Abstract
Abstract 600
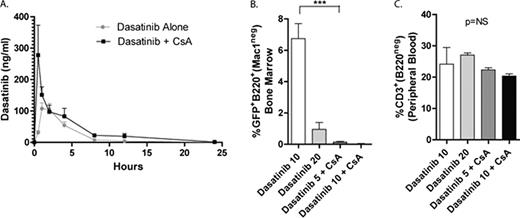
Although therapy for Bcr-Abl+ leukemia has been revolutionized by the development of the tyrosine kinase inhibitors (TKI), some patients, particularly those with advanced phase chronic myeloid leukemia or acute lymphoblastic leukemia (ALL) have unsatisfactory responses. We have demonstrated that inhibition of calcineurin with cyclosporine (CsA) increases the sensitivity of Bcr-Abl cells to TKI; furthermore, combination therapy with CsA plus dasatinib cured all mice with Bcr-Abl+ ALL, whereas all of the mice treated with dasatinib alone died with leukemia (Gregory et al, Cancer Cell, 2010). While the synergistic effect is independent of MDR1 inhibition by CsA in vitro, the possibility remains that the demonstrated survival advantage of combination therapy is due to altered pharmacokinetics (PK)and increased dasatinib exposure. We sought to determine if co-administration of CsA with dasatinib alters dasatinib PK, if differences in PK are sufficient to explain differences in response to therapy in vivo, and if combination therapy appears to adversely affect T-cell numbers. Bl6 mice were treated with dasatinib (20mg/kg/d), cyclosporine (25mg/kg/d), or both by oral gavage. Serum from peripheral blood was obtained at 0, 1, 2, 4, 8, 12, 24, and 48 hours after single doses and after one week of therapy (trough, 1, 2, 4, 8, and 12 hours). Dasatinib levels were determined by LC/LC-MS/MS. Pharmacokinetic (PK) analyses indicate that after 1 week of therapy, co-administration of CsA with dasatinib increases the Cmax and AUCinf of dasatinib as compared to dasatinib alone (277.4 v. 107.7 ng/ml and 916.8 v 487.4 ng/ml*hr, respectively; Figure A). The PK profiles suggest that co-administration of CsA with dasatinib enhances enteric absorption of dasatinib, but has little effect on its systemic elimination. Next, Arf-/-Bcr-Abl+GFP+ leukemia cells (Williams et al, Genes Dev, 2007) were transferred into unirradiated recipients which were treated with dasatinib (10 or 20 mg/kg/d) or with combination therapy (CsA 25mg/kg/d with dasatinib 5 or 10 mg/kg/d) for 7 days. Leukemia bearing mice were euthanized and the bone marrow (BM), peripheral blood (PBL) and spleens (SPL) were assessed for leukemia burden by flow cytometry. Combined CsA and dasatinib results in better disease control, even at doses predicted to result in similar exposure to dasatinib alone (i.e. half). For example, the mean percentage of GFP+B220+ BM cells was 6.7% in mice treated with dasatinib 10mg/kg/d as compared to 0.1% in mice treated with dasatinib 5mg/kg/d plus CsA (p<0.05; ANOVA/Bonferroni; Figure B). These data suggest that the synergistic effects of combining calcineurin inhibition and TKI are not due to altered PK alone. Since CsA is an immunosuppressant and there is data to suggest an immunosuppressive effect of dasatinib (Schade et al, Blood, 2008), we also measured the percentages of T-cells in the PBL and SPL of mice treated with dasatinib or CsA plus dasatinib. In this short-term experiment, there was little effect of combined therapy on the percentages of PBL and SPL T-cells as compared to dasatinib alone. For example, there was no significant difference in the percentage of CD3+B220neg cells in the PBL among the 4 groups of treated mice (p=NS; ANOVA; Figure C). Ongoing studies are designed to define the PK of dasatinib at the lower doses when combined with CsA, the effect of combined therapy on T-cell function, and long-term outcomes in mice with Bcr-Abl+ leukemia treated with CsA and lower doses of dasatinib as compared to dasatinib alone. These data support the development of early phase studies of combined therapy for Bcr-Abl+ leukemia.
Off Label Use: The use of cyclosporine as adjunctive therapy for Bcr-Abl+ leukemia will be suggested.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal