 1 patient bendamustin/prednisolone, in 6 patients bortezomib/Dex, in 6 patients lenalidomide/Dex, in 1 patient M-Dex, and in 1 patient HDM after cardiac transplantation was used.29
Toxicity
The melphalan dose had to be reduced in 12 patients because of a hematologic toxicity CTC grade greater than 2 or worsening kidney function during previous cycles. Median minimal white blood count during the first 3 cycles of M-Dex was 3.8/nL (range, 1.0-11.0/nL), and median minimal platelet count was 197/nL (range, 8-486/nL). During the following 3 cycles leukocyte counts (median, 3.0/nL; range, 0.4-7.0/nL; P = .07) as well as platelet counts (median, 134/nL; range, 18-404/nL; P = .02) decreased. In 4 patients who did not develop a hematologic nadir, the melphalan dosage was increased. Dex dose was reduced in 7 patients and increased in 3 patients, depending on the patient's tolerance and extent of fluid retention.
Median nonhematologic toxicity was CTC grade 3 during the first 3 cycles and CTC grade 2 during cycles 4 to 6. Three patients became dependent on dialysis after start of M-Dex. No treatment-related myelodysplastic syndrome or acute myeloid leukemia was observed during the follow-up period.
Overall 20 patients (33%) died while receiving M-Dex (17 patients as the result of a cardiac event, 1 patient as the result of bleeding, and 2 patients as the result of liver failure). Median time until death under therapy was 1.9 months (range, 0.4-8.3 months). Patients predominantly died because of organ progression in very advanced cardiac amyloidosis. Of note, no patient died of neutropenic sepsis or other infectious complications.
Survival
Estimated median follow-up from the start of treatment of all patients was 27.3 months (95% CI 24.3-34.6 months). Twenty-five patients were alive at last follow-up, and estimated median OS was 17.5 months (95% CI 8.1-31.5; Figure 1A). Estimated median EFS was 6.8 months (95% CI 3.6-9.3 months; Figure 1B). As first events (for definition, see “Outcome measures”) we observed 32 deaths, 6 hematologic progressions or relapses, 6 organ progressions, and 6 second-line chemotherapies.
Outcome measures. (A) OS (median 17.5 months) of all patients. (B) EFS (median 6.8 months) of all patients. Confidence intervals are given in thin lines.
Outcome measures. (A) OS (median 17.5 months) of all patients. (B) EFS (median 6.8 months) of all patients. Confidence intervals are given in thin lines.
Predictors of OS before start of treatment
For parameters determined before start of M-Dex in univariate analysis the amount of circulating iFLC (P = .003; hazard ratio for a 2-fold increase, 1.27), NT-proBNP plasma levels (P = .001; hazard ratio for a 2-fold increase, 1.45), cTNT levels (P = .02; hazard ratio for a 2-fold increase, 1.23), and KI (P = .01; hazard ratio, 2.45, KI < 80% vs KI ≥80%) turned out to be statistically significant predictors for OS. Instead of using NT-proBNP as continuous variable, we performed a second analysis, which revealed a statistically significant cutoff of 4380 ng/dL with respect to OS (P < .001; hazard ratio, 4.1; estimated median OS, 3.6 vs 34.7 months). Other parameters such as age, sex, type of light chain, number of involved organs, creatinine clearance, and presence of heavy chain failed to predict for OS.
Multivariate analysis could confirm these results and revealed iFLC (P = .008; hazard ratio, 1.63), NT-proBNP plasma levels (P = .005), cTNT plasma levels (P = .003), and KI (P < .001; hazard ratio, 5.37; KI < 80% vs KI ≥80%) at the beginning of M-Dex as significant predictors for OS (Table 4). For NT-proBNP and cTNT plasma levels, a significant interaction effect was identified (P = .001) so that the effect of both factors on OS was not independent of the effect of the other. In addition, kappa light chain was identified as a positive prognostic factor for OS (P = .02; hazard ratio, 0.21, kappa vs lambda). However, the number of involved organs was only of borderline significance (P = .08; HR, 1.54; 95% CI 0.95-2.50) in our cohort of patients who were ineligible for transplant at diagnosis (of note, only 5 patients had 1 organ involved).
Multivariate Cox regression analysis for OS (n = 52)
| . | Effect . | Hazard ratio . | Lower .95 . | Upper .95 . | P . |
|---|---|---|---|---|---|
| Age | 10 years | 0.75 | 0.43 | 1.30 | .30 |
| NT-proBNP* | 2-fold increase | 2.36 | 1.30 | 4.30 | .005 |
| cTNT* | 2-fold increase | 0.99 | 0.65 | 1.49 | .003 |
| abs. iFLC | 2-fold increase | 1.63 | 1.14 | 2.34 | .008 |
| Karnofsky index | < 80% vs ≥ 80% | 5.37 | 2.04 | 14.13 | < .001 |
| Kappa/lambda | Kappa: lambda | 4.69 | 1.29 | 17.05 | .02 |
| Heavy chain | Yes: no | 0.67 | 0.23 | 1.96 | .47 |
| Creatinine clearance | 10 mL/min increase | 0.92 | 0.76 | 1.12 | .42 |
| Number of organs | 1 additional organ | 1.54 | 0.95 | 2.50 | .08 |
| . | Effect . | Hazard ratio . | Lower .95 . | Upper .95 . | P . |
|---|---|---|---|---|---|
| Age | 10 years | 0.75 | 0.43 | 1.30 | .30 |
| NT-proBNP* | 2-fold increase | 2.36 | 1.30 | 4.30 | .005 |
| cTNT* | 2-fold increase | 0.99 | 0.65 | 1.49 | .003 |
| abs. iFLC | 2-fold increase | 1.63 | 1.14 | 2.34 | .008 |
| Karnofsky index | < 80% vs ≥ 80% | 5.37 | 2.04 | 14.13 | < .001 |
| Kappa/lambda | Kappa: lambda | 4.69 | 1.29 | 17.05 | .02 |
| Heavy chain | Yes: no | 0.67 | 0.23 | 1.96 | .47 |
| Creatinine clearance | 10 mL/min increase | 0.92 | 0.76 | 1.12 | .42 |
| Number of organs | 1 additional organ | 1.54 | 0.95 | 2.50 | .08 |
abs iFLC indicates absolute involved free light chain concentration; cTNT, cardiac troponin T; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; and OS, overall survival.
NT-proBNP and cTNT values did not independently predict for OS (P = .001). Therefore, hazard ratios for these factors were adjusted to the median level of the other factor.
Predictors of OS after start of treatment
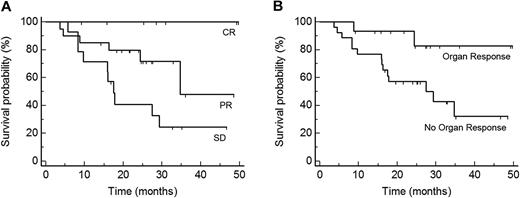
As of this writing, none of the patients who achieved a CR has died. HR was a highly significant predictor for OS (P = .01; hazard ratio for responders, 0.27; 95% CI 0.10-0.74; Figure 2A). Organ response was also associated with an OS advantage (P = .03; hazard ratio for responders, 0.23; 95% CI 0.05-1.0; Figure 2B).
Predictors of OS after start of treatment. (A) OS of patients according to the degree of best hematologic response: CR, PR, and SD (P = .004, n = 41). (B) OS of patients according to organ response (P = .03, n = 41). Best HR and organ response increase OS significantly.
Predictors of OS after start of treatment. (A) OS of patients according to the degree of best hematologic response: CR, PR, and SD (P = .004, n = 41). (B) OS of patients according to organ response (P = .03, n = 41). Best HR and organ response increase OS significantly.
Achievement of CR after the third cycle of M-Dex (n = 5) was already predictive for OS (P = .08; hazard ratio, 0.3; 95% CI 0.10-1.17). Moreover, we also have determined NT-proBNP and cTNT plasma levels after 3 cycles of M-Dex. Thirty-five patients who were not on dialysis and alive were included in this analysis. An increase of NT-proBNP (P = .02; hazard ratio, 2.81 for a fold change of 2) as well as of cTNT (P < .001; hazard ratio, 2.04 for a fold change of 2) were significantly associated with a shortened OS. The same analysis was performed for those patients who achieved a PR (n = 14) after 3 cycles of M-Dex. The results were comparable in effect estimation, but because of the small sample size, no longer statistically significant.
Patients on dialysis during treatment with M-Dex
Six patients were on dialysis before start of M-Dex; 2 died before evaluation of HR. One patient achieved CR, 2 patients PR, and 1 patient did not respond. Hematologic response rates, OS, and hematologic and nonhematologic toxicity were comparable in patients who were on dialysis and those who were not (data not shown). Furthermore, 3 patients became dependent on dialysis while on M-Dex and were evaluable for HR. One of them died before response evaluation, 1 patient had a PR, and 1 a SD.
Treatment modification and outcome measures
The starting dose of melphalan was adapted to the kidney function in 28 patients (46%), which had no influence on HR (P = .75; odds ratio, 1.24; 95% CI 0.28-5.52). Thirty-six patients (59%) received 20 mg instead of 40 mg of Dex because of advanced heart involvement or nephrotic syndrome with severe edemata. Dex dose reduction did not influence best HR (P = .65; odds ratio, 1.40 with 95% CI 0.32-6.10) or OS (P = .46; hazard ratio, 1.2; 95% CI 0.65-2.48) or EFS (P = .79; hazard ratio, 0.92; 95% CI 0.51-1.67).
Discussion
We report on a large group of well-characterized patients with systemic AL who were not eligible for HDM and therefore treated with M-Dex as first-line therapy. Median OS in our study was only 18 months. These results are poor compared with the OS of 61 months reported by Palladini et al7,8 and 57 months reported by Jaccard et al9 (Table 5).7-9,30 This difference was attributable to a high early death rate of 28% in the first 3 months. Overall, death during M-Dex therapy occurred in 33% of the patients at a median time of only 1.9 months, mostly as the result of advanced cardiac amyloidosis. Our patient cohort represents a high-risk population with advanced AL (53% with severe cardiac disease according to Mayo Clinic stage III, more than 50% with 3 or more organs involved and 10% on dialysis).
Review of M-Dex treatment
| Study and reference(s) . | No. of patients . | Recruitment dates . | Median follow-up . | Patient description . | Median age, y . | M-Dex dosage . | Median no. of cycles . | Toxicity CTC grade > 3, % of patients . | HR/CR/OR, % (intention to treat) . | Median OS, mo/no. death within 3 mo, % of patients . |
|---|---|---|---|---|---|---|---|---|---|---|
| Palladini7,8 | 46 | 1999-2002 | 20 mo (living patients) | Untreated; not eligible for HDM | 62 | M: 0.22 mg/kg; Dex: 40 mg | 4 | 11 | 67/33/48 | Not reached/4% |
| Jaccard9 | 50 | 2000-2005 | n.a. | 48 patients untreated; eligible for HDM | 59 | M: 10 mg/m2; Dex: 40 mg | 12 | 15 | 52/24/34 | 57/10% |
| Lebovic30 | 40 | 2004-2007 | n.a. | Untreated; not eligible for HDM | 67 | M: 0.22 mg/kg;* Dex: 20 mg/m2 | 4 | n.a. | 58/13/n.a | 10.5†/23% |
| Current study | 61 | 2004-2007 | 27 months | Untreated; not eligible for HDM | 65 | M: 16 mg/m2, day 1 (IV)‡; Dex: 20-40 mg | 4 | 33 | 44/11/25 | 17.5/28% |
| Study and reference(s) . | No. of patients . | Recruitment dates . | Median follow-up . | Patient description . | Median age, y . | M-Dex dosage . | Median no. of cycles . | Toxicity CTC grade > 3, % of patients . | HR/CR/OR, % (intention to treat) . | Median OS, mo/no. death within 3 mo, % of patients . |
|---|---|---|---|---|---|---|---|---|---|---|
| Palladini7,8 | 46 | 1999-2002 | 20 mo (living patients) | Untreated; not eligible for HDM | 62 | M: 0.22 mg/kg; Dex: 40 mg | 4 | 11 | 67/33/48 | Not reached/4% |
| Jaccard9 | 50 | 2000-2005 | n.a. | 48 patients untreated; eligible for HDM | 59 | M: 10 mg/m2; Dex: 40 mg | 12 | 15 | 52/24/34 | 57/10% |
| Lebovic30 | 40 | 2004-2007 | n.a. | Untreated; not eligible for HDM | 67 | M: 0.22 mg/kg;* Dex: 20 mg/m2 | 4 | n.a. | 58/13/n.a | 10.5†/23% |
| Current study | 61 | 2004-2007 | 27 months | Untreated; not eligible for HDM | 65 | M: 16 mg/m2, day 1 (IV)‡; Dex: 20-40 mg | 4 | 33 | 44/11/25 | 17.5/28% |
A 28-day cycle was used in all M-Dex studies. Dex was given on days 1-4. All studies used oral melphalan (day 1-4) except ours. Different HR criteria/HDM exclusion criteria as follows: Palladini et al7,8 : HR without FLC assessment more than 2 organs involved, severe cardiac involvement, creatinine greater than 2 mg/dL, abnormal respiratory function test, older than 65 years of age. Jaccard et al9 : HR only in n = 19 patients with FLC assessment/older than 70 years of age, Eastern Cooperative Oncology Group greater than 2. Lebovic et al30 : HR with FLC assessment/older than 60 years of age, more than 3 organs involved, advanced cardiac disease. Current study: HR with FLC assessment/older than 70 years of age, greater than NYHA stage II, PS greater than 2, systolic blood pressure less than 90 mm Hg, symptomatic pleural effusions, factor X deficiency less than 10%.
CR indicates complete remission; CTC, Common Terminology Criteria; FLC, free light chain assay; HDM, high-dose melphalan; HR, hematologic remission; IV, intravenous; M-Dex, melphalan and dexamethasone; mo, months; OR, organ response; OS, overall survival; and PS, performance status.
Creatinine greater than 2: M reduced to 0.18 mg/kg, patients older than 70 years, 50% dosage.
Since diagnosis.
Adapted to creatinine clearance.
In our opinion, the main reason for the different survival rates of M-Dex trials is the proportion of patients with severe amyloid (cardiac) disease and different eligibility criteria for HDM. Given that our criteria were not as strict as those of the center in Pavia and permit the inclusion of patients with NYHA stage II heart failure irrespective of involved organ number and kidney function (Table 5), our patient cohort receiving M-Dex is negatively selected. On the contrary, the French trial had included mostly patients eligible for HDM; as a consequence, they were younger than our patients. Unfortunately, cardiac biomarkers were not available in both studies; therefore, the Mayo staging system could not be applied to estimate the severity of cardiac amyloidosis. Our patient cohort might be best comparable with that reported by Lebovic et al,30 who treated patients with severe cardiac involvement with M-Dex resulting in a high early death rate of 23% and a short median OS of 10.5 months after diagnosis. In this analysis, the Mayo staging system was also not used (they analyzed BNP instead of NT-proBNP).
Progression-free survival (PFS) or EFS curves are not reported routinely in AL. PFS was analyzed in the Italian study30 and was longer than in our study with a median of 46 months, probably because of our high early death rate and different definition of events. For the sake of better comparability of retrospective analysis and clinical phase 2 trials, these terms should be clearly defined by a consensus panel of amyloidosis experts because EFS or PFS might reflect the efficacy of any treatment better than OS.
On an intention-to-treat basis we observed HR in 44% and CR in 11% of patients in our study. The CR rate seems to be worse compared with the studies of Jaccard et al9 and Palladini et al.7,8 This difference has to be attributed to 3 main points: first, to the high rate of early deaths; second, to the lower number of applied M-Dex cycles; and third, to the use of more stringent CR criteria, including FLC, in our study. Again, our CR rate is approximately the same as that in Lebovic et al,30 who used also FLC for hematologic assessment.
Our regimen differed in some ways from the protocols administered by other groups; however, we do not deem these differences as crucial for toxicity, survival, and response. First, we administered melphalan intravenously adapted to kidney function. This was not associated with a high rate of grade 3 or 4 hematologic toxicity; we observed no deaths of neutropenic infections. Because of the improved bioavailability it was not necessary to increase the melphalan dosage in following cycles in more than 90% of the patients. In our view, the high amount of CTC grade 3/4 nonhematologic toxicity and the high early death rate was not a consequence of the form of administration of M-Dex but of the high proportion of very sick patients in our study. Of note, the early death rate in the study of Lebovic et al30 was equal, although they used oral melphalan. Second, Dex dosage was cut in half in patients with NYHA stage III and severe fluid retention. Because a subgroup analysis showed equal remission rates and same OS for Dex 20 mg compared with 40-mg groups, the reduction in Dex dose was not accountable for the poorer outcome in our study and appears as a reasonable option to reduce toxicity. Taken together, we consider the poor OS in our study a reflection of the high risk features of our study cohort, whereas modifications of the chemotherapy protocol do not appear to have played a decisive role. However, the use of intravenous melphalan did not unequivocally improve outcomes compared with oral melphalan.
To identify patients who benefit from M-Dex treatment, we have looked for prognostic factors for HR and OS. In multivariate analysis, we could show that patients with greater levels of iFLC had a lower probability to achieve HR (Table 3). Consecutively, patients with low levels of iFLC had an improved OS (Table 4). Patients with kappa AL showed also an improved OS. The reason for this phenomenon is unclear; in our study lambda light chain type was not associated with more severe heart involvement (data not shown) or a greater amount of FLC (Table 1). Taken together with our recently published data,14 the height of iFLC before start of treatment influences disease severity, response to therapy, and OS.
In accordance with previous reports we observed that patients with chemotherapy-sensitive disease had significant better OS than those who did not respond.7-9,31 All patients with CR were alive, whereas 6 (30%) of 20 patients with PR and 10 (71%) of 14 patients with SD died. HR was significantly associated with OR, which was also a prognostic factor for OS.
As previously published,20-23 we could also demonstrate that patients with severe heart involvement determined by elevated cTNT and NT-ProBNP plasma levels and a poor KI before start of treatment are at high risk for early death (Table 4). Moreover, we show for the first time that patients who did not further deteriorate with their cardiac biomarkers as early as 3 months after start of M-Dex had a significant better OS. Therefore, serial measurement of the cardiac biomarkers might be a powerful tool to identify patients who are most likely to benefit from M-Dex at an early time point.
In summary, these observations demonstrate that treatment with M-Dex is not able to overcome the poor OS of patients with advanced cardiac involvement. Patients with severe heart involvement determined on the basis of clinical parameters as KI (eg, less than 80%) and cardiac biomarkers (eg, NT-proBNP > 4400 ng/L) do not appear to benefit from this regimen because toxicity in these patients is high and M-Dex is probably not capable to induce fast and deep HRs in the majority of the patients. New drugs such as bortezomib with low-dose Dex as first-line treatment represent a potential alternative.32 However, once M-Dex is started, achievement of CR after the third cycle is a very good predictor for an excellent prognosis. Further prospective trials will be necessary to identify the optimal time point of cardiac biomarker measurement and HR assessment to decide about change or continuation of therapy in patients without CR. We conclude that exclusion from HDM treatment because of advanced cardiac amyloid disease is not a valid criterion to recommend M-Dex therapy as alternative to every patient with newly diagnosed AL amyloidosis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the hematologists at outside hospitals and private practices for the excellent collaboration. We thank our patients and their families for their trust in our work.
Authorship
Contribution: S.D., S.O.S., T.B., and U.H. wrote the paper; A.B. was responsible for statistical analysis; S.O.S., T.B., A.V.K., J.B., E.H., and U.H. tended to the patients; M.Z. was responsible for the laboratory examinations; and S.D., S.O.S., T.B. H.G., A.D.H., and U.H. designed research.
Conflict-of-interest disclosure: We thank Binding Site for supplying the Free-lite test at no charge for this study. The authors declare no other competing financial interests.
Correspondence: Ute Hegenbart, MD, Amyloidosis Center, University Hospital, Im Neuenheimer Feld 410, D-69120 Heidelberg, Germany; ute.hegenbart@med.uni-heidelberg.de.
References
Author notes
S.D. and S.O.S. contributed equally to this work.