Abstract
Abstract 522
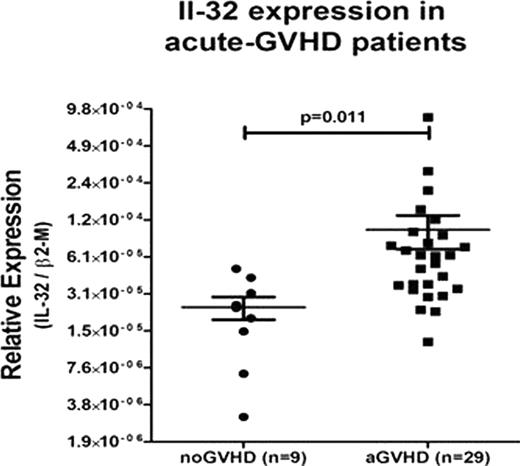
Graft-versus-host disease (GVHD) is an important complication of allogeneic hematopoietic cell transplantation (HCT). The role of various cell populations, cytokines and chemokines, present pre and post-transplantation, in the development of GVHD has been studied extensively.We investigated the potential role of Interleukin (IL)-32 in alloreactivity and GVHD. IL-32 is the protein product of the NK4 transcript first reported in IL-2 activated T lymphocytes and natural killer cells. IL-32 has pro-inflammatory and pro-apoptotic properties and induces expression of TNF-α in several cell targets. We used one-way mixed lymphocyte cultures (MLC) as a simple in vitro model of GVHD to determine IL-32 expression upon alloactivation. The α and γ isforms of (IL)-32 protein were 2-fold upregulated in allogeneic MLC compared to autologous controls (n=4, p=0.037). Concurrently, the concentrations of TNF-α, IL-6 and IL-8 in the allogeneic MLC supernatants were, as expected, upregulated significantly. This finding led us to evaluate IL-32 expression as a potential marker for GVHD after allogeneic HCT in 45 patients with either active acute GVHD or chronic GVHD, and 16 patients who did not show clinical evidence of GVHD. IL-32 mRNA levels in peripheral blood unsorted white blood cells, correlated with acute GVHD (RT-PCR values expressed as mean +/− SEM of the ratio of expression of IL-32/GUS-B = 1.01, p=0.011, vs control values IL-32/GUS-B = 0.23) but not with chronic GVHD, (IL-32/GUS-B = 0.26, p=0.16) (Figure 1). As the serine protease neutrophil proteinase 3 (PR3) has been shown to serve as activator of IL-32, and to process several inflammatory cytokines, including IL-32, TNF-α and IL-8, we postulated that the addition of the serine protease inhibitor α-1 anti-trypsin (AAT), would interfere with the processing of IL-32 by PR3, and as a result would lead to decreased proliferation of cells in MLC, and reduced cytokine production. To test this hypothesis, MLCs were treated with AAT at concentrations of 1–20 ug/ml and expression of IL-32 and PR3 were determined. In MLCs treated with AAT at the optimal concentration of 5 ug/ml, added to cultures on alternate days for a period of 7 days T lymphocyte proliferation was suppressed when compared to vehicle-treated MLC, (mean CPM=33.000 versus CPM=67.000; p=0.012). Concurrently there was a 2.5 fold decrease in IL-32 and PR3 protein levels (n=4, p=0.023). CONCLUSION: IL-32 is upregulated in patients with acute GVHD. Determination of IL-32 in patients with suspected GVHD may support the diagnosis and the decision to treat.AAT interferred with T cell activation and the release of cytokines, including IL-32, suggesting that administration of AAT may have therapeutic potential in patients with acute GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal