Abstract
Abstract 3555
Cytogenetic status represents a major prognostic factor for individuals with AML. However, approximately 50% of individuals have CN-AML and, within this cohort, genetic features have important prognostic and therapeutic implications. Mutations in the FLT3 receptor are found in 30–40% of patients with CN-AML. While the FLT3-internal tandem duplication (ITD) is associated with a poor DFS and OS when compared to CN-AML patients with a wtFLT3R, the prognostic significance of the FLT3-tyrosine kinase domain (TKD) mutation is less clear. There is considerable debate over the best postremission treatment for CN-AML patients who have a FLT3ITD. Our primary objective was to determine the relationship between different postremission therapies and outcome in CN-AML patients with FLT3 mutations.
We retrospectively reviewed the outcomes of newly diagnosed AML patients at the Massachusetts General Hospital and Dana-Farber Cancer Institute/Brigham and Women Hospital between 2002–2008 who were younger than 60 years of age, had a normal karyotype, and had a FLT3ITD and/or TKD mutation at diagnosis.
The method of Kaplan and Meier was used to estimate DFS and OS; groups were compared using the log-rank test. A p-value less than .05 was interpreted as statistically significant.
At diagnosis, there were 37 patients with an ITD mutation (7 also had a TKD mutation) and 19 patients with an isolated TKD mutation at diagnosis. Patients who received an allogeneic SCT were not included in the analysis.
ITD positive patients with an allelic ratio (AR) of mutant to wt FLT3 >.2 had a significantly worse DFS and OS than those with an AR<.2 (p=.014 and .043); ITD length was not associated with survival.
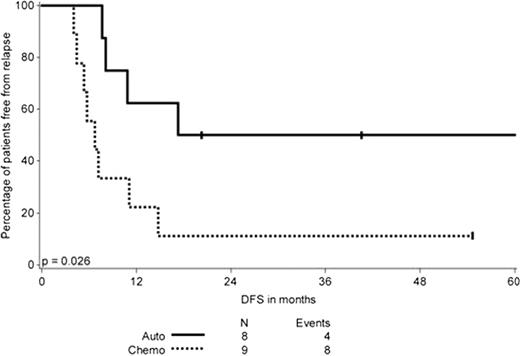
ITD positive patients who underwent an autologous SCT in CR1 had an improved DFS (Figure 1) but similar OS compared to those who received a minimum of three cycles of consolidation chemotherapy (n=8 and 9, respectively) (p=.026 for DFS and .19 for OS). In contrast, for individuals with an isolated TKD mutation, DFS and OS were similar for those who received an autologous SCT (n=6) or consolidation chemotherapy (n=8) (p=.78 for DFS and .25 for OS, respectively).
DFS in months of patients with a FLT3ITD according to the type of postremission therapy, only including patients who completed postremission therapy.
DFS in months of patients with a FLT3ITD according to the type of postremission therapy, only including patients who completed postremission therapy.
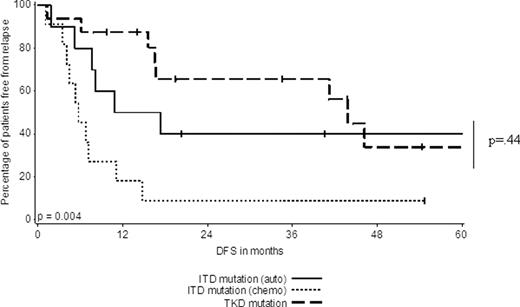
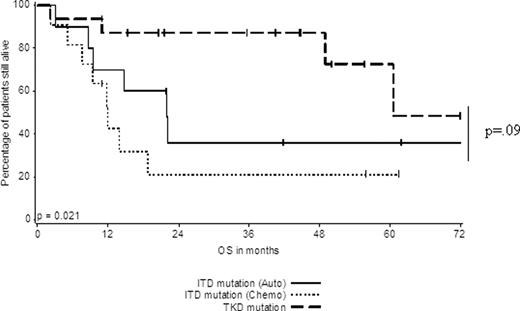
Among patients who initiated postremission therapy, we observed that individuals with an isolated TKD (n=16 for auto SCT or chemotherapy) had an improved DFS and OS compared to ITD patients (n=11) treated with chemotherapy. However, the DFS and OS of TKD patients (n=16 for auto SCT or chemotherapy) versus ITD patients (n=10) who initiated auto SCT was similar (p=.44 for DFS and .09 for OS) (Figure 2A and B, respectively).
DFS of FLT3ITD patients who initiated auto SCT or consolidation chemotherapy in comparison to patients with a TKD (who initiated postremission therapy).
DFS of FLT3ITD patients who initiated auto SCT or consolidation chemotherapy in comparison to patients with a TKD (who initiated postremission therapy).
OS of FLT3ITD patients who initiated auto SCT or consolidation chemotherapy in comparison to patients with a TKD (who initiated postremission therapy).
OS of FLT3ITD patients who initiated auto SCT or consolidation chemotherapy in comparison to patients with a TKD (who initiated postremission therapy).
Our findings suggest that patients with isolated TKD have a similar prognosis regardless of the type of postremission therapy utilized, and that their prognosis is superior to that of patients with ITD. For patients with ITD, autologous SCT in CR1 is associated with improved DFS compared to consolidation chemotherapy alone and is similar to that of patients with TKD. Our data raise the question as to whether autologous SCT should be preferred over consolidation chemotherapy for patients with ITD who are not undergoing allogeneic SCT in CR1 or therapy with a FLT3 inhibitor.
DeAngelo:Deminimus: Consultancy. Stone:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal