Abstract
Pentraxin 3 (PTX3) is a soluble pattern recognition molecule playing a nonredundant role in resistance against Aspergillus fumigatus. The present study was designed to investigate the molecular pathways involved in the opsonic activity of PTX3. The PTX3 N-terminal domain was responsible for conidia recognition, but the full-length molecule was necessary for opsonic activity. The PTX3-dependent pathway of enhanced neutrophil phagocytic activity involved complement activation via the alternative pathway; Fcγ receptor (FcγR) IIA/CD32 recognition of PTX3-sensitized conidia and complement receptor 3 (CR3) activation; and CR3 and CD32 localization to the phagocytic cup. Gene targeted mice (ptx3, FcR common γ chain, C3, C1q) validated the in vivo relevance of the pathway. In particular, the protective activity of exogenous PTX3 against A fumigatus was abolished in FcR common γ chain-deficient mice. Thus, the opsonic and antifungal activity of PTX3 is at the crossroad between complement, complement receptor 3-, and FcγR-mediated recognition. Because short pentraxins (eg, C-reactive protein) interact with complement and FcγR, the present results may have general significance for the mode of action of these components of the humoral arm of innate immunity.
Introduction
Innate immunity plays a key role as a first line of resistance against pathogens and in the activation and orientation of adaptive immunity. Similarly to adaptive immunity, innate immunity is based on cellular- and humoral-mediated mechanisms. The humoral pattern recognition receptors include collectins, ficolins, and pentraxins.
Pentraxins are phylogenetically conserved proteins1 characterized by a multimeric structure and divided in short (C-reactive protein [CRP] and serum amyloid P component) and long pentraxins. The long pentraxin 3 (PTX3) is the prototype of the long pentraxin subfamily; it shares similarities with the short pentraxins but differs for the presence of an unrelated, long N-terminal domain, as well as for gene organization, cellular source, inducing stimuli, and recognized ligands. PTX3 is produced and released by a variety of cell types, including phagocytes, dendritic cells, fibroblasts, and endothelial cells in response to primary inflammatory signals and Toll-like receptor (TLR) engagement. In neutrophils (polymorphonuclear neutrophils [PMNs]), PTX3 is stored in a ready-made form in secondary granules and is secreted in response to recognition of microbial moieties and inflammatory signals localizing in neutrophil extracellular traps.2
Recombinant PTX3 binds a variety of fungi, bacteria, and viruses, including several species of Aspergillus, and has opsonic activity, facilitating phagocytosis,2-4 innate immune cell activation in terms of cytokine and nitric oxide production,5 and orienting the development of adaptive immune response.3 Consistently, studies in vivo in Ptx3-deficient mice suggest that the role played by PTX3 in innate resistance is nonredundant and relevant in selected fungal, bacterial, and viral infections, in particular Aspergillus fumigatus,3,6-8 and recombinant PTX3 has therapeutic effects in murine models of fungal infections in immunocompetent and immunodeficient mice.9,10 PTX3 has also been observed to have a regulatory role on inflammation by acting as a feedback mechanism of inhibition of leukocyte recruitment.11
PTX3 binds also the first component of the classical complement cascade C1q, interacting with the C1q globular head.12-14 This interaction results in activation of the classical complement cascade when C1q is immobilized, a situation that mimics C1q bound to a microbial surface, or in inhibition of C1q haemolytic activity, when interaction occurs in the fluid phase, by competitive blocking of relevant sites.13 In addition, similarly to CRP,15,16 PTX3 interacts with Factor H, the main soluble regulator of the alternative pathway of complement activation, promoting Factor H deposition on PTX3-coated surfaces and preventing an exaggerated complement activation.17 Finally, PTX3 interacts with ficolin-2, enhancing complement deposition on Aspergillus fumigatus conidia.18 PTX3 recognition of the bacterial component outer membrane protein A (OMP-A) from Klebsiella pneumoniae triggers a pro-inflammatory response, based on complement activation.19,20 All these properties suggest that this long pentraxin behaves as a bona fide predecessor of antibodies.
Aspergillus fumigatus, an opportunistic ubiquitary fungus, is associated with a wide spectrum of diseases in humans, ranging from severe infections to allergy in immunocompromised and atopic subjects, respectively.21 In particular, aspergillosis is a major life-threatening infection in patients with defective phagocytosis, for instance, during chemotherapy or radiotherapy-induced neutropenia and monocytopenia.21 The innate immune system represents the first line of defense against A fumigatus.22 The complement system is activated on inhaled conidia via the alternative pathway and results in C3 deposition and cleavage.23,24 Recently, a mannose-binding lectin–dependent C2 bypass mechanism, which directly activates C3 and the alternative pathway on A fumigatus conidia, has been proposed.25,26 Opsonization with complement proteins leads to phagocytosis by PMNs and macrophages, which are major players in the innate resistance toward this fungus.24,27 PMNs represent a ready-to-use reservoir of PTX3 and release it in response to microbial or inflammatory signals, and macrophages or dendritic cells rapidly produce it in an nuclear factor κB–dependent manner. In vitro and in vivo data indicate that PTX3 expressed by PMNs is essential to control phagocytosis and fungal growth.2
PMN interaction with foreign microbes is facilitated by the recognition of opsonized material via the complement receptors CR1 (CD35), CR3 (CD11b/CD18, αMβ2), and CR4 (CD11c/CD18, αXβ2), which recognize antigen-bound complement components such as C3b or C4b.28-30 PMNs also express Fcγ receptor (FcγR)IIA (CD32) and FcγRIIIB (CD16) and can be induced to express FcγRI (CD64).31 FcγRs are also involved in the mobilization and activation of CD11b/CD18 in the phagocytic cup.32
The present study was designed to investigate the mechanisms of PTX3 as an endogenous neutrophil-stored opsonin and as an exogenously administered therapeutic agent.9,10 Therefore, we investigated the involvement of complement components, complement receptors, and FcγRs, which have been proposed as pentraxin receptors.33,34 Here, we report that PTX3 acts as an opsonin, facilitating conidia recognition and phagocytosis in an FcγR- and C-dependent manner. In particular, our results in vitro and in vivo suggest that the opsonic activity of PTX3 is mediated through FcγRII-dependent CR3 activation, which is involved in the phagocytosis of C3-opsonized A fumigatus conidia.
Methods
Reagents
Recombinant human PTX3 and its C- and N-terminal fragments were purified from Chinese hamster ovary cells, as described previously.12,35 Recombinant PTX3 contained < 0.125 endotoxin units/mL as checked by the Limulus amebocyte lysate assay (BioWhittaker Inc). Antibodies, complement depleted sera, complement components, and animals used are listed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
A fumigatus was obtained from a fatal case of pulmonary aspergillosis.36 Heat inactivated or viable conidia were used as specified.
PMN isolation and phagocytosis assay
Human PMNs were isolated from fresh whole blood obtained from healthy volunteers, as described previously.2 The amount of 2.5 × 106 PMNs (95% pure, based on morphology) were plated in 24-well plate in 0.5 mL RPMI with 1%, 3%, 10% normal human serum (NHS), heat inactivated serum (HHS; 30 minutes at 56°C), or complement depleted sera and 2.5 × 107/well A fumigatus conidia. When mentioned, the experiment was performed in the absence of NHS but in the presence of C3 (125 μg/mL), Factor B (20 μg/mL), Factor H (50 μg/mL), Factor I (3.4 μg/mL), and Factor D (0.14 μg/mL; eg, physiological concentrations present in 10% of NHS). In selected experiments, PMNs were pre-incubated for 1 hour on ice in the presence of blocking antibodies to CD11b, CD11c, CD32, CD16, or isotype controls (10 μg/106 cells). Opsonization was performed by incubating A fumigatus conidia in Ca2+/Mg2+ phosphate-buffered saline (PBS) with 50 μg/mL PTX3 (1.1 μM, assuming a molecular mass of 45 kDa for the PTX3 protomer), or the N-terminal-PTX3, or the cross-linked C-terminal-PTX3 (1.1μM) for 1 or 2 hours at room temperature. In some experiments, after opsonization, conidia were washed to remove unbound PTX3. After a 30 minutes incubation at 37°C on an orbital shaker at 150 rpm, phagocytosis was blocked by adding NaF (Sigma-Aldrich, final concentration 0.2M). Cytospins were stained with Diff Quick (Dade, Biomap). At least 200 PMNs per sample were counted under oil immersion microscopy (100×). Results are expressed as phagocytic index (PI), the average number of conidia phagocytosed per 100 neutrophils, or as percentage of phagocytosis, the percentage of neutrophils containing at least 1 conidium. Normality of data were assessed where necessary. The statistical analysis (mean, SEM, and Student paired t test) was performed on the PI values obtained from 3-6 different donors for experimental condition and from independent experiments. PTX3 released by PMNs (8 × 106/mL) was measured in the supernatant after incubation for 30, 60, or 120 minutes with conidia (3 × 108) in the presence of 0%, 3%, or 10% NHS, as described previously.2
Phagocytosis assay and PMN activation in whole blood
For phagocytosis assays in vivo or in whole blood or with murine bone marrow cells, conidia were labeled with fluorescin 5(6)-isothiocynate (FITC; Sigma-Aldrich; 5 mg/mL in dimethyl sulfoxide [DMSO]). An amount of 108 FITC-conidia eventually opsonized with PTX3 (50 or 100 μg/mL) were added to 1 mL of whole blood and incubated for 30 minutes at 37°C. Samples were placed on ice to block phagocytosis, and red cells were lysed by adding 10 mL of cold ammonium chloride lysis solution pH 7.2. An amount of 100 μL of each sample were fixed with 1% paraformaldehyde (PFA) and analyzed by flow cytometry. PTX3 binding to A fumigatus conidia, PMN activation, and C3 deposition assays are described in supplemental Methods.
Confocal microscopy
After activation in whole blood, human PMNs were treated and incubated with anti-PTX3 rabbit polyclonal antibody and biotin-conjugated anti-CD32 (Serotec) or anti-activated CD11b mAb (BioLegend) followed by Alexa Fluor 488-goat anti–rabbit immunoglobulin (Ig)G and Alexa Fluor 647-streptavidin, or Alexa Fluor 647-goat anti–mouse IgG (Molecular Probes), as described in supplemental Methods.
Blood collected from C57BL/6J, Ptx3-, or FcR common γ chain (FcRγ)–deficient mice was treated as described in supplemental Methods and incubated with anti–mouse CD11b (BD Biosciences), followed by anti–rat 488 (Alexa Fluor). Cells were analyzed with a laser scanning confocal microscope (FluoView FV1000; Olympus). Images (1024 × 1024 pixels) were acquired with an oil immersion objective (100×/1.4 NA Plan-Apochromat; Olympus).
In vivo phagocytosis and infection
Mice were injected intratracheally with 8 × 107 heat inactivated FITC-labeled conidia opsonized with PTX3 and killed 4 hours later. All procedures involving animals conformed with institutional guidelines in compliance with national and international law and policies on the care and use of laboratory animals. Bronchoalveolar lavage (BAL) leukocytes were analyzed by fluorescence-activated cell sorting (FACS) with peridinin chlorophyll protein complex (PerCP) anti-CD45, phycoerythrin (PE) anti-Ly6G, allophycocyanin (APC) anti-CD11b, or cytospun and stained with Diff Quick for microscopic analysis.
C57BL/6 and FcRγ-deficient mice were anesthetized with 2.5% avertin (Sigma Chemical Co) before intranasal infection with 2 × 107/20 μL viable, unopsonized A fumigatus conidia and treated with 1 mg/kg intranasally of PTX3 as previously described9,10 or sterile saline for 5 consecutive days, starting the day of the infection. Mice were killed 1 day after PTX3 treatment to count colony forming units (CFU) in the lung and brain. For histology, paraffin-embedded sections were stained with periodic acid-Schiff.
Results
The PTX3 opsonizing activity is serum dependent
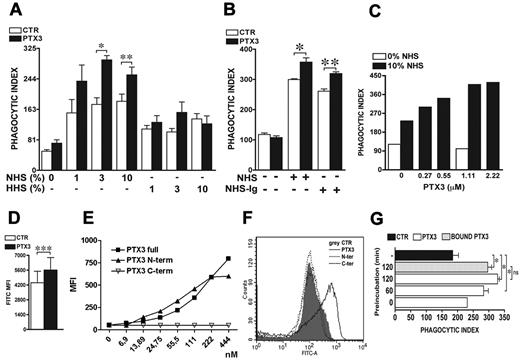
As shown in Figure 1A, PTX3 (1.11μM) amplifies PMN phagocytic activity in a serum dependent manner. In the absence of serum, phagocytosis of A fumigatus conidia occurred at very low levels (PI 50) and increased in the presence of NHS. The effect was dose-dependent, as the PI increased to 153 ± 3, 175 ± 2, and 183 ± 2 with 1%, 3%, or 10% NHS, respectively. The opsonization of conidia with PTX3 only marginally or inconsistently increased the PI in the absence of serum (PI 72 ± 9). In the presence of 1%, 3%, or 10% NHS, PTX3 significantly increased the PI to 237 ± 42, 294 ± 11, and 254 ± 20, respectively (P = .03 and P = .007 with 3% and 10% NHS, respectively). Similar results were obtained when the percentage of phagocytosis was analyzed.
PTX3-mediated increase of A fumigatus conidia phagocytosis is serum and complement dependent but not Ig-dependent. (A) For the phagocytic assay, A fumigatus conidia were preincubated or not (CTR) with 1.1μM PTX3 and then incubated for 30 minutes with neutrophils in the presence of 1%, 3%, or 10% NHS or HHS. Results are mean ± SEM of 3-6 pooled experiments. (B) The phagocytosis assay was performed with 10% NHS or Ig-depleted human serum (NHS-Ig). Results are mean ± SEM of 3 pooled experiments. (C) The phagocytosis assay was performed with A fumigatus conidia pre-incubated with different doses of PTX3 (0.27-2.22μM) in the presence or not of 10% of NHS. Results are representative of 1 of 2 experiments. (D) FACS analysis of PTX3-opsonized FITC-conidia phagocytosis. MFI results are mean ± SEM (n = 7 donors). (E) Full-length PTX3 (PTX3full), N-terminal domain (PTX3N-term), and C-terminal domain of PTX3 (PTX3C-term) were incubated with A fumigatus conidia at different concentrations (6.9-444nM). The binding was detected as described in supplemental Methods. MFI results are representative of 1 of 3 independent experiments. (F) FACS analysis of FITC-conidia phagocytosis after opsonization with full-length PTX3, N-terminal, or C-terminal domains. Results (neutrophil MFI) are representative of 1 of 3 independent experiments. (G) Conidia were pre-incubated (white and gray bars) or not (black bar) with 1.1 μM PTX3 for the indicated time (0, 60, 120 minutes) and eventually washed to eliminate unbound PTX3 before the phagocytosis assay (gray bar) in the presence of 10% NHS. Results are mean ± SEM of 2-6 pooled experiments. *P ≤ .05; **P < .01; ***P = .0005, Student paired t test.
PTX3-mediated increase of A fumigatus conidia phagocytosis is serum and complement dependent but not Ig-dependent. (A) For the phagocytic assay, A fumigatus conidia were preincubated or not (CTR) with 1.1μM PTX3 and then incubated for 30 minutes with neutrophils in the presence of 1%, 3%, or 10% NHS or HHS. Results are mean ± SEM of 3-6 pooled experiments. (B) The phagocytosis assay was performed with 10% NHS or Ig-depleted human serum (NHS-Ig). Results are mean ± SEM of 3 pooled experiments. (C) The phagocytosis assay was performed with A fumigatus conidia pre-incubated with different doses of PTX3 (0.27-2.22μM) in the presence or not of 10% of NHS. Results are representative of 1 of 2 experiments. (D) FACS analysis of PTX3-opsonized FITC-conidia phagocytosis. MFI results are mean ± SEM (n = 7 donors). (E) Full-length PTX3 (PTX3full), N-terminal domain (PTX3N-term), and C-terminal domain of PTX3 (PTX3C-term) were incubated with A fumigatus conidia at different concentrations (6.9-444nM). The binding was detected as described in supplemental Methods. MFI results are representative of 1 of 3 independent experiments. (F) FACS analysis of FITC-conidia phagocytosis after opsonization with full-length PTX3, N-terminal, or C-terminal domains. Results (neutrophil MFI) are representative of 1 of 3 independent experiments. (G) Conidia were pre-incubated (white and gray bars) or not (black bar) with 1.1 μM PTX3 for the indicated time (0, 60, 120 minutes) and eventually washed to eliminate unbound PTX3 before the phagocytosis assay (gray bar) in the presence of 10% NHS. Results are mean ± SEM of 2-6 pooled experiments. *P ≤ .05; **P < .01; ***P = .0005, Student paired t test.
Conidia phagocytosis in the presence of HHS was higher than in the absence of serum, but the opsonic activity of PTX3 was abolished (Figure 1A). By contrast, depletion of IgG from serum did not modify PTX3 activity (P = .04 and P = .007 before or after IgG depletion; Figure 1B).
Based on dose-response experiments (0.27-2.22μM), we observed that the PI increased starting from 0.27μM, reaching a plateau at 1.11μM (Figure 1C). Even at 1.11μM, PTX3 had no effect in the absence of NHS. The following experiments were performed with 1.11μM. Similar stimulation by PTX3 (Figure 1D; P = .0005) was observed when FITC-labeled A fumigatus phagocytosis in whole blood was analyzed.
We then mapped the domain(s) involved in the opsonic activity of PTX3 (Figure 1E). The N-terminal domain of PTX3 binds to A fumigatus conidia but has no opsonic activity. Thus, recognition is mediated by the N-terminal domain but phagocytosis requires the whole molecule.
As shown in Figure 1G, the addition of PTX3 in the assay (0 minutes preincubation) marginally modified the PI in a 30-minute phagocytosis assay (231 vs 184 ± 2, in the presence and absence of PTX3, respectively), while preincubation of conidia with PTX3 increased the PI from 231 (without pre-incubation) to 283 ± 15 (60 minutes preincubation) and 328 ± 6 (120 minutes preincubation). When conidia were washed after pre-incubation to eliminate unbound PTX3, the PI was comparable to the PI in the presence of unbound PTX3 (296 ± 12 vs 328 ± 6, P = nonsignificant [NS]). These results suggest that conidia opsonization by PTX3 and not phagocyte activation by unbound PTX3 is responsible of increased phagocytosis.
Role of complement
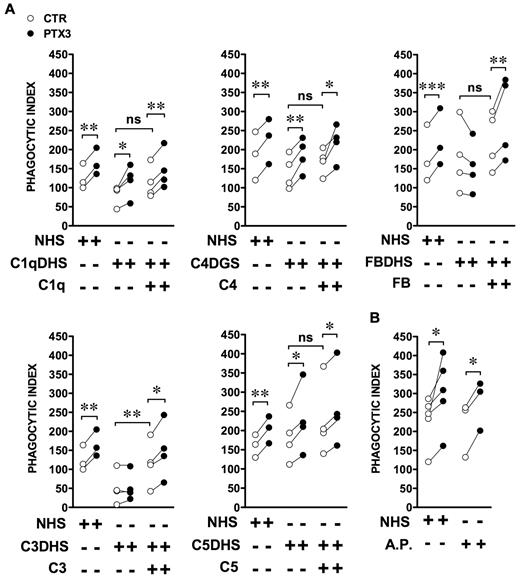
The following experiments were performed with commercially available sera depleted of specific complement components. Figure 2A shows that the amplification mediated by PTX3 occurred independently of the presence of C1q in the assay (P = .04 in the absence and P = .005 in the presence of C1q). PTX3 activity was also independent of the presence of C4, which is implicated in both the classical and lectin pathways (P = .006 in the absence and P = .03 in the presence of C4). To address the role of the alternative pathway, Factor B–depleted serum was used (Figure 2A); the amplification of PI and percent of phagocytosis were completely abrogated and were restored by the addition of recombinant Factor B (P = .004). In the presence of C3-depleted serum, the phagocytosis was compromised, consistently with the fact that the 3 pathways of C activation were inactive and C3 fragments (C3b, iC3b) are major opsonins recognized by C receptors (CR3 and CR4). As shown in Figure 2A, in the absence of C3, the PI was very low and was increased to normal levels by the reconstitution of serum with recombinant C3. PTX3-mediated amplification was abrogated in the absence of C3 and restored after the addition of recombinant C3 (P = .04). Finally, to address the role of the C terminal pathway C5-9 and of the C5a anaphylotoxin in PTX3-mediated activity, C5-depleted serum was used. As shown in Figure 2A, PTX3 activity occurred independently of the absence of C5 (P = .04 in the absence and P = .01 in the presence of C5). Finally, we performed the phagocytosis assay in the absence of serum but in the presence of the recombinant components of the alternative pathway (C3, Factor B, Factor H, Factor D, and Factor I) at the concentration present in 10% NHS. As shown in Figure 2B, alternative pathway components reconstituted a phagocytic activity comparable to NHS, and PTX3 maintained its opsonizing activity (P = .04 with NHS, P = .01 with alternative pathway components).
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on the alternative pathway of complement. (A) The phagocytosis assay was performed in the presence of 10% C1q-, C3-, C5-, or Factor B–depleted human serum (C1qDHS, C3DHS, C5DHS, and FBDHS) or C4-depleted guinea pig serum (C4DGS). Sera were reconstituted with the respective purified human proteins (C1q, C3, C4, C5, Factor B). (B) The phagocytosis assay was performed in the presence of C3, Factor B, Factor H, Factor D, or Factor I at the concentration present in 10% NHS. Results are paired PI values of independent donors in the absence or presence of recombinant PTX3. *P ≤ .05; **P < .01; ***P < .0001, Student paired t test.
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on the alternative pathway of complement. (A) The phagocytosis assay was performed in the presence of 10% C1q-, C3-, C5-, or Factor B–depleted human serum (C1qDHS, C3DHS, C5DHS, and FBDHS) or C4-depleted guinea pig serum (C4DGS). Sera were reconstituted with the respective purified human proteins (C1q, C3, C4, C5, Factor B). (B) The phagocytosis assay was performed in the presence of C3, Factor B, Factor H, Factor D, or Factor I at the concentration present in 10% NHS. Results are paired PI values of independent donors in the absence or presence of recombinant PTX3. *P ≤ .05; **P < .01; ***P < .0001, Student paired t test.
Role of CD11b
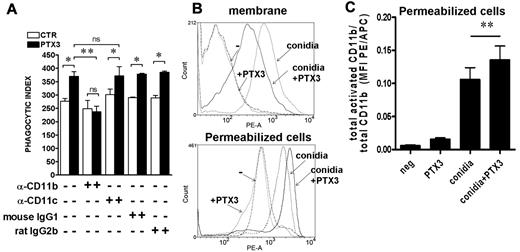
Given the role of C3 in PTX3 activity, we next analyzed the role of iC3b and C3b receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18). To this aim, the phagocytosis assay was performed in the presence of blocking antibodies to CD11b or CD11c. As shown in Figure 3A, in the presence of anti-CD11b, PTX3 activity was abolished (P = NS), whereas the anti-CD11c had no effect (P = .04 in the presence vs absence of PTX3). Irrelevant isotype controls did not influence PTX3 activity. Next, we analyzed C3 and iC3b deposition on A fumigatus conidia by FACS analysis in the presence of NHS at different concentrations (from 3- 50%) and PTX3 (1.11 μM) at different time points (2-40 minutes). Under these experimental conditions, we did not observe a PTX3-dependent increase of C3 fragment deposition using available reagents (not shown), although we cannot exclude a different kinetics of deposition or a minor but functionally important increase. Increased activity or affinity of CR3 for conidia might also be responsible of the increase in phagocytic efficiency mediated by PTX3.37 To address this point, we analyzed the active form of CD11b on PMNs in the presence of conidia opsonized or not by PTX3 using the CBRM1/5 antibody recognizing an activation epitope of CD11b.37,38 In 9 different donors analyzed, the active form of CD11b decreased on the cell membrane in the presence of PTX3-opsonized conidia compared with the absence of PTX3 (mean fluorescence intensity [MFI] without PTX3: 555 ± 65; MFI with PTX3: 453 ± 55; P = .009). However, the total activated CD11b, evaluated after permeabilization of cells, was significantly higher in the presence of PTX3-opsonized conidia compared with nonopsonized conidia (MFI without PTX3: 1473 ± 205; MFI with PTX3: 1639 ± 228; P = .006), suggesting increased internalization. Figure 3B shows FACS analysis results on activated CD11b on the membrane and in permeabilized cells of 1 representative donor of 9 tested. The addition of PTX3 alone did not modify CD11b activation (see also Figure 4B). Figure 3C and supplemental Figure 1 show the ratio between activated and total CD11b MFI PE/APC and percentage, respectively, in permeabilized cells in a further 6 different donors. This analysis suggests that expression of the CD11b activation epitope is induced by conidia and that location of the activated integrin on the cell membrane or inside the cell (in the phagocytic cup) reflects the process of translocation in the phagocytic cup.
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on CD11b and is associated to CD11b activation. (A) The phagocytosis assay was performed in the presence of 10% NHS and anti-CD11b (M1/70) or anti-CD11c (3.9) blocking antibodies or control IgGs. Results are mean ± SEM of 3-5 pooled experiments. *P ≤ 0.05; **P < .01; Student paired t test. (B) FACS analysis of activated CD11b associated with the membrane or present in permeabilized cells (membrane and intracellular) on neutrophils after incubation of whole blood with PTX3-opsonized conidia for 15 minutes. The histogram shows 1 of 9 donors analyzed with similar results. (C) Ratio of activated and total CD11b MFI PE/APC in permeabilized cells after incubation for 15 minutes with conidia or PTX3-opsonized conidia (n = 6).
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on CD11b and is associated to CD11b activation. (A) The phagocytosis assay was performed in the presence of 10% NHS and anti-CD11b (M1/70) or anti-CD11c (3.9) blocking antibodies or control IgGs. Results are mean ± SEM of 3-5 pooled experiments. *P ≤ 0.05; **P < .01; Student paired t test. (B) FACS analysis of activated CD11b associated with the membrane or present in permeabilized cells (membrane and intracellular) on neutrophils after incubation of whole blood with PTX3-opsonized conidia for 15 minutes. The histogram shows 1 of 9 donors analyzed with similar results. (C) Ratio of activated and total CD11b MFI PE/APC in permeabilized cells after incubation for 15 minutes with conidia or PTX3-opsonized conidia (n = 6).
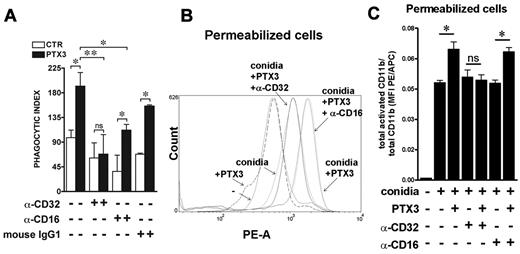
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on CD32, which regulates CD11b activation. (A) The phagocytosis assay was performed in the presence of 10% NHS and anti-CD16 (LNK16) or anti-CD32 (AT10 or IV.3) blocking antibodies or control IgGs. Results are mean ± SEM of 3-5 pooled experiments. *P ≤ .05; Student paired t test. (B) FACS analysis of membrane and intracellular activated CD11b in permeabilized neutrophils defined as FSC-Ahigh/SSC-Ahigh, after incubation of whole blood with PTX3-opsonized conidia for 15 minutes, in the presence of F(ab′)2 blocking anti–human CD16 (3G8) and CD32 (7.3). The histogram shows 1 of 7 donors analyzed with similar results. (C) Ratio of activated and total CD11b MFI PE/APC in permeabilized cells after incubation for 15 minutes with conidia or PTX3-opsonized conidia in the presence of anti-CD16 (3G8) or anti-CD32 (IV.3) blocking antibodies.
PTX3-mediated increase of A fumigatus conidia phagocytosis depends on CD32, which regulates CD11b activation. (A) The phagocytosis assay was performed in the presence of 10% NHS and anti-CD16 (LNK16) or anti-CD32 (AT10 or IV.3) blocking antibodies or control IgGs. Results are mean ± SEM of 3-5 pooled experiments. *P ≤ .05; Student paired t test. (B) FACS analysis of membrane and intracellular activated CD11b in permeabilized neutrophils defined as FSC-Ahigh/SSC-Ahigh, after incubation of whole blood with PTX3-opsonized conidia for 15 minutes, in the presence of F(ab′)2 blocking anti–human CD16 (3G8) and CD32 (7.3). The histogram shows 1 of 7 donors analyzed with similar results. (C) Ratio of activated and total CD11b MFI PE/APC in permeabilized cells after incubation for 15 minutes with conidia or PTX3-opsonized conidia in the presence of anti-CD16 (3G8) or anti-CD32 (IV.3) blocking antibodies.
Role of FcγRs
It has been reported that pentraxins, including PTX3, interact with FcγRs and mediate part of their biological activity through the activation of these receptors.33,34 Several studies demonstrated that FcγR are also involved in the mobilization and activation of CD11b/CD18 in the phagocytic cup.32 Thus, we addressed the involvement of PMN FcγRs in CD11b activation by PTX3-opsonized conidia by adding blocking anti-FcγRs antibodies in the phagocytic assay. As shown in Figure 4A, PTX3 prophagocytic activity was inhibited by the presence of anti-CD32 (P = NS in the presence vs absence of PTX3), whereas in the presence of anti-CD16 or irrelevant antibodies, PTX3 activity was maintained (P = .04 and .03, respectively). Moreover, in 7 different donors analyzed, the increase in the activated CD11b in permeabilized cells observed in the presence of PTX3-opsonized conidia (MFI without PTX3: 556 ± 139; MFI with PTX3: 715 ± 167; P = .02) was significantly inhibited by the presence of F(ab′)2 blocking anti-CD32 (MFI with PTX3 and anti-CD32: 563 ± 134; P = .0004) but not by anti-CD16 (MFI with PTX3 and anti-CD16: 633 ± 141; P = NS). Figure 4B shows FACS analysis results on activated CD11b of 1 representative donor of 7 tested, and Figure 4C shows the ratio between activated and total CD11b MFI PE/APC in permeabilized cells in a further 3 different donors. All together, these results suggest that PTX3-dependent activation of CD11b could be mediated by inside-out signaling through FcγRIIA/CD32.
Recruitment of CR3 and CD32 in the phagocytic cup
To visualize the cellular localization of CR3 and CD32 during the phagocytic process, highly resolved confocal images were acquired on plated (Figure 5A-C) or cytospun PMNs (Figure 5D). Resting PMNs or PMNs undergoing A fumigatus conidia phagocytosis were permeabilyzed and stained for the active form of CD11b, CD32, and PTX3. As shown in Figure 5A, in resting PMNs, endogenous PTX3, CD11b, and CD32 were localized in granules. As expected, activated CD11b immunostaining was very faint in resting PMNs.37 In the presence of unopsonized conidia (Figure 5B, left panels), endogenous PTX3 was localized in granules and in part in the phagocytic cup. CD32 and CD11b were distributed in the cell and in part in the phagocytic cup, where they colocalized with endogenous PTX3. When conidia were opsonized with PTX3 (Figure 5B-D, right panels), both CD32 and CD11b were mostly localized in the phagocytic cup around PTX3-opsonized conidia. Therefore, interaction with PTX3-sesitized conidia results in an increase in total activated CD11b, with localization and sequestration of activated CD11b and CD32 to the phagocytic cup.
The opsonization of conidia with PTX3 increases the colocalization of CD11b and CD32 in the neutrophil phagocytic cup. (A-C) Confocal microscopy analysis (FluoView FV1000; Olympus) of PTX3, CD11b, and CD32 in resting cells (A), CD11b and PTX3 (B), or CD32 and PTX3 (C) colocalization by double staining. After phagocytosis of nonopsonized conidia (B-C left) or PTX3-opsonized conidia (B-C right), cells were fixed with 4% PFA and stained for PTX3 (green) and CD11b (red; B) or PTX3 and CD32 (red; C). DNA labeling is also shown (DAPI [4,6 diamidino-2-phenylindole]). Panels from top to bottom show single staining for CD11b (A left, B) or CD32 (A right, C); for PTX3, double fluorescence for PTX3 and CD11b (A left, B) or PTX3 and CD32 (A right, C); double fluorescence and differential interference contrast (Nomarski; inset, Normaski and DAPI). (D) Triple staining for CD11b, CD32, and PTX3 in cytospun neutrophils by confocal microscopy. After phagocytosis of nonopsonized conidia (left) or PTX3-opsonized conidia (right), cells were cytospun and fixed with 4% PFA and stained for human PTX3 (green), CD11b (blue), and CD32 (red). Panels from top to bottom show double fluorescence for PTX3 and CD11b; CD11b and CD32; PTX3 and CD32; triple staining for PTX3, CD11b, and CD32; triple fluorescence and differential interference contrast (Nomarski). Images (1024 × 1024 pixels) were acquired with an oil immersion objective (100× 1.4 NA Plan-Apochromat; Olympus). One or more internalized conidia per cell are indicated by asterisks or arrows. Bars indicate magnification.
The opsonization of conidia with PTX3 increases the colocalization of CD11b and CD32 in the neutrophil phagocytic cup. (A-C) Confocal microscopy analysis (FluoView FV1000; Olympus) of PTX3, CD11b, and CD32 in resting cells (A), CD11b and PTX3 (B), or CD32 and PTX3 (C) colocalization by double staining. After phagocytosis of nonopsonized conidia (B-C left) or PTX3-opsonized conidia (B-C right), cells were fixed with 4% PFA and stained for PTX3 (green) and CD11b (red; B) or PTX3 and CD32 (red; C). DNA labeling is also shown (DAPI [4,6 diamidino-2-phenylindole]). Panels from top to bottom show single staining for CD11b (A left, B) or CD32 (A right, C); for PTX3, double fluorescence for PTX3 and CD11b (A left, B) or PTX3 and CD32 (A right, C); double fluorescence and differential interference contrast (Nomarski; inset, Normaski and DAPI). (D) Triple staining for CD11b, CD32, and PTX3 in cytospun neutrophils by confocal microscopy. After phagocytosis of nonopsonized conidia (left) or PTX3-opsonized conidia (right), cells were cytospun and fixed with 4% PFA and stained for human PTX3 (green), CD11b (blue), and CD32 (red). Panels from top to bottom show double fluorescence for PTX3 and CD11b; CD11b and CD32; PTX3 and CD32; triple staining for PTX3, CD11b, and CD32; triple fluorescence and differential interference contrast (Nomarski). Images (1024 × 1024 pixels) were acquired with an oil immersion objective (100× 1.4 NA Plan-Apochromat; Olympus). One or more internalized conidia per cell are indicated by asterisks or arrows. Bars indicate magnification.
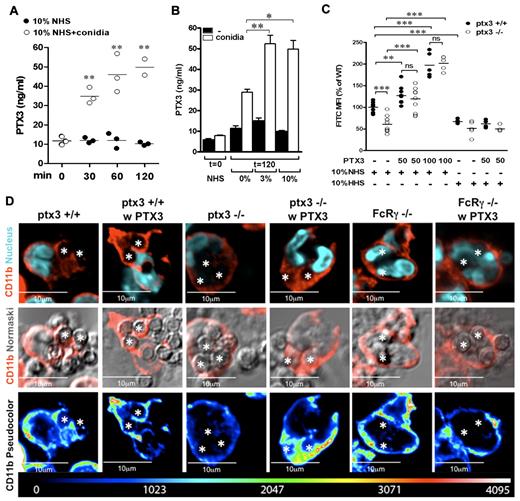
Mode of action of PMN-stored PTX3
Figure 6A shows the kinetics of PTX3 release by human PMNs during phagocytosis. PTX3 levels in PMN supernatant were already significantly increased by the presence of conidia and 10% NHS upon 30 minutes of incubation (11.6 ± 1.2 ng/mL vs 34.7 ± 2.4 ng/mL, P = .001). In the absence of the pathogen, we did not observe spontaneous release of PTX3. Moreover, PTX3 release was favored by the combined presence of conidia and NHS, which suggests that facilitated recognition, due to the concerted effect of complement and PTX3, further increases degranulation (Figure 6B).
Endogenous PTX3 is released by PMNs during conidia phagocytosis and contributes to internalization through the molecular mechanisms used by recombinant PTX3. (A) Kinetics of PTX3 release from human PMNs in the presence of 10% NHS and conidia. PTX3 levels were measured in the supernatants of 8 × 106/mL PMNs at different time points of incubation (0, 30, 60, and 120 minutes) with conidia and 10% NHS or NHS alone. (B) PTX3 levels were measured in PMN supernatants upon 2 hours of incubation with or without conidia and different concentrations of NHS (0%, 3%, 10%). (C) FACS analysis of FITC-conidia phagocytosis by wild-type (Ptx3+/+) and Ptx3-deficient (−/−) bone marrow PMNs (CD45+, Ly6Ghigh, CD11bhigh) in the presence of 10% NHS or HHS. When indicated, conidia were pre-opsonized with recombinant PTX3 (50 or 100 μg/mL). Experiments were performed in duplicate with PMNs collected from 4-10 Ptx3+/+ and Ptx3−/− mice. Data were normalized and expressed as percentage of the mean in wild-type PMNs. *P ≤ .05; **P < .01; ***P < .0001; Student unpaired t test. (C) Confocal analysis of total CD11b location in wild-type, Ptx3−/−, and FcRγ-deficient (FcRγ−/−) PMNs during phagocytosis of conidia eventually opsonised with recombinant PTX3. (Top) CD11b (red) and nucleus (DAPI). (Middle) CD11b (red) and Normarski. (Bottom) CD11b in pseudocolor scale. Two or 3 internalized conidia per cell are indicated with asterisks. Note that location of CD11b in the phagocytic cup is impaired in Ptx3−/− and FcRγ−/− PMNs and is rescued by recombinant PTX3 in Ptx3−/− PMNs but not in FcRγ−/− PMNs.
Endogenous PTX3 is released by PMNs during conidia phagocytosis and contributes to internalization through the molecular mechanisms used by recombinant PTX3. (A) Kinetics of PTX3 release from human PMNs in the presence of 10% NHS and conidia. PTX3 levels were measured in the supernatants of 8 × 106/mL PMNs at different time points of incubation (0, 30, 60, and 120 minutes) with conidia and 10% NHS or NHS alone. (B) PTX3 levels were measured in PMN supernatants upon 2 hours of incubation with or without conidia and different concentrations of NHS (0%, 3%, 10%). (C) FACS analysis of FITC-conidia phagocytosis by wild-type (Ptx3+/+) and Ptx3-deficient (−/−) bone marrow PMNs (CD45+, Ly6Ghigh, CD11bhigh) in the presence of 10% NHS or HHS. When indicated, conidia were pre-opsonized with recombinant PTX3 (50 or 100 μg/mL). Experiments were performed in duplicate with PMNs collected from 4-10 Ptx3+/+ and Ptx3−/− mice. Data were normalized and expressed as percentage of the mean in wild-type PMNs. *P ≤ .05; **P < .01; ***P < .0001; Student unpaired t test. (C) Confocal analysis of total CD11b location in wild-type, Ptx3−/−, and FcRγ-deficient (FcRγ−/−) PMNs during phagocytosis of conidia eventually opsonised with recombinant PTX3. (Top) CD11b (red) and nucleus (DAPI). (Middle) CD11b (red) and Normarski. (Bottom) CD11b in pseudocolor scale. Two or 3 internalized conidia per cell are indicated with asterisks. Note that location of CD11b in the phagocytic cup is impaired in Ptx3−/− and FcRγ−/− PMNs and is rescued by recombinant PTX3 in Ptx3−/− PMNs but not in FcRγ−/− PMNs.
We next analyzed conidia phagocytosis by murine wild-type and Ptx3-deficient bone marrow PMNs in the presence of 10% NHS or HHS (Figure 6C). In agreement with previous studies,2 we observed defective phagocytosis by Ptx3-deficient PMNs compared with wild-type PMNs in the presence of NHS (P < .0001). In the presence of HHS, the defect was marginal (P = .11). Phagocytosis by wild-type PMNs was impaired in the presence of HHS, compared with NHS (P = .0002). When conidia were pre-opsonized with recombinant PTX3, phagocytosis was significantly increased in wild-type cells (P = .004 and P < .0001, with 50 μg/mL and 100 μg/mL, respectively), and the defect of Ptx3-deficient PMNs was completely rescued (P < .0001 with 50 μg/mL and 100 μg/mL) in the presence of NHS but not HHS.
Finally, we analyzed by confocal microscopy CD11b localization in wild-type, Ptx3-deficient, and FcRγ-deficient PMNs upon interaction with conidia. As shown in Figure 6D, in wild-type PMNs, immunostaining for CD11b was localized on the cell membrane and around unopsonised phagocytosed conidia; in Ptx3- and FcRγ-deficient PMNs, immunostaining for CD11b remained localized on the membrane, and no immunostaining was observed around phagocytosed conidia. When conidia were pre-opsonized by recombinant PTX3, CD11b immunostaining around phagocytosed conidia or in the phagocytic cup was increased in wild-type as well as in Ptx3-deficient PMNs but not in FcRγ-deficient PMNs. All together, these results indicate that the prophagocytic activity of endogenous PMN-stored PTX3, which is released by PMNs during the phagocytosis assay, depends on the presence of fresh serum and thus of complement and is associated with the recruitment of CD11b in the phagocytic cup.
In vivo mechanism of action
To demonstrate the molecular mechanisms involved in PTX3-mediated activity, wild-type, C1q-, C3-, and FcRγ-deficient mice, as well as sCR1-treated mice, were injected intratracheally with FITC-labeled A fumigatus conidia and killed 4 hours later to assess phagocytosis by BAL Ly6G+CD11b+ cells by FACS analysis and microscopic count. As shown in Table 1, showing 1 of 2-3 experiments performed with similar results, in wild-type mice, both the MFI and the percentage of FITC-positive Ly6G+CD11b+ cells and the PI of PMNs were significantly increased by pre-opsonization of conidia with PTX3 (P = .03, p = .03 and P = .04, respectively). PTX3 activity was conserved in C1q-deficient mice (P = .03, P = .04, and P = .007, for MFI, %, and PI, respectively), whereas it was lost in sCR1-treated mice, in C3-deficient mice, and in FcRγ-deficient mice. Finally, PTX3 activity was conserved in severe combined immunodeficiency (SCID) and recombination activating gene (Rag) 2–deficient mice (P = .03 and P = .004 for MFI, respectively), which suggests that Igs are not involved in the interplay among PTX3, FcγRs, complement, and CR3.
In vivo phagocytosis of FITC-conidia by alveolar neutrophils
| . | MFI in Ly6G+CD11b+ . | Percent in Ly6G+CD11b+ . | MFI in CD45+ . | PI in PMNs . |
|---|---|---|---|---|
| WT | ||||
| CTR (n =5) | 3407 ± 742 | 34 ± 5 | 5642 ± 1495 | 269 ± 33 |
| PTX3 (n = 5) | 6233 ± 852 | 51 ± 5 | 9552 ± 1067 | 356 ± 21 |
| P | .03 | .03 | .06 | .04 |
| C1q KO | ||||
| CTR (n = 6) | 5377 ± 356 | 55 ± 3 | 6662 ± 335 | 299 ± 9 |
| PTX3 (n = 6) | 7077 ± 579 | 63 ± 2 | 7138 ± 453 | 349 ± 12 |
| P | .03 | .04 | .41 | .007 |
| sCR1-treated | ||||
| CTR (n = 5) | 3909 ± 1021 | 35 ± 5 | 10336 ± 1258 | 238 ± 8 |
| PTX3 (n = 5) | 4695 ± 639 | 41 ± 4 | 11075 ± 1153 | 242 ± 17 |
| P | .53 | .40 | .68 | .83 |
| C3 KO | ||||
| CTR (n = 4) | 2761 ± 640 | 40 ± 6 | 4249 ± 984 | 271 ± 11 |
| PTX3 (n = 4) | 2531 ± 475 | 40 ± 5 | 3285 ± 565 | 246 ± 13 |
| P | .78 | .93 | .42 | .20 |
| FcR[gamma] KO | ||||
| CTR (n = 3) | 1627 ± 473 | 50 ± 5 | 2385 ± 968 | 232 ± 10 |
| PTX3 (n = 3) | 1693 ± 148 | 50 ± 2 | 1942 ± 279 | 241 ± 16 |
| P | .89 | .99 | .68 | .67 |
| SCID | ||||
| CTR (n = 6) | 2849 ± 186 | 59 ± 3 | 3012 ± 206 | 162 ± 8 |
| PTX3 (n = 5) | 4328 ± 637 | 69 ± 4 | 3996 ± 222 | 209 ± 14 |
| P | .03 | .06 | .01 | .01 |
| Rag2 KO | ||||
| CTR (n = 8) | 2051 ± 186 | 49 ± 4 | 3233 ± 410 | 179 ± 6 |
| PTX3 (n = 8) | 3110 ± 250 | 58 ± 3 | 4582 ± 410 | 210 ± 2 |
| P | .004 | .11 | .03 | .001 |
| . | MFI in Ly6G+CD11b+ . | Percent in Ly6G+CD11b+ . | MFI in CD45+ . | PI in PMNs . |
|---|---|---|---|---|
| WT | ||||
| CTR (n =5) | 3407 ± 742 | 34 ± 5 | 5642 ± 1495 | 269 ± 33 |
| PTX3 (n = 5) | 6233 ± 852 | 51 ± 5 | 9552 ± 1067 | 356 ± 21 |
| P | .03 | .03 | .06 | .04 |
| C1q KO | ||||
| CTR (n = 6) | 5377 ± 356 | 55 ± 3 | 6662 ± 335 | 299 ± 9 |
| PTX3 (n = 6) | 7077 ± 579 | 63 ± 2 | 7138 ± 453 | 349 ± 12 |
| P | .03 | .04 | .41 | .007 |
| sCR1-treated | ||||
| CTR (n = 5) | 3909 ± 1021 | 35 ± 5 | 10336 ± 1258 | 238 ± 8 |
| PTX3 (n = 5) | 4695 ± 639 | 41 ± 4 | 11075 ± 1153 | 242 ± 17 |
| P | .53 | .40 | .68 | .83 |
| C3 KO | ||||
| CTR (n = 4) | 2761 ± 640 | 40 ± 6 | 4249 ± 984 | 271 ± 11 |
| PTX3 (n = 4) | 2531 ± 475 | 40 ± 5 | 3285 ± 565 | 246 ± 13 |
| P | .78 | .93 | .42 | .20 |
| FcR[gamma] KO | ||||
| CTR (n = 3) | 1627 ± 473 | 50 ± 5 | 2385 ± 968 | 232 ± 10 |
| PTX3 (n = 3) | 1693 ± 148 | 50 ± 2 | 1942 ± 279 | 241 ± 16 |
| P | .89 | .99 | .68 | .67 |
| SCID | ||||
| CTR (n = 6) | 2849 ± 186 | 59 ± 3 | 3012 ± 206 | 162 ± 8 |
| PTX3 (n = 5) | 4328 ± 637 | 69 ± 4 | 3996 ± 222 | 209 ± 14 |
| P | .03 | .06 | .01 | .01 |
| Rag2 KO | ||||
| CTR (n = 8) | 2051 ± 186 | 49 ± 4 | 3233 ± 410 | 179 ± 6 |
| PTX3 (n = 8) | 3110 ± 250 | 58 ± 3 | 4582 ± 410 | 210 ± 2 |
| P | .004 | .11 | .03 | .001 |
FACS analysis (MFI and percent) and microscopic analysis (PI) of internalized FITC-conidia. Student t test. Data show 1 out of 2-3 experiments performed for each group with similar results.
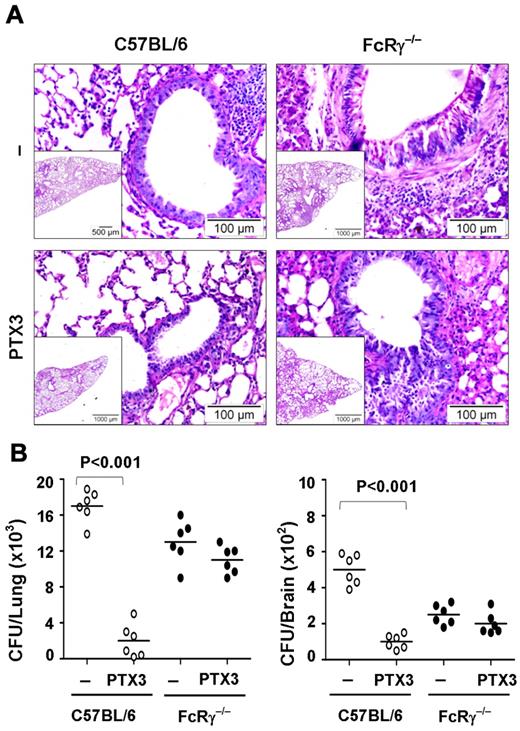
Role of FcRγ in vivo
Finally, we analyzed the therapeutic potential of PTX3 in an in vivo model of pulmonary aspergillosis in wild-type mice compared with FcRγ-deficient mice. FcRγ-deficient mice are more susceptible than wild-type mice to the inflammatory pathology associated with the pulmonary infection (Figure 7A). Few infiltrates of inflammatory mononuclear cells scattered in an otherwise intact lung parenchyma were present in C57BL/6 mice as opposed to the abundant infiltration of inflammatory cells and signs of diffuse interstitial pneumonia observed in FcRγ-deficient mice. This may have compensated for the defective phagocytic activity of FcRγ-deficient mice and thus explains the relative ability of these mice to restrain the fungal growth (Figure 7B). PTX3 limited inflammation (Figure 7A) and restrained the fungal growth (Figure 7B) both in lung and brain in wild-type mice but was totally ineffective in FcRγ-deficient mice.
The therapeutic potential of PTX3 in aspergillosis is abrogated in FcRγ-deficient mice. (A) Periodic acid-Schiff–stained medial sections of lungs from C57BL/6 or FcRγ−/− mice intranasally infected with 2 × 107/20 μL viable, unopsonized Aspergillus fumigatus conidia and treated with 1 mg/kg/intranasally of PTX3 or saline for 5 consecutive days and assessed 1 day after the end of treatment. Note the presence of few infiltrates of inflammatory mononuclear cells scattered in an otherwise intact lung parenchyma in C57BL/6 mice as opposed to the abundant infiltration of inflammatory cells and signs of diffuse interstitial pneumonia in FcRγ−/− mice and its amelioration by PTX3 treatment in C57BL/6 but not in FcRγ−/− mice. Bars indicate magnification. (B) Colony forming units (mean ± SE, n = 6) in the lung and brain of C57BL/6 or FcRγ−/− mice treated with PTX3 or saline.
The therapeutic potential of PTX3 in aspergillosis is abrogated in FcRγ-deficient mice. (A) Periodic acid-Schiff–stained medial sections of lungs from C57BL/6 or FcRγ−/− mice intranasally infected with 2 × 107/20 μL viable, unopsonized Aspergillus fumigatus conidia and treated with 1 mg/kg/intranasally of PTX3 or saline for 5 consecutive days and assessed 1 day after the end of treatment. Note the presence of few infiltrates of inflammatory mononuclear cells scattered in an otherwise intact lung parenchyma in C57BL/6 mice as opposed to the abundant infiltration of inflammatory cells and signs of diffuse interstitial pneumonia in FcRγ−/− mice and its amelioration by PTX3 treatment in C57BL/6 but not in FcRγ−/− mice. Bars indicate magnification. (B) Colony forming units (mean ± SE, n = 6) in the lung and brain of C57BL/6 or FcRγ−/− mice treated with PTX3 or saline.
All together, these results confirm in vitro data indicating that PTX3-mediated phagocytic activity depends on complement but not on the classical pathway. Moreover, they demonstrate the nonredundant role of FcγRs in PTX3 opsonizing activity and therapeutic potential in aspergillosis.
Discussion
The present study was designed to investigate the mechanisms of PTX3 as an endogenous neutrophil-stored opsonin and as an exogenously administered therapeutic agent.9,10 Therefore, we investigated the molecular mechanisms underlying PTX3-mediated opsonic activity in a model of A fumigatus conidia phagocitosis by human PMNs in vitro. In particular, we addressed the involvement of complement components, complement receptors, and FcγRs. Specifically, we now report the role of the alternative pathway of the complement, CD11b activation induced by PTX3 opsonized conidia, the functional involvement of FcγRs in PTX3-dependent activities, and finally that PTX3 activity in innate defense is lost in C3- or FcRγ-deficient mice. PTX3 activates different effector pathways possibly involved in innate resistance to this opportunistic pathogen, including the classic, the alternative, and the lectin pathway of complement activation by binding C1q, Factor H, and ficolin-2, respectively,12,13,17,18 or the promotion of phagocytosis by interacting with an as yet unidentified cellular receptor(s),3 possibly FcγRs, which have been proposed as pentraxin receptors.34
The data presented here confirm previous reports on the role of complement in conidia phagocytosis.23,24 Actually, phagocytosis was significantly increased in the presence of fresh serum as source of complement compared with the absence of serum or in the presence of HHS. The experiments performed in the presence of complement component-deficient sera indicate that C3 in particular plays a key role in Aspergillus conidia phagocytosis, whereas the deficiency of C5 is less relevant, suggesting that C3-mediated opsonization of conidia and not the C5a-mediated activation of phagocytes is mainly involved in this process. Dumestre-Pérard et al26 demonstrated that the alternative pathway is activated by different Aspergillus species, whereas neither the classical nor the lectin pathways through C4 and C2 cleavage are activated. In our experimental conditions, C1q-deficiency marginally reduced the phagocytic activity in vitro but severely impaired resistance to lung infection,3 and the reconstitution of C4-, C5- and Factor B–deficient sera with the recombinant proteins marginally increased the PI, suggesting that possibly all pathways are implicated in conidia opsonization, at least of this clinical strain. In this scenario, the opsonization of conidia with recombinant PTX3 in in vitro assays amplified the complement-dependent effects on phagocytosis. Actually, PTX3-facilitated phagocytosis was observed only in the presence of NHS, and in particular, in the presence of C3-sufficient serum. Similarly, in in vivo phagocytosis, complement inhibition with sCR1 treatment abolished PTX3 effects. Phagocytosis assays performed with wild-type and Ptx3-deficient PMNs in the presence of fresh serum as source of complement indicate that also the prophagocytic activity of endogenous PTX3 depends on complement. Concerning the pathways of complement activation relevant for PTX3 activity, we observed that in the absence of C1q or C4 in vitro and C1q in vivo, PTX3 maintained its facilitating activity. Only Factor B, thus the alternative pathway, was necessary for PTX3 activity. Actually, the in vitro reconstitution of the alternative pathway (C3, Factor B, Factor I, Factor H) was sufficient for PTX3 activity. Finally, C5-deficiency was irrelevant, suggesting that PTX3 acts through amplification of C3-dependent opsonization and not through C5a-dependent cell activation. In agreement with the in vitro and in vivo data presented here, recombinant PTX3 played a therapeutic role in aspergillosis in C1q-deficient mice, rescuing their defective resistance to the infection,3 thus suggesting that PTX3 plays its role in facilitating conidia phagocytosis independently of the interaction with C1q. Aspergillus immune evasion through interaction with Factor H and C4b binding protein has been recently demonstrated.39,40 Given the PTX3 interaction with both Aspergillus conidia and Factor H, we speculate that PTX3 could play a role in counterbalancing these immune evasion systems on complement.
Experiments performed with integrin-blocking antibodies, as well as FACS and confocal analysis, indicated that in the presence of PTX3-opsonized conidia, CD11b activation, internalization, recruitment to the phagocytic cup, and CD11b-dependent phagocytosis were increased. Previous studies have provided evidence that FcγR-derived signals induce activation of CR3.41 Specifically, FcγR stimulation in macrophages promotes CR3 clustering into high-avidity complexes in phagocytotic cups by a mechanism involving release of integrins from their cytoskeletal constraints, thus enhancing their lateral diffusion. A similar mechanism of inside-out activation of CR3 integrin by CD44 ligation has recently been demonstrated.42 In particular, CD44 ligation led to increased mobility of CR3, increased recruitment of the high-affinity state of CR3 to the phagocytic cup, and finally increased phagocytosis. The experiments performed here in the presence of FcγR-blocking antibodies as well as confocal and FACS analysis strongly suggest that upon opsonization of conidia with PTX3, FcγRIIA/CD32 mediates inside-out activation of CD11b and consequently phagocytosis of C3b-opsonized A fumigatus conidia (supplemental Figure 2). Human and murine FcγRs differ in many aspects including the affinity for the antibody Fc-fragment and the signaling pathway induced. Thus, it is difficult to extrapolate data from animal studies to the human system. However, the results obtained with FcRγ-deficient mice, which lack signaling from any functional activating FcRs, demonstrate their involvement in PTX3 therapeutic activity toward Aspergillus. On the same line, CD11b recruitment in the phagocytic cup was defective in Ptx3- and FcRγ-deficient PMNs and was rescued by recombinant PTX3 in Ptx3-deficient PMNs but not FcRγ-deficient PMNs. These results suggest that endogenous, neutrophil-stored, and exogenous (eg, in a therapeutic setting) PTX3 acts via the same complement/CD11b/FcγR-dependent pathway.
As shown in previous studies2 and confirmed here, endogenous PTX3 stored in neutrophil granules plays an essential role in recognition and disposal of conidia. Therefore, the margin for increased efficiency in the phagocytosis of conidia by PMNs in the presence of exogenous PTX3 is limited. This could explain the significant but far from spectacular enhancement observed in the presence of recombinant PTX3 in vitro, compared with the more impressive effect observed in wild-type versus Ptx3-deficient mice.
Moreover, additional mechanisms could be involved in PTX3-dependent protection from fungal infection in vivo. These include PTX3-dependent amplification of the antifungal role of other cell types, such as macrophages, dendritic cells, epithelial cells; induction of other mediators such as β-defensins and cathelicidins; development of protective T-helper (Th)1 adaptive responses through up-regulation of interleukin-12 in dendritic cells; and modulation of inflammatory responses.3,9-11 Therefore, mechanisms other than increased phagocytosis contribute to the antifungal activity of PTX3 in vivo. PMNs may actually feed on some of these (for instance, activation of Th1 and Th17 responses).43 Finally, the in vivo experiments in SCID and Rag2-deficient mice, which lack T and B cells, indicate that low-affinity natural antibodies, which could bind conidia or FcγRs, do not contribute to the interplay among PTX3, complement, CR3, and FcγR-mediated recognition described here.
Several phagocyte pattern recognition receptors (PRRs) cooperate when they sense whole organisms through different pathogen-associated molecular patterns or with particular pathogen-associated molecular patterns able to interact with multiple PRRs. For instance, CD36 mediates binding and internalization of Gram-positive bacteria and cooperates with TLR2 and TLR6, which induce cytokine production.44 Similarly, OMP-A interacts with LOX-1 and scavenger receptor endothelial cell (SREC)–I, activates phagocytes through TLR2, and binds to PTX3, thus leading to amplification of the innate response to this moiety.19,20 This cooperation among simultaneously engaged PRRs leads to activation of diverse cellular signaling pathways and of the humoral arm of the innate immune system, resulting in synergy and amplification of the innate responses to pathogens. The results presented here demonstrate that co-existence of FcγR ligands (PTX3) and CR3 ligands (C3bi) on opsonized conidia initiates FcγRII-dependent CR3 mobilization and activation, leading to amplification of A fumigatus conidia recognition and phagocytosis. Thus, PTX3 is a fluid phase PRM whose opsonic activity is at the crossroad between complement, CR3, and FcγR-mediated recognition. Because classic short pentraxins, such as CRP, interact with complement components (Factor H and C1q) and with FcγRs,34 the results reported here may have broad implications for the mode of action of this ancient class of functional ancestors of antibodies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Emanuela Morenghi for assistance in statistical analysis.
This work was supported by the European Commission (ERC project HIIS, “MUGEN” LSHG-CT-2005-005203, “MUVAPRED” LSHP-CT-2003-503240), Ministero dell'Istruzione, Università e della Ricerca (MIUR; project FIRB), Telethon (Telethon grant n. GGP05095), fondazione CARIPLO (project Nobel), and Italian Cystic Fibrosis Research Foundation (Festa per l'80° compleanno del Presidente Faganelli).
Authorship
Contribution: F.M. performed research and interpreted data; A.D., L.D., T.Z., and S.Z. performed research; B.B. and L.R. analyzed data; A.M. designed research, interpreted data, wrote the manuscript; and C.G. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no completing financial interests.
Correspondence: Dr Cecilia Garlanda, Lab Ricerche in Immunologia e Infiammazione, Istituto Clinico Humanitas, via Manzoni 113, 20089 Rozzano (Milan) Italy; e-mail: cecilia.garlanda@humanitasresearch.it.
References
Author notes
A.M. and C.G. contributed equally to this study.

![Figure 5. The opsonization of conidia with PTX3 increases the colocalization of CD11b and CD32 in the neutrophil phagocytic cup. (A-C) Confocal microscopy analysis (FluoView FV1000; Olympus) of PTX3, CD11b, and CD32 in resting cells (A), CD11b and PTX3 (B), or CD32 and PTX3 (C) colocalization by double staining. After phagocytosis of nonopsonized conidia (B-C left) or PTX3-opsonized conidia (B-C right), cells were fixed with 4% PFA and stained for PTX3 (green) and CD11b (red; B) or PTX3 and CD32 (red; C). DNA labeling is also shown (DAPI [4,6 diamidino-2-phenylindole]). Panels from top to bottom show single staining for CD11b (A left, B) or CD32 (A right, C); for PTX3, double fluorescence for PTX3 and CD11b (A left, B) or PTX3 and CD32 (A right, C); double fluorescence and differential interference contrast (Nomarski; inset, Normaski and DAPI). (D) Triple staining for CD11b, CD32, and PTX3 in cytospun neutrophils by confocal microscopy. After phagocytosis of nonopsonized conidia (left) or PTX3-opsonized conidia (right), cells were cytospun and fixed with 4% PFA and stained for human PTX3 (green), CD11b (blue), and CD32 (red). Panels from top to bottom show double fluorescence for PTX3 and CD11b; CD11b and CD32; PTX3 and CD32; triple staining for PTX3, CD11b, and CD32; triple fluorescence and differential interference contrast (Nomarski). Images (1024 × 1024 pixels) were acquired with an oil immersion objective (100× 1.4 NA Plan-Apochromat; Olympus). One or more internalized conidia per cell are indicated by asterisks or arrows. Bars indicate magnification.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/24/10.1182_blood-2009-12-258376/4/m_zh89991061780005.jpeg?Expires=1769857420&Signature=Zwc9Ppe5KD7ND-EHT98m4luN2-LgXrXqWTaCwwyTYdUJFIB2~TvEgzXoqHHQuyDPL9ALdu9ZIh~TD1KsYeWJgC-3kfrGVRPfmE-NsL7U-qQYKtd~tvZLz~ceTQKXxn-Vh03QlDMdShTSd8xBL0q9USLJluzD19rJwYv0nu~vwrZ-7GIoAr29rRiFctlEJyENm1DxDj2saMnFpoXPUpX-TiE~8MhUIU8-kEz2BDL6V8FVv6NTEUSG2njaRi3MvkbSMel1i1QYS-XQKTAHCDlBkJ3OI53PFXHGULzf4J~AxTUNCo3bEZAMnga2FwPd3seasZ6I9ZB1Bqd2M2zRQIriCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal