Abstract
Interleukin-2 (IL-2) and IL-21 share activities in the control of T- and B-cell maturation, proliferation, function, and survival. However, opposing roles for IL-2 and IL-21 have been reported in the development of regulatory T cells. To dissect unique, redundant, and opposing activities of IL-2 and IL-21, we compared T- and B-cell development and function in mice lacking both IL-2 receptor α (IL-2Rα) and IL-21R (double knockouts [DKO]) with single knockout and wild-type (WT) mice. Similarly to il2ra−/− mice, DKO showed reduced numbers of regulatory T cells and, consequently, hyper-activation and proliferation of T cells associated with inflammatory disease (ie, colitis), weight loss, and reduced survival. The absence of IL-2Rα resulted in overproduction of IL-21 by IFN-γ–producing CD4+ T cells, which induced apoptosis of marginal zone (MZ) B cells. Hence, MZ B cells and MZ B-cell immunoglobulin M antibody responses to Streptococcus pneumoniae phosophorylcholine were absent in il2ra−/− mice but were completely restored in DKO mice. Our results highlight key roles of IL-2 in inhibiting IL-21 production by CD4+ T cells and of IL-21 in negatively regulating MZ B-cell survival and antibody production.
Introduction
Cytokines with receptors sharing the common gamma chain (γc) including interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 are important mediators of adaptive immune responses. IL-2 is the first and probably the best-characterized member of this family and has been shown to support growth, proliferation, and survival of T and B cells. Although some of its activities seem to be nonessential in vivo, key functions of IL-2 are the expansion of memory T cells in recall responses as well as the maintenance and development of natural FoxP3+ regulatory T cells (Tregs).1,2 Mice lacking IL-2 or components of the IL-2R (ie, IL-2Rα and IL-2Rβ) come down with fatal multiorgan inflammation including inflammatory bowel disease3-5 due to reduced numbers of Tregs and, consequently, hyperactivation and expansion of T cells.1 The deregulated activation of CD4 T cells in these mice also leads to the extensive differentiation of B cells into antibody secreting plasma cells, causing elevated levels of immunoglobulins in the serum and exhaustion of B cells.3-6 IL-21 is the most recently described member of the type I cytokine family and has been implicated in the regulation of T- and B-cell function. IL-21 plays an important role in T helper 2 (Th2) effector responses to airway allergens and nematode infection.7,8 In addition, several recent reports demonstrated a pivotal role of IL-21 in preventing CD8 T-cell exhaustion during chronic viral infection.9-11 In vitro, IL-21 has been shown to inhibit TGF-β–induced differentiation of naive CD4+ T cells to inducible regulatory T cells (iTregs) and to promote Th17 differentiation instead.12 However, a critical role of IL-21 in the control of Th17 and Treg cells in vivo remains questionable considering that IL-21 and IL-21R knockouts developed severe autoimmune disease and had unaltered frequencies of Tregs and Th17 cells in models of autoimmune encephalitis and myocarditis.13,14 IL-21 has different effects on B cells.15 In vitro experiments and IL-21 transgenic mice highlighted a potentially dual role of IL-21 on B cells. On the one hand, it can promote apoptosis of resting B cells, and, on the other hand, drives isotype switching and plasma cell differentiation dependent on the context of stimulation.16,17 However, B-cell homeostasis is unaffected in il21r−/− mice, while germinal center formation and IgG1 responses are severely impaired.18 IL-21R is highly expressed on CD23+ CD21int follicular (FO) B cells, which represent the most abundant subset of B cells. They circulate in the periphery and are found in all secondary lymphoid organs. Upon antigen encounter, FO B cells undergo activation, somatic hypermutation and class-switching, which depends on help provided by CD4+ T cells. IL-21 produced by follicular helper T cells (TFH) has been suggested to promote germinal center formation and immunoglobulin (Ig) class switching.19,20 CD23lo CD21hi marginal zone (MZ) B cells represent a more innate type of B cells.21 In rodents, MZ B cells are exclusively located in the spleen. They are rapidly activated without help of CD4+ T cells, which allows prompt production of low-affinity unswitched IgM antibodies in response to blood borne antigens.21,22 The immune complexes generated by this rapid low- affinity IgM response are then transported onto follicular dendritic cells, thereby facilitating the response of FO B cells.23 The study presented here highlights the importance of IL-2 and IL-21 in determining the balance and the functionality of marginal zone and follicular B cells.
Methods
Mice and antibodies
C57BL/6, il2ra−/− (B6.129S4-Il2ratm1Dw/J)4 and il21r−/− mice (C57BL/6 N7)7 were bred and maintained under specific pathogen-free conditions at Biosupport, Schlieren. For experiments, age-matched mice in the age of 6-9 weeks were used. For the experiment depicted in Figure 5C, C57BL/6 mice were purchased from Charles River Inc. Swiss federal and local animal ethics committees approved the described animal experiments.
The following antibodies were used: fluorescein isothiocyanate–labeled anti-CD21, anti-CD44 and anti–IL-17A; phycoerythrin-labeled anti-B220, anti-CD5, anti-CD21, and anti-CD62L; peridinin chlorophyll protein complex-labeled anti-CD4; allophycocyanin-labeled anti-CD8, anti-CD19, anti-IFNγ and anti-FoxP3; biotin-labeled anti-CD23. For secondary staining of biotin-labeled antibodies, phycoerythrin-, peridinin chlorophyll protein complex-, or allophycocyanin-labeled streptavidin was used. For nonviable cell exclusion, 7-amino-actinomycin D (7-AAD) viability staining solution was used. All of the listed reagents were purchased from eBioscience.
Histopathology of intestines
Intestinal tissue specimens were fixed in 4% paraformaldehyde for subsequent embedding in paraffin. After preparation of tissue sections, deparaffinized sections were stained with hematoxylin and eosin for histological analysis. To compare the spontaneous histopathological alterations in the large intestine, a scoring system ranging from 0 (no alterations) to 15 (most severe signs of colitis) was established, including the following parameters: (1) infiltration of the lamina propria (LP) of the large intestine (score from 0 to 3), (2) mucin depletion/ loss of goblet cells (score from 0 to 3), (3) crypt abscesses (score from 0 to 3),(4) epithelial erosion (score from 0 to 1), (5) hyperemia (score from 0 to 2), and (6) thickness of the colonic mucosa (score from 0 to 3).
Cell preparation
Single-cell suspensions from spleen, peripheral (inguinal) lymph nodes, and Peyer patches were prepared by pressing the organs through 40-μm pore size strainers (BD Biosciences) in phosphate-buffered saline (PBS) supplemented with 2% fetal calf serum. For the isolation of lamina propria lymphocytes, the colon was cut into small pieces and incubated 30 minutes under agitation at 37°C in HEPES-buffered saline 2% fetal calf serum (FCS; Ca/Mg free) containing 5mM EDTA (ethylenediaminetetraacetic acid) and 2mM dithiothreitol. After thorough vortexing, the supernatant, containing intraepithelial lymphocytes, was discarded. The procedure was repeated 2 more times without dithiothreitol to remove further epithelial cells. The remaining tissue was then digested 80 minutes under agitation at 37°C in HBS supplemented with 1 mg/mL collagenase IV (Sigma). The mixture was vortexed and the lamina propria lymphocyte-containing supernatant was filtered through 40-μm pore size strainers, washed and resuspended in PBS 2% FCS.
Immunofluorescent stainings and analysis
For surface stainings, the cells were resuspended in PBS 2% FCS, briefly incubated with Fc receptor-blocking monoclonal antibody (clone 2.4G2) and subsequently incubated at 4°C with the relevant fluorescently labeled surface antibodies. After 15 minutes cells were washed and resuspended in PBS 2% FCS for flow cytometric analysis using a FACSCalibur (BD Biosciences). FACS data were then analyzed using FlowJo Version 8.8.6 (TreeStar Inc).
In vitro restimulation and intracellular staining
To detect cytokine production, cells were incubated 4 hours at 37°C with phorbol 12-myristate 13-acetate (PMA; 10−7M, Sigma-Aldrich), ionomycin (1 μg/mL, Sigma-Aldrich) and monensin (2 μg/mL, Sigma-Aldrich). For intracellular cytokine staining, restimulated cells were surface stained, then fixed with 2% formalin for 10 minutes. For FoxP3 staining, unstimulated cells were incubated 10 minutes with FACS lysing solution (BD Biosciences). After permeabilization with PBS 2% FCS supplemented with 0.5% saponin, cells were incubated with fluorescently labeled antibodies for 30 minutes at room temperature, washed twice, and resuspended in PBS 2% FCS for analysis. For intracellular IL-21 staining, IL-21R/ human Fc fusion protein was produced and used as previously described.10
Immunizations
Groups of mice (n = 4) were injected intravenously each with 1 × 108 colony-forming units of heat-inactivated Streptococcus pneumoniae (strain D39) and bled at day 5 to determine IgM antibody responses to phosphorylcholine, Additionally groups of mice were injected intravenously with 50 μg of Qβ virus-like particles (Qβ-VLP) containing Escherichia coli-derived RNA. Capsids of the RNA phage Qβ were cloned into pQβ10 vector and purified as previously described.24 Mice were bled at day 14 to determine VLP-specific IgM and IgG serum antibody levels.
Measurement of antibodies by ELISA
Serum antibody levels in 6- to 8-week-old mice were determined by serial serum dilution on 96-well plates (Maxisorp; Nunc) coated with either (1) 1 μg/mL unlabeled goat anti–mouse IgG1 or IgM (Southern Biotech) for measurement of natural antibodies in naive mice, (2) 25 μg/mL phosphorylcholine conjugated to bovine serum albumin (PC-BSA, Biosearch Technologies), or (3) 1 μg/mL Qβ-VLP, for measurement of respective antibody responses in immunized mice
Generation of mixed-bone marrow chimeras
Bone marrow cells were obtained from flushing of femurs and tibias of donor WT CD45.1, il2ra−/− CD45.2 and DKO CD45.2. Lethally irradiated receiver mice (WT CD45.1) were then reconstituted by intravenous injection of equal numbers of CD45.1 and CD45.2 bone marrow cells. Receiver mice were treated with antibiotics and were analyzed 8 weeks after reconstitution.
Ex vivo follicular and marginal zone B-cell stimulation
Spleens from C57BL/6 mice (Charles River) were separately processed into single-cell suspension and were stained with CD21- and CD23-specific antibodies in the presence of Fc receptor blocking antibody. CD21int CD23hi FO B and CD21hi CD23lo MZ B cells were sorted on a FACSAria cell sorter (BD Biosciences) to a purity > 98%. Sorted cells from each spleen were cultured in complete IMDM medium supplemented with the indicated amount of recombinant IL-2 (Invitrogen) and IL-21 (R&D Systems). After 6 hours, cells were stained with 7-aminoactinomycin D (7-AAD) and analyzed on a FACSCalibur (BD Biosciences). Cell viability was determined by discrimination of size and granularity using forward and side scatter combined with 7-AAD staining. The percentage of viable cells for each condition was then normalized to the viability of cells from the same spleen incubated without cytokines. After 6 hours, the baseline viability without any cytokine was ∼ 80% for FO B cells and ∼ 55% for MZ B cells. The CD21 and CD23 expression pattern characteristic of MZ and FO B cells was unaffected by the culture conditions.
Quantitative RT-PCR
Total RNA was isolated separately from the spleens of 3 mice per group using TRI Reagent (Molecular Research Center Inc.), treated with DNase (Invitrogen), and reverse transcribed using Super Script III RT (Invitrogen). Quantitative real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed with Brilliant SYBR Green (Stratagene) on an i-Cycler (Bio-Rad Laboratories). Expression was normalized to the house keeping gene β-actin. Primers used: β-actin (fwd) 5′-TGTCATCCTGCTCTTCTTTCTC-3′ and (rev) 5′-GCACCTTG-GAAGCCCTAC-3′; IL-21 (fwd) 5′-CGCCTCCTGATTAGACTTCG-3′ and (rev) 5′-ATGCTCACAGTGCCCCTTTA-3′.
Statistical analysis
Two-tailed paired and unpaired t tests were performed using Prism 4.0 (GraphPad software). In the figures, * indicates P < .05, ** indicates P < .01, and *** indicates P < .001.
Results
Mice lacking both IL-2Rα and IL-21R show reduced numbers of Tregs and T-cell hyperactivation and develop colitis similarly to mice lacking IL-2Rα only
To investigate the interplay of IL-2 and IL-21 in the regulation of adaptive immune responses, we compared il2ra/il21r double-knockout mice (DKO) to the respective single-knockouts and WT mice. Similarly to il2ra−/− mice,4 DKO mice showed a severe loss in body weight, hemolytic anemia, and splenomegaly and had to be euthanized between 8 and 12 weeks of age according to animal protection laws (Figure 1A-B and data not shown). Frequencies and total numbers of splenic CD4+ and, in particular, CD8+ T cells were strikingly increased (Figure 1C) and displayed an effector/memory phenotype as determined by up-regulation of CD44 and down-regulation of CD62L surface expression in DKO and il2ra−/− mice compared with WT and il21r−/− mice (Figure 1D). In addition, frequency of IFN-γ producing CD4+ T cells was massively enhanced in DKO and il2ra−/− mice, whereas frequencies of IL-17A– and IL-4–producing cells were negligible and comparable in the 4 groups of mice (Figure 1E and data not shown). Similar results were obtained by analyzing lymph nodes (data not shown). Percentages of Foxp3+ Tregs were comparably reduced in spleens, lymph nodes, and Peyer patches of il2ra−/− and DKO mice and remained unchanged in il21r−/− compared with WT mice (Figure 1F). Consequently, both il2ra−/− and DKO mice showed a pronounced infiltration of CD4 and CD8 T cells in the colonic lamina propria and developed comparably severe colitis as determined by flow cytometry (Figure 1G) and histological analysis (Figure 1H), respectively. Although il21r−/− mice showed a slightly increased cellularity in the colonic lamina propria, distinct histopathological signs for a spontaneous intestinal inflammation were absent (Figure 1H). In conclusion, our results indicate that obliteration of IL-21 signaling is not sufficient to rescue the defect of regulatory T cells and hence the lymphoproliferative disorder of IL-2Rα–deficient mice.
Il2ra/il21r DKO mice show reduction of Tregs, T-cell hyperactivation, and weight loss and develop colitis similar to il2ra−/− mice. (A) Body weight of il2ra−/− (n = 4-11), il21r−/− (n = 11), DKO (n = 4-10), and WT (n = 5) mice at indicated times. Values indicate percentages of WT. Normalization was done separately for males and females before pooling the data of a group. (B-F) Analysis of the spleen of 5- to 7-week-old mice. Shown is total number of splenocytes after erythrocyte lysis (B), percentages (left panel) and absolute numbers (right panel) of splenic CD4 and CD8 T cells (C), surface expression of CD44 and CD62L on CD4+ T cells (D), frequency of CD4+ T cells producing IFNγ or IL-17 after 4-hour stimulation with PMA and ionomycin (E), and percentages of FoxP3+ CD4+ regulatory T cells (F). (G) FACS analysis of lamina propria cells of the colon isolated from 7- to 9-week-old mice as described in “Cell preparation.” Cells were stained with CD4- and CD8-specific antibodies. Shown are dot plots of individual mice representative for the group. (B,C,E,F) Values indicate averages ± SEM of 4-5 mice per group. Data shown are representative of 3 separate experiments. (H) Histopathological scoring of colons of 7- to 10-week old mice (n = 5-8/group) as described in “Histopathology of intestines.” Scores between 0 and 4 indicate no or mild colitis; scores between 5 and 9 indicate moderate colitis; and scores between 10 and 15 indicate severe colitis.
Il2ra/il21r DKO mice show reduction of Tregs, T-cell hyperactivation, and weight loss and develop colitis similar to il2ra−/− mice. (A) Body weight of il2ra−/− (n = 4-11), il21r−/− (n = 11), DKO (n = 4-10), and WT (n = 5) mice at indicated times. Values indicate percentages of WT. Normalization was done separately for males and females before pooling the data of a group. (B-F) Analysis of the spleen of 5- to 7-week-old mice. Shown is total number of splenocytes after erythrocyte lysis (B), percentages (left panel) and absolute numbers (right panel) of splenic CD4 and CD8 T cells (C), surface expression of CD44 and CD62L on CD4+ T cells (D), frequency of CD4+ T cells producing IFNγ or IL-17 after 4-hour stimulation with PMA and ionomycin (E), and percentages of FoxP3+ CD4+ regulatory T cells (F). (G) FACS analysis of lamina propria cells of the colon isolated from 7- to 9-week-old mice as described in “Cell preparation.” Cells were stained with CD4- and CD8-specific antibodies. Shown are dot plots of individual mice representative for the group. (B,C,E,F) Values indicate averages ± SEM of 4-5 mice per group. Data shown are representative of 3 separate experiments. (H) Histopathological scoring of colons of 7- to 10-week old mice (n = 5-8/group) as described in “Histopathology of intestines.” Scores between 0 and 4 indicate no or mild colitis; scores between 5 and 9 indicate moderate colitis; and scores between 10 and 15 indicate severe colitis.
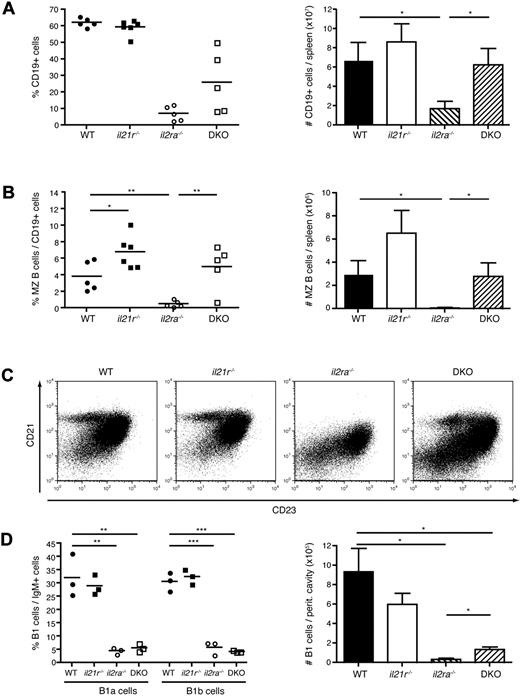
IL-2Rα-deficient mice lack MZ B cells, which are restored in il2ra/il21r DKO mice
IL-2 and IL-21 have been described to regulate the fate and function of B cells.4,6,25 As reported previously, numbers of peripheral CD19+ B cells were considerably reduced in il2ra−/− mice, probably because of exhaustive activation by CD4+ T cells, but were unchanged in il21r−/− mice. Interestingly, B cells were partially recovered but were still underrepresented in the spleen of DKO mice compared with WT (Figure 2A). When analyzing subpopulations of B cells in the spleen, we noticed a complete absence of CD23lo CD21hi MZ B cells in il2ra−/− mice. Interestingly, they were restored to WT levels in the DKO mice (Figure 2B-C) demonstrating that their disappearance in il2ra−/− mice depends on IL-21. Moreover, il21r−/− mice showed an increase in MZ B cells. We next investigated peritoneal B1 cells, including B1a and B1b cells, which represent an additional arm of “innate” natural IgM-producing B cells. Numbers were strongly reduced in both il2ra−/− and DKO mice, although the latter group was slightly less affected (Figure 2D), indicating an IL-21–independent mechanism of B1 cell loss in the absence of IL-2Rα. These data suggest a role of IL-21 in negatively regulating the homeostasis of MZ B cells.
Marginal zone B-cell loss in il2ra−/− mice is restored in DKO mice. Analysis of B-cell populations in the spleen (A-C) and peritoneum (D) of 6- to 8-week-old mice. (A) Percentages (left panel) and total numbers (right panel) of CD19+ B cells. (B) Percentages of CD21+CD23− MZ B cells among total CD19+ B cells (left panel) and total number of MZ B cells (right panel). Symbols represent individual mice and values in column plots indicate averages ± SEM of groups of mice (n = 4-5/group). (C) Dot plots of CD21 and CD23 expression gated on CD19+ B cells of individuals representative for the group. Data are representative of at least 3 independent experiments. (D) Percentages of IgMhi CD23− CD5+ B1a cells and IgMhi CD23− CD5− B1b cells (left panel) and total B1 cell numbers (right panel) in the peritoneal cavity. Three mice per group were analyzed.
Marginal zone B-cell loss in il2ra−/− mice is restored in DKO mice. Analysis of B-cell populations in the spleen (A-C) and peritoneum (D) of 6- to 8-week-old mice. (A) Percentages (left panel) and total numbers (right panel) of CD19+ B cells. (B) Percentages of CD21+CD23− MZ B cells among total CD19+ B cells (left panel) and total number of MZ B cells (right panel). Symbols represent individual mice and values in column plots indicate averages ± SEM of groups of mice (n = 4-5/group). (C) Dot plots of CD21 and CD23 expression gated on CD19+ B cells of individuals representative for the group. Data are representative of at least 3 independent experiments. (D) Percentages of IgMhi CD23− CD5+ B1a cells and IgMhi CD23− CD5− B1b cells (left panel) and total B1 cell numbers (right panel) in the peritoneal cavity. Three mice per group were analyzed.
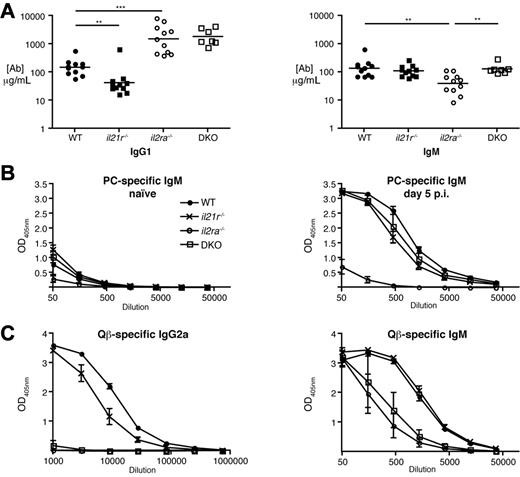
MZ B cell antibody response is absent in il2ra−/− mice and restored in DKO mice, whereas FO B-cell IgM and IgG responses are affected in both il2ra−/− and DKO mice.
Mice lacking IL-2, IL-2Rα, or IL-2Rβ have been described to develop a hyper-IgG1-syndrome at young age due to the unspecific polyclonal activation mediated by deregulated CD4 T cells; however, these mice fail to mount antigen-specific IgG responses after immunization.3-5 We found 10-fold increased IgG1 serum antibody levels in both naive il2ra−/− and DKO mice consistent with hyperactivated CD4 T cells in both groups of mice (Figure 3A). Notably, natural IgM antibody levels were reduced in naive il2ra−/− mice compared with WT and il21r−/− mice but were back to normal in the DKO mice (Figure 3A), which correlated with the loss and recovery of MZ B cells in il2ra−/− and DKO mice, respectively. These data indicate a prominent role of MZ B cells in the production of natural IgM antibodies.
Absence of MZ B-cell antibody response il2ra−/− mice is restored in DKO mice. (A) Concentration of IgG1 and IgM in the serum of naive mice. Symbols represent individual mice. (B) Mice (n = 4/group) were immunized intravenously with 108 colony forming units of heat-inactivated S pneumoniae. Blood was collected before and 5 days after immunization. Serum IgM antibody titers specific for phosphorylcholine were determined by ELISA. (C) Mice (n = 4/group) were immunized intravenously with Qβ-VLP and bled at day 14 for measurement of antibody responses. Qβ-specific IgM and IgG2a antibody titers were measured by ELISA. OD405nm values show averages ± SEM of groups at indicated serum dilutions.
Absence of MZ B-cell antibody response il2ra−/− mice is restored in DKO mice. (A) Concentration of IgG1 and IgM in the serum of naive mice. Symbols represent individual mice. (B) Mice (n = 4/group) were immunized intravenously with 108 colony forming units of heat-inactivated S pneumoniae. Blood was collected before and 5 days after immunization. Serum IgM antibody titers specific for phosphorylcholine were determined by ELISA. (C) Mice (n = 4/group) were immunized intravenously with Qβ-VLP and bled at day 14 for measurement of antibody responses. Qβ-specific IgM and IgG2a antibody titers were measured by ELISA. OD405nm values show averages ± SEM of groups at indicated serum dilutions.
We next immunized mice with heat-inactivated S pneumoniae and measured phosphorylcholine (PC)–specific IgM antibodies, which are produced mainly by MZ B cells.21 As shown in Figure 3B, il2ra−/− mice failed to generate anti-PC IgM antibodies, whereas DKO mice mounted a strong PC-specific IgM response comparable with WT and il21r−/− mice. To study antigen-specific IgM and isotype-switched IgG responses by FO B cells, we immunized mice with replication-defective Qβ-VLP containing E coli ssRNA. Qβ-VLP responses have been well characterized and shown to trigger a potent IgG2c (previously termed IgG2a) antibody response dependent on CD4 T helper (Th) cells and TLR7-MyD88 signaling together with a Th cell–independent IgM response mainly derived from FO B cells and not MZ B cells.26,27 Although the IgG2c response to VLP was only weakly reduced in il21r−/− mice, as shown recently,28 it was completely inoperable in il2ra−/− and DKO mice (Figure 3C), possibly because of defective T-cell help and germinal center reactions. Moreover, the IgM response to this antigen was substantially compromised but not completely absent in both il2ra−/− and DKO (Figure 3C). Together, these data suggest that FO B cell–derived IgM and IgG responses are affected to a similar level in il2ra−/− and DKO mice, whereas IgM responses mediated by MZ B cells are absent in il2ra−/− and restored in DKO mice.
Selective depletion of MZ B cells in il2ra−/− mice depends on T-cell hyperactivation
Previous reports indicated that extensive B-cell activation and long-term exhaustion in il2rb-deficient mice depended on the hyperactivated CD4 T cells.5 To investigate whether deregulated CD4+ T cells in il2ra−/− mice were responsible for the loss of MZ B cells, we generated mixed bone marrow chimeras with WT CD45.1 and either il2ra−/− or DKO CD45.2 bone marrow. Chimeric mice did not develop the lymphoproliferative disorder characterizing il2ra−/− mice, most probably because of the restored presence of Tregs derived mainly from the WT compartment (Figure 4C and data not shown).1 Analysis of splenic B-cell populations revealed that MZ B cells derived from il2ra−/− and DKO bone marrow developed normally, although to a slightly reduced extent compared with the WT compartment (Figure 4A-B). As shown in Figure 4D, il2ra−/− and DKO CD4 T cells were not activated, confirming the reestablished control of T cells by Tregs. These results indicate that the absence of MZ B cells in il2ra−/− mice depends on T-cell hyperactivation.
IL-2Rα is not essential for MZ B-cell development in a noninflamed environment. Mixed bone marrow chimeras were generated by reconstitution of irradiated WT C57BL/6 mice (CD45.1) with a 1:1 mixture of bone marrow cells of WT (CD45.1) and il2ra−/− (CD45.2) or WT (CD45.1) and DKO (CD45.2) mice. Two months later, the spleen was removed, and the isolated cells were analyzed by flow cytometry. (A) Cells were stained with anti-CD21, anti-CD23, anti-CD19, and anti-CD45.2 or CD45.1 monoclonal antibodies. Representative dot plots of individual samples show CD21 and CD23 expression gated on CD19+ CD45.2+ B cells (top panel) or CD19+ CD45.1+ B cells (bottom panel). (B) Shown is ratio of knockout (CD45.2) versus WT (CD45.1) CD21+CD23− MZ B cells among CD19+ cells. Values indicate averages ± SEM of 4 mice per group. (C) Percentages of FoxP3+ regulatory T cells among CD4+ cells. Symbols indicate individual mice. Notably, more than 90% of the Tregs in the chimera were derived from CD45.1 WT bone marrow (data not shown). (D) CD44 and CD62L expression on cells gated on CD4+ and CD45.2+. Shown are dot plots of individual samples representative of a group of mice.
IL-2Rα is not essential for MZ B-cell development in a noninflamed environment. Mixed bone marrow chimeras were generated by reconstitution of irradiated WT C57BL/6 mice (CD45.1) with a 1:1 mixture of bone marrow cells of WT (CD45.1) and il2ra−/− (CD45.2) or WT (CD45.1) and DKO (CD45.2) mice. Two months later, the spleen was removed, and the isolated cells were analyzed by flow cytometry. (A) Cells were stained with anti-CD21, anti-CD23, anti-CD19, and anti-CD45.2 or CD45.1 monoclonal antibodies. Representative dot plots of individual samples show CD21 and CD23 expression gated on CD19+ CD45.2+ B cells (top panel) or CD19+ CD45.1+ B cells (bottom panel). (B) Shown is ratio of knockout (CD45.2) versus WT (CD45.1) CD21+CD23− MZ B cells among CD19+ cells. Values indicate averages ± SEM of 4 mice per group. (C) Percentages of FoxP3+ regulatory T cells among CD4+ cells. Symbols indicate individual mice. Notably, more than 90% of the Tregs in the chimera were derived from CD45.1 WT bone marrow (data not shown). (D) CD44 and CD62L expression on cells gated on CD4+ and CD45.2+. Shown are dot plots of individual samples representative of a group of mice.
Enhanced IL-21 production in il2ra−/− mice directly induces MZ B-cell death
The experiments described in the previous paragraphs indicated that the lack of MZ B cells in il2ra−/− mice depended on deregulated T cell activation and IL-21 production. Indeed, quantitative PCR analysis showed that IL-21 mRNA levels were strongly (ie, ∼ 10-fold) up-regulated in spleens of naive il2ra−/− compared with WT mice (Figure 5A).
Enhanced IL-21 expression on CD4+ T cells in il2ra−/− and DKO mice is responsible for death of MZ B cells. (A) Expression of IL-21 mRNA in the spleen as determined by real-time PCR. Values show averages ± SEM of 3 mice per group. (B-D) IL-21 production measured by flow cytometry. Splenocytes of individual mice (n = 3/group) were stimulated with PMA and ionomycin for 4-hour prior staining of surface CD4 and intracellular IFN-γ and IL-21. (B) Dot plot profiles of IL-21 and IFNγ expression gated on CD4+ cells of individual samples representative for a group. Numbers indicate average percentage ± SD of IFN-γ+CD4+ cells. (C) Histograms represent the overlay of IL-21 expression stained by a soluble IL-21R-Fc gated on all CD4+ (left panel), CD4+IFNγ+ (middle panel), or CD4+IFNγ− (right panel) cells of WT (dashed line) and il2ra−/− (continuous line) splenocytes. The solid gray line indicates staining with a control-Fc. (D) Bar graph shows averages ± SEM of the geometric MFI of staining with IL-21R-Fc gated on IFNγ+ and IFNγ− cells. Data shown are representative of 2 independent experiments. The dashed line indicates the MFI obtained when staining with a control-Fc. (E) MZ and FO B cells were purified as described in “Ex vivo follicular and MZ B-cell stimulation” and cultured with indicated concentrations of cytokines for 6 hours before measurement of viability by staining with 7-AAD. The bar graph shows viability relative to a medium control in each condition. Baseline viability in the absence of cytokines was ∼80% for FO B and ∼55% for MZ B cells. Similar results were obtained in 2 independent experiments. (F) Reciprocal roles of IL-2 and IL-21 in regulating MZ and FO B cells during inflammation. IL-2 is required for Treg development and function and might directly support survival of MZ B cells. In the absence of IL-2, the lack of functional Tregs results in the deregulated activation of CD4 cells, leading to secretion of IFNγ and IL-21. The latter directly and preferentially induces death of MZ B over FO B cells.
Enhanced IL-21 expression on CD4+ T cells in il2ra−/− and DKO mice is responsible for death of MZ B cells. (A) Expression of IL-21 mRNA in the spleen as determined by real-time PCR. Values show averages ± SEM of 3 mice per group. (B-D) IL-21 production measured by flow cytometry. Splenocytes of individual mice (n = 3/group) were stimulated with PMA and ionomycin for 4-hour prior staining of surface CD4 and intracellular IFN-γ and IL-21. (B) Dot plot profiles of IL-21 and IFNγ expression gated on CD4+ cells of individual samples representative for a group. Numbers indicate average percentage ± SD of IFN-γ+CD4+ cells. (C) Histograms represent the overlay of IL-21 expression stained by a soluble IL-21R-Fc gated on all CD4+ (left panel), CD4+IFNγ+ (middle panel), or CD4+IFNγ− (right panel) cells of WT (dashed line) and il2ra−/− (continuous line) splenocytes. The solid gray line indicates staining with a control-Fc. (D) Bar graph shows averages ± SEM of the geometric MFI of staining with IL-21R-Fc gated on IFNγ+ and IFNγ− cells. Data shown are representative of 2 independent experiments. The dashed line indicates the MFI obtained when staining with a control-Fc. (E) MZ and FO B cells were purified as described in “Ex vivo follicular and MZ B-cell stimulation” and cultured with indicated concentrations of cytokines for 6 hours before measurement of viability by staining with 7-AAD. The bar graph shows viability relative to a medium control in each condition. Baseline viability in the absence of cytokines was ∼80% for FO B and ∼55% for MZ B cells. Similar results were obtained in 2 independent experiments. (F) Reciprocal roles of IL-2 and IL-21 in regulating MZ and FO B cells during inflammation. IL-2 is required for Treg development and function and might directly support survival of MZ B cells. In the absence of IL-2, the lack of functional Tregs results in the deregulated activation of CD4 cells, leading to secretion of IFNγ and IL-21. The latter directly and preferentially induces death of MZ B over FO B cells.
Moreover, FACS analysis revealed an increased frequency of CD4+ T cells producing IL-21 in splenocytes of il2ra−/− mice upon 4-hour stimulation with PMA and ionomycin in vitro (Figure 5B-C left panel). Notably, the entire population of IFNγ-secreting CD4+ T cells coproduced IL-21 (CD4+IFNγ+IL-21+ T cells), whereas we did not find any IL-21+IFNγneg CD4+ cells (Figure 5C middle and right panels). Besides the increased frequency of CD4+IFNγ+IL-21+ T cells in il2ra−/− mice, these cells produced also elevated amounts of IL-21 on a per-cell basis compared with the sister population in WT mice as indicated by comparing mean fluorescence intensity (MFI) of the staining (Figure 5D). Thus, we conclude that IL-21 production was strongly increased in IL-2Rα–deficient and DKO mice, as a net result of the increased proportion of cells coproducing IFNγ and IL-21 (Figure 5B-C) and of an enhanced IL-21-production per cell (Figure 5D). We hypothesized that IL-21 may directly affect survival of MZ and FO B cells. To address this, we sorted these populations and cultured them with different concentrations of IL-2, IL-21, or both. After 6 hours, we analyzed the fraction of surviving cells relative to the medium control based on scattering properties and 7-AAD staining. IL-2 showed a subtle prosurvival effect on MZ B cells at very high doses (100 ng/mL) and no effect on FO B cells. In contrast, IL-21 induced extensive death of MZ B cells at concentrations ≥ 10 ng/mL, whereas survival of FO B cells was moderately affected by IL-21. Notably, culture of MZ B cells with rIL-2 and IL-21 did not revert the MZ B-cell death induced by IL-21 (Figure 5E). These data indicate that IL-21 potently and directly induces death of MZ B cells.
Discussion
Mice lacking IL-2 or components of the IL-2R (IL-2Rα and IL-2Rβ) have been suggested to develop fatal lymphoproliferative and inflammatory disease due to reduction of Foxp3+ Treg cells in the thymus and peripheral lymphoid organs.1,2 IL-21 has been suggested to inhibit development of inducible Tregs.12 Therefore, it has remained possible that IL-21 contributes to the fate of Tregs and disease in mice with a defect in the IL-2/IL-2R pathway. However, we show here that il2ra/il21r DKO and il2ra single knockout mice display a comparable loss of Foxp3+ Tregs associated with T cell hyper-activation/expansion and the development of anemia, spontaneous colitis, and fatal wasting disease, suggesting that IL-2 regulates homeostasis of Tregs independently of IL-21. Notably, we found that deregulated CD4+ T cells in il2ra−/− and DKO mice coproduced IL-21 and IFN-γ but no IL-17 and no IL-4, a cytokine secretion profile distinct from Th1, Th2, and Th17 cells. We have previously described such a population of IFNγ+IL-21+IL-17− CD4+ cells during chronic LCMV infection.10 Although the T-cell phenotype was indistinguishable in il2ra/il21r DKO and il2ra single knockout mice, we identified remarkable differences in B-cell populations and responses. Il2ra−/− mice showed a considerable reduction in numbers of FO B cells, which was partially restored in DKO mice. Nevertheless, similarly to il2ra−/− mice, DKO mice failed to mount specific IgG2 antibody responses to immunization with virus-like particles most probably due to polyclonal activation of CD4+ T cells that are incapable to provide cognate help.
We saw an even more pronounced difference by studying homeostasis and function of MZ B cells. Although the MZ B-cell population was considerably increased in il21r−/− mice, it was eradicated in il2ra−/− mice due to overproduction of IL-21 by deregulated CD4+ cells as demonstrated by the complete recovery of MZ B cells in DKO mice in the presence of hyperactivated CD4+ T cells. Further evidence that IL-21 overproduction by deregulated CD4+ T cells is responsible for the obliteration of MZ B cells in il21r−/− mice has been provided by the generation of mixed bone marrow chimera. Development of IL-2Rα–deficient MZ and FO B cells was unaffected in mixed chimeras that were not inflamed due to the presence of functional Tregs derived from WT bone marrow.1 This result also demonstrates that IL-2 signaling is not directly required for MZ B-cell development. The increased frequency of MZ B cells in il21r−/− mice further indicates that even in a noninflamed context IL-21 limits MZ B-cell survival. Indeed, we found that IL-21 at concentrations as low as 1 ng/mL adversely affected survival of purified MZ B cells, and to a much lesser extent FO B cells, in vitro.
MZ B cells have been suggested to play an important role in the initial 3-5 days of an immune response to bacteria, and in particular to pneumococcal phosphorylcholine (PC),21 which is known as a T cell–independent type 2 (TI-2) antigen. Il2ra−/− mice immunized with heat-inactivated S pneumoniae failed to produce anti-PC–specific IgM antibodies, whereas the anti-PC IgM response was intact in DKO mice, confirming that the recovered MZ B cells population in the latter is indeed functional. In contrast, IgM responses to virus-like particles, which are driven mainly by follicular B cells,26 were comparably reduced in il2ra−/− and DKO mice underlining a defect in FO B-cell responses in both groups of mice.
Taken together (Figure 5F), our results highlight a crucial role of IL-21 as a negative regulator of MZ B-cell survival in homeostasis and, in particular, in a condition of autoimmunity and inflammation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Stephanie Hiltbrunner and Esther Rosenwald for technical assistance and Malgorzata Kisielow and Annette Schütz (Institute for Biomedical Technology, Eidgenössische Technische Hochschule Zürich) for cell sorting.
This project was funded by Swiss National Science Foundation 310030_124922.
Authorship
Contribution: L.T. and K.Y. performed experiments; L.T., K.Y., C.M., J.K., and M.K. designed and analyzed experiments; M.F.B. provided mice and reagents; and L.T., K.Y, J.K., and M.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Manfred Kopf, Molecular Biomedicine, Eidgenössische Technische Hochschule Zürich, Wagistrasse 27, 8952 Schlieren, Switzerland; e-mail: manfred.kopf@ethz.ch.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal