Abstract
The anti-CD20 antibody rituximab depletes human B cells from peripheral blood, but it remains controversial to what extent tissue-resident B cells are affected. In representative patients with rheumatoid arthritis, we here demonstrate that recently activated presumably short-lived plasmablasts expressing HLA-DRhigh and Ki-67 continuously circulate in peripheral blood after B-cell depletion by rituximab at 26%-119% of their initial numbers. They circulate independent of splenectomy, express immunoglobulin A (IgA), β7 integrin, and C-C motif receptor 10 (CCR10) and migrate along CCL28 gradients in vitro, suggesting their mucosal origin. These plasmablasts express somatically hypermutated VH gene rearrangements and spontaneously secrete IgA, exhibiting binding to microbial antigens. Notably, IgA+ plasmablasts and plasma cells were identified in the lamina propria of patients treated with rituximab during peripheral B-cell depletion. Although a relation of these “steady state”–like plasmablasts with rheumatoid arthritis activity could not be found, their persistence during B-cell depletion indicates that their precursors, that is, B cells resident in the mucosa are not deleted by this treatment. These data suggest that a population of mucosal B cells is self-sufficient in adult humans and not replenished by CD20+ B cells immigrating from blood, lymphoid tissue, or bone marrow, that is, B cells depleted by rituximab.
Introduction
Plasmablasts are an immediate product of B-cell activation that home either to the bone marrow or to the mucosa to secrete antibody as terminally differentiated plasma cells.1-3 In healthy persons, B cells and antibodies protect from pathogens, whereas loss of immunologic tolerance and malignant transformation result in the generation of autoreactive B cells and autoantibody production and development of lymphoma, respectively. Direct targeting of B cells by the chimeric anti–human CD20 antibody rituximab (RTX) has been developed for the treatment of rheumatoid arthritis (RA) and B-cell malignancies.4,5 However, not all patients respond to RTX therapy, and the efficiency of B-cell depletion in lymphoid tissues as well as the susceptibility of different B-cell subtypes remain of central interest, in particular related to individual unresponsiveness.
In RA, depletion of peripheral CD20+ B cells reduces, but does not eliminate, autoimmunity, indicated by subsequent flares and continuous production of autoantibodies.6 Persisting (auto-) antibodies in patients treated with RTX reflect the continued presence of antibody-secreting cells, whereas it remains unclear whether those are long-lived, CD20− plasma cells, eg, residing in the bone marrow, or whether they have been generated de novo from B cells not depleted by RTX. In this context, germinal center B cells of the Peyer patches and peritoneal B1 B cells resisted to RTX treatment in human cd20tg mice.7 Depletion of B cells from human lymphoid tissues of systemic immunity, for example, the spleen and lymph nodes by RTX has been described in individual cases.8-10 Thus, the efficiency of global or selective B-cell depletion remains of interest, in particular whether there is a distinct susceptibility of B-cell subsets to RTX within gut-associated lymphoid tissues (GALT).
Here, we demonstrate the continued presence of dividing and migratory plasmablasts expressing a mucosal phenotype in the peripheral blood of patients with RA consistent with steady-state plasmablasts1 after treatment with RTX. These data show that a subset of chronically activated mucosal B lymphocytes carrying highly mutated VH gene rearrangements and secreting antimicrobial immunoglobulin A (IgA) are not deleted by RTX. This reflects the robustness of humoral mucosal immunity during B-cell depletion with RTX and underscores the independent regulation of mucosal immune responses in steady state. The resistance of some mucosal B cells to RTX is apparently not stringently related to RA pathogenesis but could represent a mechanism underlying enhanced RTX resistance in some mucosa-associated lymphoid tissue lymphoma cases.11,12
Methods
A detailed description of the patients, samples, and methods are available as supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Patients
Blood samples were taken from 25 patients who received 2× 1 g of RTX (Roche), from 3 persons who had undergone splenectomy with or without additional RTX treatment, and healthy control subjects, from 3 of them after a tetanus booster immunization.13 Endoscopic biopsies were analyzed from patients with diffuse large B-cell lymphoma 3 months after initiation of RTX therapy (+ cyclophosphamide, hydroxydaunomycin, Oncovin, prednisone). The ethics committee of the Charité University Medicine approved the study, and the patients' informed consent was obtained before enrollment in accordance with the Declaration of Helsinki.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated and processed as described before.1 PBMCs were prepared for and analyzed by flow cytometry according to standard procedures. Antibodies used were CD19-phycoerythrin (PE; clone HD37; Dako), CD19-PE-cyanine 7 (SJ25C1; BD), CD45-peridinine chlorophyll protein (PerCp; 2D1; BD), CD27-cyanine 5 (2E4; kind gift from René van Lier, Academic Medical Center, University of Amsterdam), biotinylated κ (G20-193; BD) and λ Ig light chain (JDC-12; BD) and IgA (G20-359; BD), human leukocyte antigen (HLA)–DR coupled to fluorescein isothiocyanate (FITC; clone 30; German Rheumatism Research Center Berlin), CD20-FITC (2H7; BD), CD20-PerCp (L27; BD), β7 integrin-PE (FIB504; BD), Ki-67–FITC (MIB-1, controlled by DAK-GO1; both Dako), CD3-PacificBlue (UCHT1; BD), CD14-PacificBlue (M5E2; BD), CCR10-PE or -allophycocyanine (314305; R&D Systems), IgA-FITC (M24A; Millipore), isotype-matched control antibodies (R35-95, BD; and 54447, R&D Systems). Biotinylated antibody binding was visualized with streptavidin-PerCp (BD). α4β7 integrin and β7 integrin expression as detected by FIB504 is identical on human blood B lymphocytes, as shown and discussed previously.1,14
Transwell migration assay
Enzyme-linked immunospot assay
EliSpot technique was used to detect functional antibody-secreting cells with the use of goat anti–human IgG, IgA, and IgM (Sigma-Aldrich) for coating of MultiScreen flat-bottom 96-well plates (Millipore) and detection. Spots were visualized by streptavidin–horseradish peroxidase (Sigma-Aldrich) followed by 3-amino-9-ethylcarbazole (Sigma) dissolved in N,N-dimethylformamide (Sigma), diluted in a 0.2M sodium acetate/0.2M acetic acid buffer. Plates were read by an EliSpot reader (CTL).
In vitro antibody secretion, multiplex analysis, and enzyme-linked immunosorbent assay
PBMCs were incubated 2 days in RPMI 1640 Glutamax containing 10% fetal calf serum (Invitrogen) at 37°C/5% CO2 to allow for spontaneous secretion of antibody. Cell culture supernatants were analyzed for IgA content by Bioplex (Bio-Rad) and for IgA-rheumatoid factor by enzyme-linked immunosorbent assay (Generic Assays). The reactivity of IgA was analyzed by enzyme-linked immunosorbent assay with the use of polystyrene plates coated with the indicated antigens, and binding of IgA was detected with goat anti–human IgA alkaline phosphatase (Jackson ImmunoResearch Laboratories Inc), followed by visualization with P-nitrophenyl phosphate (Sigma) in ethanolamine buffer. Extinction was photometrically determined at 405 nm.
Single-cell sorting and reverse transcriptase polymerase chain reaction
Individual IgA+ plasmablasts from healthy controls and or RA patients depleted of B cells with RA or tetanus-specific plasmablasts from previously immunized persons15 were sorted from the PBMCs into 96-well plates with the use of a FACSAria (BD).
VHDJHC genes were amplified, sequenced according to previously published protocols,16 and analyzed with Chromas 2.33 Sequence Viewer (Chromas Technelysium), the BLAST database of the National Center for Biotechnology Information,17 and JOINSOLVER.18 Sequences containing a stop codon or an out-of-frame rearrangement were considered to be nonproductive and were not included in the analysis.
Immunohistochemistry and immunofluorescence staining
For immunostaining 2- to 3-μm sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step before incubation with antibodies against CD20 (L26), CD79a (JCB117), MUM1 (MUM1p), Ki-67 (MIB1), and polyclonal rabbit anti–human IgA (all Dako). For detection the streptavidin PO kit (K5001; Dako) was used with diaminobenzidine. For immunofluorescence labeling, sections were incubated with rabbit anti–IgA antibody followed by Alexa Fluor 555–conjugated anti–rabbit antibody (Invitrogen) and subsequent incubation with mouse anti–Ki-67 followed by Alexa Fluor 488–conjugated anti–mouse antibody (Invitrogen). Nuclei were counterstained with DAPI (4′-6′-diamidine-2-phenylindole; Roche).
Statistical analysis
Data were analyzed with FlowJo Version 7.2.5 (TreeStar) and GraphPad Prism Version 4 (GraphPad Software). Statistical significance was calculated with the Mann-Whitney test for comparisons between patient groups at different time points. The Wilcoxon test was used to compare paired observations (both 95% confidence interval [CI], 2-tailed). Spearman rank correlation analysis (2-tailed, 95% CI) was performed in Figure 2.
Results
Circulation of CD20−/CD27high plasmablasts and plasma cells during depletion of peripheral CD20+ naive and memory B cells
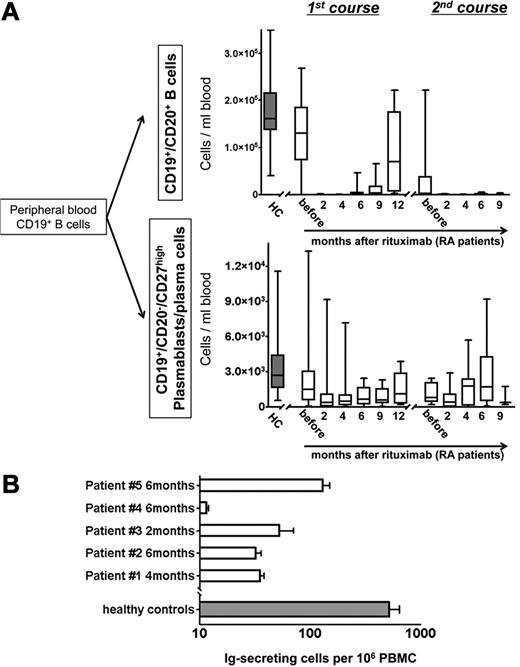
Twenty-five patients with RA were analyzed before and after anti-CD20 antibody therapy with RTX for total CD19+ B cells and the underlying subpopulations of naive and antigen-experienced “memory” B cells (CD20+/CD27+/−)19 and antibody-secreting cells (CD20−/CD27high) comprising plasmablasts and plasma cells.1,13 Although naive and memory B cells were fully depleted from the peripheral blood after RTX, antibody-secreting plasmablasts expressing HLA-DRhigh and Ki-67 were continuously detectable throughout the duration of B-cell depletion. Before therapy, the total number of plasmablasts/plasma cells, but not of CD20+ B cells, was significantly reduced in patients with RA compared with healthy controls (Figure 1; supplemental Table 1).
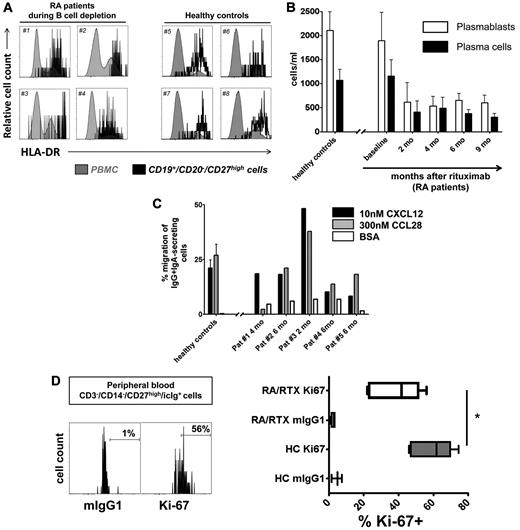
Continuous detection of circulating antibody-secreting cells during B-cell depletion with RTX. (A) Peripheral blood CD19+/CD20+ naïve and memory B-cell counts and CD19+/CD20−/CD27high plasmablast and plasma cell counts in RTX-treated patients with rheumatoid arthritis (RA; open boxes). Healthy, untreated controls (HC; n = 41; gray boxes) are shown for comparison. (B) Detection of functional immunoglobulin (Ig)–secreting cells from peripheral blood of patients with RA during B-cell depletion after RTX treatment with the use of an EliSpot assay (5 samples from 4 independent experiments; open boxes; error bars indicate SD of duplicates). The gray bar depicts corresponding values obtained from 10 healthy persons (error bars indicate SD of 10 samples tested).
Continuous detection of circulating antibody-secreting cells during B-cell depletion with RTX. (A) Peripheral blood CD19+/CD20+ naïve and memory B-cell counts and CD19+/CD20−/CD27high plasmablast and plasma cell counts in RTX-treated patients with rheumatoid arthritis (RA; open boxes). Healthy, untreated controls (HC; n = 41; gray boxes) are shown for comparison. (B) Detection of functional immunoglobulin (Ig)–secreting cells from peripheral blood of patients with RA during B-cell depletion after RTX treatment with the use of an EliSpot assay (5 samples from 4 independent experiments; open boxes; error bars indicate SD of duplicates). The gray bar depicts corresponding values obtained from 10 healthy persons (error bars indicate SD of 10 samples tested).
Two until 4 months after RTX treatment, depletion of circulating CD20+ B cells was observed in all patients (Figure 1A). Before B-cell depletion therapy, median counts of CD20+ B cells were 130.13 cells/μL, dropping significantly to < 2 cells/μL after 2-6 months and increased to 3.18 cells/μL after 9 months, and 70.34 cells/μL after 12 months. Repopulation of CD20+ B cells was not observed earlier than 6 months after RTX infusion. At this time, in 3 of 20 patients CD20+ B cells occurred in the blood at levels > 6 cells/μL. Two months after therapy, CD20+ B-cell counts were reduced on average by 99.6% ± 0.3% compared with baseline. At the same time and throughout B-cell depletion therapy, plasmablasts and plasma cells remained detectable at 26%-119% of their median numbers before therapy. Their median number before treatment (1.48 cells/μL) was reduced to 0.39-1.12 cells/μL during the depletion phase between 2 and 12 months after RTX, with significant reductions at 2-4 months after treatment (Figure 1A; supplemental Table 1).
At the time when the second course of B-cell depletion was initiated, CD20+ B-cell counts were very low (< 6 cells/μL) in 6 of 10 patients, whereas in the remaining 4 patients, B cells were detectable at counts > 6 cells/μL. After the second RTX treatment, CD20+ B-cell counts remained at or were repeatedly reduced to low levels of < 1 cell/μL for ≥ 9 months, within the detection limit of one B cell in 3 × 105-2.5 × 106 PBMCs. At the same time, blood plasmablast/plasma cell counts were stably maintained at median numbers between 0.39 and 1.78 cells/μL throughout the second treatment course (Figure 1A; supplemental Table 1). For flow cytometric analyses, up to 2.5 × 106 events were collected per analysis, from which T cells, monocytes, and dead cells were excluded to achieve accurate quantification and characterization of B cells and plasmablasts/plasma cells even at low frequencies (supplemental Figure 1). The detection of intracellular immunoglobulin in PBMCs expressing CD19+ and CD27high during B-cell depletion provided characteristic evidence for the persistence of plasmablasts/plasma cells (7 samples; data not shown).
The detection of functional antibody-secreting cells in blood during in vivo B-cell depletion was confirmed by spontaneous secretion of antibodies by these cells in an EliSpot assay, yielding a mean frequency of 53 antibody-secreting cells among 106 PBMCs (range, 12-130 cells/106 PBMCs; 5 donors; Figure 1B) compared with an average of 520 antibody-secreting cells per 106 PBMCs from 10 healthy donors.
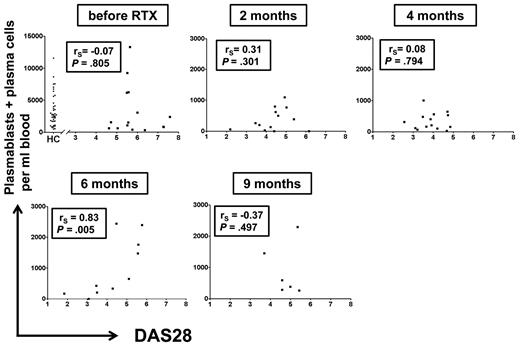
To delineate a potential association of circulating plasmablast/plasma cell numbers with RA disease activity before and during B-cell depletion, individual plasmablast/plasma cell numbers were plotted against RA disease activity with the use of DAS28. Except 6 months after RTX treatment, no significant correlations were observed between these parameters (Figure 2).
Total numbers of CD19+/CD20−/CD27high plasmablasts/plasma cells do not consistently correlate with RA disease activity. Numbers of peripheral plasmablasts and plasma cells were plotted against RA disease activity index DAS28 at indicated time points. Each dot represents one patient. Except for 6 months after therapy, no significant correlation was detected between disease activity and plasmablast/plasma cell numbers (Spearman rank correlation analysis), suggesting that this peripheral cell population is not closely linked to disease activity. The first graph includes plasmablast/plasma cell numbers of 41 untreated healthy controls (HCs) for comparison.
Total numbers of CD19+/CD20−/CD27high plasmablasts/plasma cells do not consistently correlate with RA disease activity. Numbers of peripheral plasmablasts and plasma cells were plotted against RA disease activity index DAS28 at indicated time points. Each dot represents one patient. Except for 6 months after therapy, no significant correlation was detected between disease activity and plasmablast/plasma cell numbers (Spearman rank correlation analysis), suggesting that this peripheral cell population is not closely linked to disease activity. The first graph includes plasmablast/plasma cell numbers of 41 untreated healthy controls (HCs) for comparison.
These data indicate the stable presence of apparently RTX-resistant antibody-secreting cells in the blood of patients with RA during the long-term depletion of circulating CD20+ B cells.
Circulating plasmablasts and plasma cells during B-cell depletion therapy express a mucosal phenotype
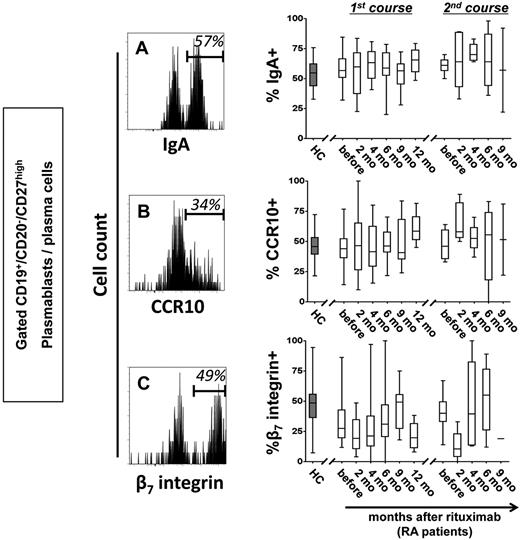
We previously identified a distinct subset of antibody-secreting cells in the peripheral blood of healthy persons during steady state with the phenotype consistent with their generation in mucosal immune responses.1 In this context, RTX-resistant cells in patients with RA were further characterized before and during B-cell depletion. Similar to healthy controls, more than one-half of CD19+/CD20−/CD27high plasmablasts/plasma cells detectable before and throughout B-cell depletion therapy expressed IgA, the mucosal cell adhesion molecule β7 integrin and the mucosal chemokine receptor CCR10. These data suggest that RTX-resistant plasmablasts may arise from mucosal immune reactions and thus share important similarities with steady-state plasmablasts/plasma cells (Figure 3; supplemental Table 2). Of note, the average frequency of β7 integrin+ plasmablasts/plasma cells was significantly lower in patients with RA before RTX therapy compared with healthy controls (P = .0072).
Circulating plasmablasts and plasma cells during B-cell depletion express IgA and receptors for mucosal homing. Representative cytometric detection of IgA (A), CCR10 (B), and β7 integrin (C) on plasmablasts and plasma cells during RTX treatment are shown. Frequencies of IgA+, CCR10+, and β7 integrin+ cells were determined in 44 healthy controls (HCs; gray boxes) and in RTX-treated patients with RA throughout therapy (open boxes). Box plots depict minimum, maximum, and median values and upper and lower quartiles.
Circulating plasmablasts and plasma cells during B-cell depletion express IgA and receptors for mucosal homing. Representative cytometric detection of IgA (A), CCR10 (B), and β7 integrin (C) on plasmablasts and plasma cells during RTX treatment are shown. Frequencies of IgA+, CCR10+, and β7 integrin+ cells were determined in 44 healthy controls (HCs; gray boxes) and in RTX-treated patients with RA throughout therapy (open boxes). Box plots depict minimum, maximum, and median values and upper and lower quartiles.
Deeper analyses of steady-state plasmablasts/plasma cells showed that with the exception of a significant coexpression of CCR10 and IgA, the 3 molecules showed an independent expression pattern. Of interest, 72%-86% (mean, 80%) of these plasmablasts/plasma cells expressed at least 1 of the mucosal markers, IgA, CCR10, or β7 integrin (supplemental Figure 2), indicating that RTX-resistant plasmablasts stably express a mucosal phenotype in healthy and RTX-treated persons.
Splenectomy does not abrogate the steady-state production of IgA+ plasmablasts
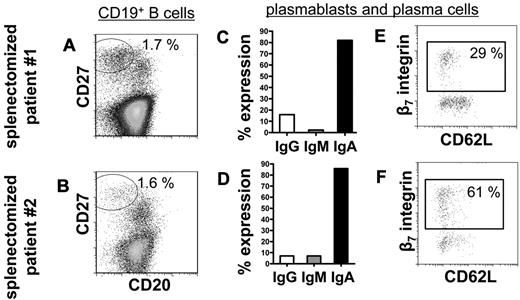
To substantiate the hypothesis that peripheral blood IgA+ plasmablasts in steady state before and after RTX treatment derive from mucosal immune reactions in vivo, peripheral blood from 3 persons who had undergone splenectomy (one of them treated with RTX) was analyzed for steady-state plasmablasts. Comparable with healthy persons,1 CD19+/CD20−/CD27high plasmablasts/plasma cells made up 1.7% and 1.6% of CD19+ B cells in the blood of 2 persons who had undergone splenectomy who were not treated with RTX. Almost all of these cells expressed IgA (82% and 86%) and 29% and 61%, respectively, expressed β7 integrin (Figure 4). The third patient who had undergone splenectomy treated with RTX displayed abundant presence of circulating IgA+, CCR10+, and β7 integrin+ plasmablasts 6 months after treatment (2 cells/μL), whereas CD20+ B cells were reduced to 3 cells/μL. This dataset showed that the spleen is not a regular induction or maintenance site of IgA+ plasmablasts circulating during steady state and B-cell depletion and additionally supports their mucosal origin.
The frequency of circulating plasmablasts and plasma cells is not impaired in persons who have undergone splenectomy. Normal frequencies of CD19+/CD20−/CD27high plasmablasts and plasma cells1 among CD19+ B cells were detectable in 2 persons who underwent a splenectomy (A-B). These plasmablasts and plasma cells show characteristics of homeostatic mucosal plasmablasts in that they produce of IgA (C-D) and express β7 integrin but rarely CD62L (E-F). Frequencies indicate proportions of gated cells among the total cell number shown in each dot plot.
The frequency of circulating plasmablasts and plasma cells is not impaired in persons who have undergone splenectomy. Normal frequencies of CD19+/CD20−/CD27high plasmablasts and plasma cells1 among CD19+ B cells were detectable in 2 persons who underwent a splenectomy (A-B). These plasmablasts and plasma cells show characteristics of homeostatic mucosal plasmablasts in that they produce of IgA (C-D) and express β7 integrin but rarely CD62L (E-F). Frequencies indicate proportions of gated cells among the total cell number shown in each dot plot.
Detection of dividing and migratory HLA-DRhigh expressing plasmablasts during B-cell depletion
Subsequent analysis sought to identify whether circulating RTX-resistant antibody-secreting cells represent plasmablasts of recent generation or established, recirculating plasma cells. Before and during B-cell depletion, at least one-half of these cells showed a phenotype of recently generated plasmablasts1,13 in that they expressed HLA-DRhigh. Proportions of plasmablasts versus plasma cells were similar in patients with RA compared with healthy controls without significant changes over the time of B-cell depletion, indicative of their stable turnover and their equal resistance toward RTX (Figure 5A-B; supplemental Table 2).
Generation of migratory and proliferating plasmablasts during B-cell depletion. (A) Cytometric detection of HLA-DRhigh plasmablasts in peripheral blood samples of 4 patients with RA during B-cell depletion (#1 to #4) at 4 and 6 months, respectively, after a first cycle of RTX therapy and 2 and 4 months, respectively, after a second cycle of RTX treatment, compared with 4 healthy controls (#5 to #8). (B) Kinetics of HLA-DRhigh plasmablast and HLA-DRlow plasma cell counts13 during peripheral blood CD20+ B-cell depletion. Data from 16 healthy controls are shown for comparison. (C) Plasmablasts from 4 healthy controls and 5 patients treated with RTX were tested for in vitro migration toward 300nM CCL28 and 10nM CXCL12, respectively, in 4 independent experiments. Migration toward bovine serum albumin (BSA) was used as a control and yielded 2%-7% migration of antibody-secreting cells. Mean values and SEM are depicted in panels B and C. (D) CD27high/intracellular Ighigh plasmablasts were analyzed for cell proliferation as detected by Ki-67 expression. Ki-67+ expressing plasmablasts were significantly reduced in RTX-treated patients with RA (RA/RTX; 7 samples; open boxes; 3 independent experiments) compared with healthy controls (HCs; 6 samples; gray boxes; P = .0156 by Mann-Whitney test). Mouse (m)IgG1 of irrelevant specificity was used to control Ki-67 staining. Box plots depict minimum, maximum, and median values and upper and lower quartiles.
Generation of migratory and proliferating plasmablasts during B-cell depletion. (A) Cytometric detection of HLA-DRhigh plasmablasts in peripheral blood samples of 4 patients with RA during B-cell depletion (#1 to #4) at 4 and 6 months, respectively, after a first cycle of RTX therapy and 2 and 4 months, respectively, after a second cycle of RTX treatment, compared with 4 healthy controls (#5 to #8). (B) Kinetics of HLA-DRhigh plasmablast and HLA-DRlow plasma cell counts13 during peripheral blood CD20+ B-cell depletion. Data from 16 healthy controls are shown for comparison. (C) Plasmablasts from 4 healthy controls and 5 patients treated with RTX were tested for in vitro migration toward 300nM CCL28 and 10nM CXCL12, respectively, in 4 independent experiments. Migration toward bovine serum albumin (BSA) was used as a control and yielded 2%-7% migration of antibody-secreting cells. Mean values and SEM are depicted in panels B and C. (D) CD27high/intracellular Ighigh plasmablasts were analyzed for cell proliferation as detected by Ki-67 expression. Ki-67+ expressing plasmablasts were significantly reduced in RTX-treated patients with RA (RA/RTX; 7 samples; open boxes; 3 independent experiments) compared with healthy controls (HCs; 6 samples; gray boxes; P = .0156 by Mann-Whitney test). Mouse (m)IgG1 of irrelevant specificity was used to control Ki-67 staining. Box plots depict minimum, maximum, and median values and upper and lower quartiles.
Functional tests of RTX-resistant steady-state cells showed, similar to previous data obtained from healthy controls, that they expressed functionally active chemokine receptors, CXCR4 and CCR10, permitting in vitro migration toward the homeostatic chemokine CXCL12 and mucosal tissue-expressed chemokine CCL28, respectively. Plasmablasts circulating during B-cell depletion therapy migrated toward 300nM CCL28 at a mean frequency of 19% (range, 2%-38%). At the same time, plasmablasts also migrated toward 10nM CXCL12 (mean frequency, 21%; range, 8%-48%; 5 donors). This equals the magnitude of in vitro migration observed when healthy control PBMC samples were tested, yielding mean frequencies of migrating plasmablasts of 21% (10nM CXCL12) and 25% (300nM CCL28), respectively (Figure 5C). Consistent with previous reports,20 we demonstrate that CCL28 selectively attracts IgA-expressing plasmablasts coexpressing CCR10 (supplemental Figure 3).
Further, RTX-resistant plasmablasts also proliferated in vivo, as identified by Ki-67 expression. During B-cell depletion therapy, 22%-56% (mean, 42%; 7 donors) of circulating antibody-secreting cells stained positively for the proliferation marker Ki-67, whereas the same cells were not stained by control antibody (mean, 3%; P = .0156, Wilcoxon test). Six healthy control samples showed a median of 62% Ki-67+ plasmablasts, indicating a maintained proliferative capacity of the RTX-resistant plasmablasts, however, at lower levels (Figure 5D).
These phenotypical and functional data indicate that during comprehensive depletion of CD20+ B cells from peripheral blood by RTX, activated; that is, proliferating and migratory plasmablasts are frequently detectable and suggest either chronic activation and differentiation of RTX-resistant precursors or the maintenance of the observed plasmablast population by continuous cell division.
Human lamina propria CD20+ B cells, but not IgA+ plasmablasts and plasma cells, are depleted by RTX
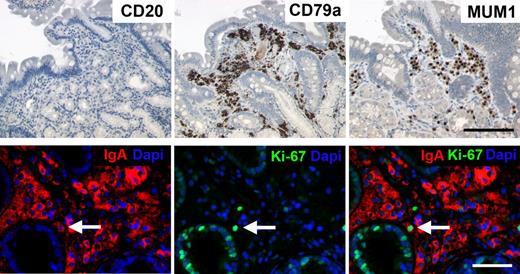
Because the bulk of the data above indicated a mucosal nature of the circulating RTX-resistant plasmablasts, we now addressed directly whether tissue-resident CD20+ B cells and IgA-secreting cells are affected by RTX therapy in vivo in the lamina propria. Therefore, we analyzed endoscopic biopsies taken from the gastrointestinal tract of 3 patients with diffuse large B-cell lymphoma during RTX treatment for CD20+ and IgA+ cells. All 3 samples did not stain with an antibody specific for human CD20, indicating the complete absence of CD20+ B cells in these mucosal tissue samples. By contrast, stainings for CD79a, MUM1, and IgA detected numerous positive cells in the lamina propria, predominantly beneath the surface epithelium. In combination with the plasmacytoid cell morphology of IgA+, MUM1+, or CD79+ cells and the absence of CD20 expression, the labeled cells represent IgA-secreting plasma cells. Moreover, proliferating Ki-67+/IgA+ plasmablasts were also detected at very low frequency: 2 cells of total 270 IgA+ plasma cells per 10 high-power fields, 1 of 167 cells and 0 of 45 cells in the tree specimen, respectively (Figure 6). These data show for the first time the resistance of human lamina propria IgA+ plasmablasts and plasma cells toward RTX treatment, whereas CD20+ B cells were entirely depleted at the same site.
Detection of IgA+ plasmablasts and plasma cells, but not CD20+ cells within human mucosal biopsies during RTX treatment. Three patients with diffuse large B-cell lymphoma were treated with RTX plus cyclophosphamide, hydroxydaunomycin, Oncovin, prednisone, and control biopsies were taken after 3 months from the duodenum or stomach. A representative duodenal biopsy is shown with absence of CD20+ B cells, whereas numerous CD79a, MUM1, and IgA+ cells were detected. Virtually all cells that were stained by these markers showed plasma cell morphology, including IgA+/Ki67+ plasmablasts detectable at a low frequency (white arrow). Original magnifications, ×200 (top) and ×400 (bottom). Black bar indicates 100 μm; white bar, 50 μm. Microscope was Olympus AX70 and AxioImager Z1 (Zeiss); numeric aperture of objective lenses were ×20, 0.70 mm; ×40, 0.90 mm. Stains used were immunoperoxide, Alexa Fluor 488/555, and DAPI (4′-6′-diamidine-2-phenylindole). Camera used was JVC KY-F70 and CCD camera (AxioCam MRm; Zeiss); acquisition software was DISKUS and Axiovision (Zeiss); and software used for image processing was Adobe Photoshop 7.0 (Adobe Systems).
Detection of IgA+ plasmablasts and plasma cells, but not CD20+ cells within human mucosal biopsies during RTX treatment. Three patients with diffuse large B-cell lymphoma were treated with RTX plus cyclophosphamide, hydroxydaunomycin, Oncovin, prednisone, and control biopsies were taken after 3 months from the duodenum or stomach. A representative duodenal biopsy is shown with absence of CD20+ B cells, whereas numerous CD79a, MUM1, and IgA+ cells were detected. Virtually all cells that were stained by these markers showed plasma cell morphology, including IgA+/Ki67+ plasmablasts detectable at a low frequency (white arrow). Original magnifications, ×200 (top) and ×400 (bottom). Black bar indicates 100 μm; white bar, 50 μm. Microscope was Olympus AX70 and AxioImager Z1 (Zeiss); numeric aperture of objective lenses were ×20, 0.70 mm; ×40, 0.90 mm. Stains used were immunoperoxide, Alexa Fluor 488/555, and DAPI (4′-6′-diamidine-2-phenylindole). Camera used was JVC KY-F70 and CCD camera (AxioCam MRm; Zeiss); acquisition software was DISKUS and Axiovision (Zeiss); and software used for image processing was Adobe Photoshop 7.0 (Adobe Systems).
Circulating plasmablasts during B-cell depletion therapy spontaneously secrete antimicrobial IgA
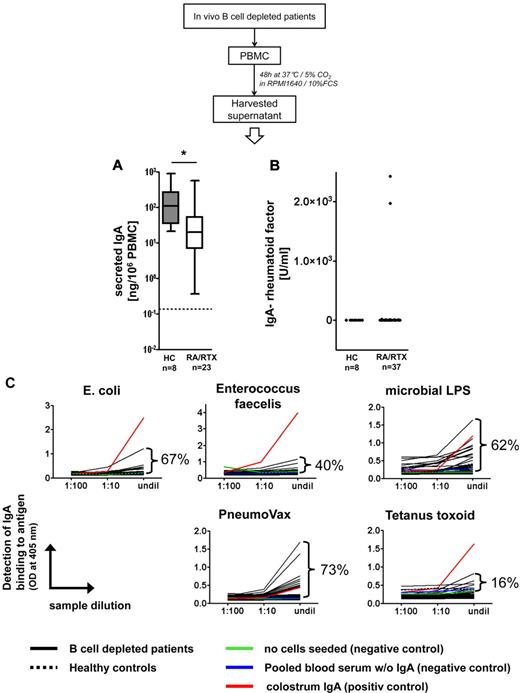
In autoimmune patients, circulating autoreactive plasmablasts can contribute to systemic autoantibody production. Therefore, we analyzed the nature of IgA secreted by steady-state plasmablasts after RTX therapy. PBMCs from patients depleted of B cells or healthy controls were allowed to spontaneously secrete antibody for 2 days in vitro, and supernatants were subjected to further analyses.
All samples obtained from 8 healthy controls and 23 patients depleted of B cells spontaneously secreted IgA in vitro. PBMCs from persons depleted of B cells secreted 72.3% less IgA than control PBMCs, on average 59 ng IgA/106 PBMC versus 213 ng IgA/106 PBMCs, respectively (P = .0112, Mann-Whitney test; Figure 7A). IgA-rheumatoid factor was detected in 5% (2 of 37) PBMC supernatants from patients depleted of B cells and not in healthy control samples (Figure 7B). By contrast, all PBMC supernatants from patients depleted of B cells and control donors contained IgA capable of binding to at least 1 of the microbes or microbial antigens tested (Escherichia coli, Enterococcus faecalis, microbial lipopolysaccharides, and PneumoVax, ie, mixed pneumococcal polysaccharides; Figure 7C). Ninety-two percent of the same samples bound to > 1 of these antigens.
Plasmablasts generated during B-cell depletion secrete antimicrobial IgA. (A) Spontaneous in vitro production of IgA from PBMCs of RTX-treated patients with RA (RA/RTX; open box) and healthy controls (HCs; gray box). Dotted line indicates the detection limit. Box plots depict minimum, maximum, and median values and upper and lower quartiles. IgA produced patient samples (B) analyzed (5%), whereas all samples bound to ≥ 1 of the 4 microbes and microbial antigens tested (C). PBMC supernatants were subjected to enzyme-linked immunosorbent assay at different dilutions with the indicated antigens for coating and IgA-specific detection. Frequencies of samples from RTX-treated patients (n = 37; black lines) yielding optical densities exceeding negative control samples at the highest concentration (undil) are indicated and reflect proportions of samples that showed binding to a particular antigen. Almost all samples tested showed concentration-dependent binding. Binding to irrelevant protein antigen (tetanus toxoid) was used as a control assay on the same samples, yielding infrequent binding of the secreted IgA compared with microbial antigens. Colostrum IgA isolated from breast milk was used as an internal positive control, pooled blood sera depleted of IgA, and medium cultured without PBMCs as internal negative controls. All PBMC supernatants from B-cell depleted and control donors bound to ≥ 1 of the bacteria or microbial antigens. Dotted black lines indicate pooled data from 8 healthy controls.
Plasmablasts generated during B-cell depletion secrete antimicrobial IgA. (A) Spontaneous in vitro production of IgA from PBMCs of RTX-treated patients with RA (RA/RTX; open box) and healthy controls (HCs; gray box). Dotted line indicates the detection limit. Box plots depict minimum, maximum, and median values and upper and lower quartiles. IgA produced patient samples (B) analyzed (5%), whereas all samples bound to ≥ 1 of the 4 microbes and microbial antigens tested (C). PBMC supernatants were subjected to enzyme-linked immunosorbent assay at different dilutions with the indicated antigens for coating and IgA-specific detection. Frequencies of samples from RTX-treated patients (n = 37; black lines) yielding optical densities exceeding negative control samples at the highest concentration (undil) are indicated and reflect proportions of samples that showed binding to a particular antigen. Almost all samples tested showed concentration-dependent binding. Binding to irrelevant protein antigen (tetanus toxoid) was used as a control assay on the same samples, yielding infrequent binding of the secreted IgA compared with microbial antigens. Colostrum IgA isolated from breast milk was used as an internal positive control, pooled blood sera depleted of IgA, and medium cultured without PBMCs as internal negative controls. All PBMC supernatants from B-cell depleted and control donors bound to ≥ 1 of the bacteria or microbial antigens. Dotted black lines indicate pooled data from 8 healthy controls.
Thus, circulating plasmablasts detectable during B-cell depletion secrete antimicrobial IgA in virtually all healthy persons and patients with RA, whereas IgA-rheumatoid factor is produced by the same cell population only in isolated patients. The overall data are consistent with the notion that these cells are generated by homeostatic mucosal immune responses while lacking a clear implication in autoimmunity.
IgA+ plasmablasts generated during steady-state express mutated VHDJH gene rearrangements
The microbial binding capabilities of IgA secreted by RTX-resistant circulating plasmablasts and the previous observation that the peritoneum is protective against RTX-mediated B-cell depletion in mice7 lead us to speculate that these cells might originate from a B1-like B-cell compartment. If they were, they should carry weakly hypermutated VH gene rearrangements.21 Therefore, we analyzed VHDJH gene rearrangements to calculate the frequency of somatic hypermutation in RTX-resistant IgA+ plasmablasts in a total of 34 circulating IgA+ plasmablasts obtained from 2 healthy controls, 66 IgA+ plasmablasts from 3 patients after RTX treatment, as well as 69 antigen-specific IgG+ plasmablasts from 3 donors after tetanus vaccination.13,15 All cells carried somatically hypermutated VH gene rearrangements with comparable mutational frequencies in the 3 populations analyzed: IgA+ plasmablasts from healthy persons showed 3-39 mutations within the VH region with a mean mutational frequency of 8.5% (range, 1.3%-17.0%), whereas RTX-resistant IgA+ plasmablasts exhibited 7-48 mutations with a mean mutational frequency of 9.9% (range, 3.0%-20.3%). For comparison, antigen-specific IgG+ plasmablasts induced by a tetanus vaccination13 yielded a mutational frequency of the same magnitude (mean mutational frequency, 9.7%). These data indicate that B-cell activation and differentiation resulting in circulating IgA+ plasmablasts in healthy persons and in patients with RA after RTX treatment do not differ in their mutational frequency. These results do not support the view that circulating IgA+ plasmablasts may derive from B1-like B cells.
Discussion
B-cell depletion by RTX has become an important option in treating RA and non-Hodgkin lymphoma, although still some of its mechanisms of action remain unclear. Although comprehensive depletion by RTX of B cells from peripheral blood is widely documented, the efficiency of B-cell removal from lymphoid tissues is of critical importance to understand potential reasons for partial or complete lack of response.
This study provides evidence for continued circulation of migratory and dividing plasmablasts with high degree of similarity with previously characterized plasmablasts circulating during steady state1 during B-cell depletion after RTX treatment. As a consequence, this observation suggests the persistence of a distinct population of noncirculating, RTX-resistant B cells. At least one-half of these cells showed the phenotype of recently generated plasmablasts1,13 before and during B-cell depletion, in that they expressed HLA-DRhigh and Ki-67, and were migratory in vitro toward ligands of CXCR4 and CCR10, thus sharing important characteristics with plasmablasts circulating in healthy persons during steady state.1 According to the emergence of antigen-specific plasmablasts in the blood after systemic or mucosal vaccination, the age of such circulating plasmablasts can be estimated with ≤ 2 weeks.13,22 However, the current data do not formally exclude that some older resident plasma cells may reenter cell cycle and recirculate, although the currently available data clearly do not support this view.2 Indicative of their generation in mucosal immune responses and very similar to plasmablasts circulating during steady state,1 more than one-half of plasmablasts/plasma cells detectable before and throughout B-cell depletion therapy expressed IgA, and the same cells predominantly coexpressed CCR10 and to a lesser extent α4β7 integrin.23,24 The IgA secreted by these plasmablasts in patients with RA showed rheumatoid factor reactivity only in single patients, whereas secretion of antimicrobial antibodies was detected in all patients as well as controls. In this regard, commensal microbes specifically induce secretory IgA production in the intestine of normal mice.25 Because IgA is the predominant antibody isotype produced in the gut and secreted at mucosal surfaces26 and is dimeric when spontaneously secreted by normal PBMC in steady state,27 the detection of IgA+ plasmablasts in patients after B-cell depletion indicates that anti-CD20 therapy has a limited effect on this measure of mucosal immunity.
In addition, the expression of the mucosal homing receptor α4β7 integrin by plasmablasts allow their interaction with mucosal addressin cell adhesion molecule-1 (MadCAM-1), which is expressed in the high endothelial venules of GALT,3,28 mediating their firm arrest to gut vessel endothelium. As a result, antibody-secreting cells in the lamina propria express α4β7 integrin.3,29 Further, CCR10 expression by IgA+ plasmablasts mediates their migration toward CCL28,20,30 which is expressed in gut- and airway-associated lymphoid tissues.31 Consistently, CCR10 is abundantly expressed by IgA-secreting cells residing within human mucosal effector sites.32 Of critical importance, IgA+ plasmablasts were identified in mucosal biopsies obtained after peripheral B-cell depletion, being apparently equally resistant to RTX compared with circulating plasmablasts. The expression of α4β7 integrin and CCR10 by circulating plasmablasts not only reflects their migrational capacity toward mucosal tissues but also suggest that they originate from mucosal immune reactions, because Rotavirus-specific plasmablasts express the same phenotype during acute Rotavirus infection.33 As plasmablasts observed here express combinations of IgA, CCR10, and α4β7 integrin, and because the absence of the spleen as another potential source of plasmablasts does not extinguish circulating IgA+ plasmablasts, we assume that these are induced within mucosal immune reactions. Migration of plasmablasts toward CXCL12 shows functionally active CXCR4 expression, as described before for plasmablasts induced in both systemic and mucosal induced immune responses.2,3,34
Hence, the underlying chronic B-cell activation most likely occurs within mucosal tissues in response to microbial stimuli and could reflect a RTX-resistant B-cell subset that exists in human inductive GALT. Coherently, antibody-mediated immunity at mucosal surfaces appears to be largely intact in RTX-treated patients, because (1) IgA-secreting plasma cells were abundantly present in lamina propria specimens patients depleted of B cells and (2) only few patients experienced complications related to impaired mucosal immunity during B-cell depletion in agreement with safety data of clinical studies.5,35 The resistance of B cells to RTX-mediated depletion in immune tissues has been studied before. In mouse models of B-cell depletion, B cells of the peritoneal cavity are not or less rapidly depleted,36,37 and germinal center B cells of the Peyer patches can be resistant to RTX.7 In man, depletion of tissue-based B cells by RTX was observed to a large extent in spleen, bone marrow, and kidney8-10,38-40 and is reflected by the inability of patients who have undergone B-cell depletion to mount a sufficient immune response upon parenteral vaccination.41,42
The efficiency of B-cell depletion within human GALT has not been addressed so far except for a single case, reporting successful B-cell depletion after anti-CD20 therapy in the appendix.43 The present study is the first to provide data indicative of RTX-resistant mucosal B cells in man. Such a self-sufficient mucosal plasmablast generation could significantly contribute to the human mucosal plasma cell population, as reflected by the detection of IgA+ plasmablasts in the lamina propria. Our data are further consistent with a previous report suggesting that lamina propria B cells do not contribute to the human (intestinal) IgA response,44 because we did not detect any CD20+ B cells but proliferating IgA+ plasmablasts in the lamina propria.
In accordance with mouse data45 and the self-sufficiency of mucosal plasmablast production in steady state, their generation and/or maintenance was not abrogated in persons who had undergone a splenectomy. Splenectomized mice further lack peritoneal cavity B1a cells,46 suggesting that an equivalent population would not contribute to circulating IgA+ plasmablasts in man. Consistent with this notion, all circulating IgA+ plasmablasts analyzed during the steady state of healthy persons and RTX-treated patients expressed highly mutated VHDJH gene rearrangements. The expression of those supports their differentiation from antigen-experienced B cells as precursors of mucosal plasmablasts circulating in steady state and B-cell depletion.
In this regard, sessile mucosal memory B cells have been described in the mucosal epithelium47 and beneath microfold (M) cells within mucosal isolated lymphoid follicles.48 Of note, malignant mucosa-associated lymphoid tissue B cells also express mutated VH rearrangements49 and can survive RTX treatment within epithelial cell aggregates,12 suggesting a possible link between RTX-resistant mucosal B cells as a source for plasmablast production in steady state. With respect to RA, no stringent numerical or functional correlation was identified between residual disease activity and the persistent generation of IgA+ plasmablasts in these patients, so that persisting autoantibody titers observed during RTX treatment6 appear to be provided by long-lived plasma cells surviving the treatment in bone marrow and synovia.38,40
Although scientific and clinical implications require additional research, the present data of this study point toward a RTX-resistant subset of human mucosal B cells chronically differentiating into circulating mucosal IgA+ plasmablasts.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank T. Kaiser, K. Raba, and J. Kirsch for excellent assistance with cell sorting and Drs J. Benckert and H. Wardemann for kind provision of bacteria. We thank Simone Spieckermann for excellent technical assistance. We also thank all rheumatologists involved in the study.
This work was supported by the Deutsche Forschungsgemeinschaft through grants SFB421/B13 (T.D.), SFB650/TP16 (T.D.), and Z3 (C.L.), DFG Do7-1 (T.D.), SPP Immunobone Do 8-1 (T.D. and H.E.M.), by the Deutsche Gesellschaft für Rheumatologie through the DGRh start-up grant (H.E.M.), by the Charité University Medicine, and the Berlin Senate.
Authorship
Contribution: H.E.M., D.F., C.G., C.L., K.R., and S.S. performed research and analyzed results; H.E.M. and C.L. made the figures; H.E.M. and T.D. designed research; H.E.M., T.D., and A.R. discussed results and wrote the manuscript; and H.-P.T., C.D., and E.F. discussed results and provided vital material for the study.
Conflict-of-interest disclosure: T.D. and E.F. have received support for conducting clinical studies and speakers' honoraria (< $10 000) from Roche. The remaining authors declare no competing financial interests.
Correspondence: Henrik E. Mei, B Cell Memory Group, Deutsches Rheumaforschungszentrum (DRFZ), Charitéplatz 1, 10117 Berlin, Germany; e-mail: mei@drfz.de.