Abstract
Abstract 3554
The role of SCT in the management of Ig light chain (AL) amyloidosis remains undefined.
We report 422 patients (Table 1) who received SCT for AL amyloidosis to compare outcomes of patients treated before January 2006 with those of patients treated from January 2006 until December 1, 2009.
Patient Characteristics
| . | Patient Group . | . | |
|---|---|---|---|
| Characteristic . | Pre-2006 SCT (n=265) . | Post-2006 SCT (n=157) . | P Value . |
| Age, y | 56.8 (49.6–63.3) | 58.2 (52.2–63.4) | .08 |
| Men | 157 (59.2) | 94 (59.8) | .92 |
| Amyloid involvement | |||
| Renal | 179 (67.5) | 114 (72.6) | .33 |
| Cardiac | 132 (49.8) | 79 (50.3) | .54 |
| Liver | 43 (16.2) | 15 (9.6) | .06 |
| IVS thickness, mm | 12 (10–14) | 12 (10–15) | >.99 |
| EF, % | 65 (60–70) | 64 (60–68) | .16 |
| No. of organs involved | .62 | ||
| 1 | 128 (48) | 69 (44) | |
| 2 | 99 (37) | 66 (42) | |
| >2 | 38 (14) | 22 (14) | |
| Conditioning dose <200 mg/m2 melphalan | 84 (31.7) | 60 (38.2) | .20 |
| Albumin, g/dL | 2.8 (1.9–3.3) | 2.6 (2.1–3.0) | .15 |
| Creatinine, mg/dL | 1.1 (0.9–1.3) | 1.0 (0.9–1.3) | .12 |
| NT–proBNP, pg/mL | 671 (181–3,425) | 717 (165–1,976) | .47 |
| Troponin T, ng/mL | 0.01 (0.01–0.02) | 0.01 (0.01–0.03) | .42 |
| Alkaline phosphatase, U/L | 93 (72–145) | 84 (67–107) | .001 |
| b2M, mg/L | 2.51 (1.94–3.26) | 2.75 (2.25–3.96) | <.001 |
| . | Patient Group . | . | |
|---|---|---|---|
| Characteristic . | Pre-2006 SCT (n=265) . | Post-2006 SCT (n=157) . | P Value . |
| Age, y | 56.8 (49.6–63.3) | 58.2 (52.2–63.4) | .08 |
| Men | 157 (59.2) | 94 (59.8) | .92 |
| Amyloid involvement | |||
| Renal | 179 (67.5) | 114 (72.6) | .33 |
| Cardiac | 132 (49.8) | 79 (50.3) | .54 |
| Liver | 43 (16.2) | 15 (9.6) | .06 |
| IVS thickness, mm | 12 (10–14) | 12 (10–15) | >.99 |
| EF, % | 65 (60–70) | 64 (60–68) | .16 |
| No. of organs involved | .62 | ||
| 1 | 128 (48) | 69 (44) | |
| 2 | 99 (37) | 66 (42) | |
| >2 | 38 (14) | 22 (14) | |
| Conditioning dose <200 mg/m2 melphalan | 84 (31.7) | 60 (38.2) | .20 |
| Albumin, g/dL | 2.8 (1.9–3.3) | 2.6 (2.1–3.0) | .15 |
| Creatinine, mg/dL | 1.1 (0.9–1.3) | 1.0 (0.9–1.3) | .12 |
| NT–proBNP, pg/mL | 671 (181–3,425) | 717 (165–1,976) | .47 |
| Troponin T, ng/mL | 0.01 (0.01–0.02) | 0.01 (0.01–0.03) | .42 |
| Alkaline phosphatase, U/L | 93 (72–145) | 84 (67–107) | .001 |
| b2M, mg/L | 2.51 (1.94–3.26) | 2.75 (2.25–3.96) | <.001 |
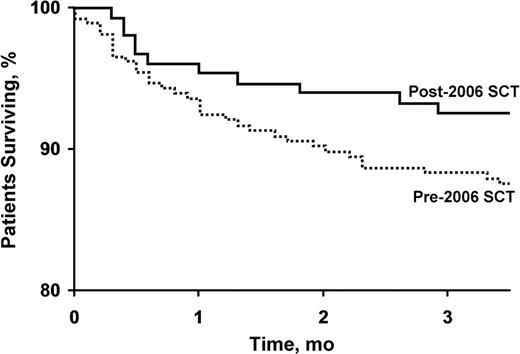
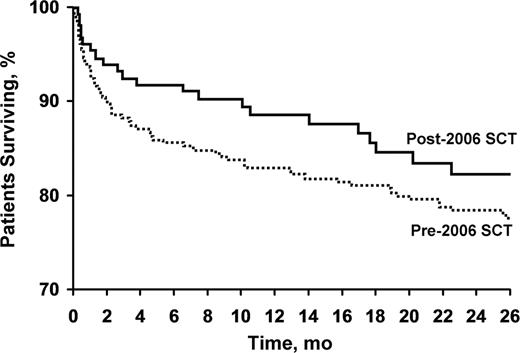
Day 100 all-cause mortality decreased over this time period from 12% to 7% (P=.09) figure 1. Survival at 2 years increased from 78% to 82%. Figure 2 The major determinants of early mortality (before day 100) were the presence of cardiac involvement with amyloid and increased levels of cardiac biomarkers, lower serum albumin, higher serum creatinine, and a higher number of organs involved. (Table 2) On multivariate survival analysis, higher levels of serum troponin T and N-terminal pro–brain natriuretic peptide were the only predictors of early mortality after SCT.
Survival through d 100 in patients with AL who underwent SCT before and after 2006 (P=.09).
Survival through d 100 in patients with AL who underwent SCT before and after 2006 (P=.09).
Survival through 24 mo. in patients with AL who underwent SCT before and after 2006 (P=.09).
Survival through 24 mo. in patients with AL who underwent SCT before and after 2006 (P=.09).
Group Differences by Survival Duration
| . | Survival Group . | . | |
|---|---|---|---|
| Characteristic . | Early Death (n=43) . | Survived 100 d (n=379) . | P Value . |
| Age, y | 56.2 (49.4–64.6) | 57.5 (51.4–63.2) | .78 |
| Men | 27 (63) | 224 (59) | .74 |
| Amyloid involvement | |||
| Renal | 33 (77) | 260 (69) | .30 |
| Cardiac | 32 (74) | 179 (47) | .001 |
| Liver | 10 (23) | 48 (13) | .06 |
| IVS thickness, mm | 14 (12–16) | 12 (10–14) | .003 |
| EF, % | 66 (56–70) | 65 (60–69) | .94 |
| No. of organs involved | .001 | ||
| 1 | 10 (23) | 187 (49) | |
| 2 | 21 (49) | 144 (38) | |
| >2 | 12 (28) | 48 (13) | |
| Conditioning dose <200 mg/m2 melphalan | 22 (51.1) | 122 (32) | .02 |
| Albumin, g/dL | 2.3 (1.4–3.0) | 2.7 (2.0–3.3) | .01 |
| Creatinine, mg/dL | 1.2 (1.0–1.7) | 1.1 (0.9–1.3) | .004 |
| NT–proBNP, pg/mL | 3,061 (878–4,446) | 579 (165–1,976) | .004 |
| Troponin T, ng/mL | 0.03 (0.01–0.08) | 0.01 (0.01–0.02) | .001 |
| Alkaline phosphatase, U/L | 88 (74–182) | 88 (69–127) | .21 |
| b2M, mg/L | 3.05 (2.47–4.25) | 2.51 (2.03–3.38) | .004 |
| Urine total protein, g/d | 6.1 (0.56–8.99) | 3.5 (0.23–7.25) | .08 |
| . | Survival Group . | . | |
|---|---|---|---|
| Characteristic . | Early Death (n=43) . | Survived 100 d (n=379) . | P Value . |
| Age, y | 56.2 (49.4–64.6) | 57.5 (51.4–63.2) | .78 |
| Men | 27 (63) | 224 (59) | .74 |
| Amyloid involvement | |||
| Renal | 33 (77) | 260 (69) | .30 |
| Cardiac | 32 (74) | 179 (47) | .001 |
| Liver | 10 (23) | 48 (13) | .06 |
| IVS thickness, mm | 14 (12–16) | 12 (10–14) | .003 |
| EF, % | 66 (56–70) | 65 (60–69) | .94 |
| No. of organs involved | .001 | ||
| 1 | 10 (23) | 187 (49) | |
| 2 | 21 (49) | 144 (38) | |
| >2 | 12 (28) | 48 (13) | |
| Conditioning dose <200 mg/m2 melphalan | 22 (51.1) | 122 (32) | .02 |
| Albumin, g/dL | 2.3 (1.4–3.0) | 2.7 (2.0–3.3) | .01 |
| Creatinine, mg/dL | 1.2 (1.0–1.7) | 1.1 (0.9–1.3) | .004 |
| NT–proBNP, pg/mL | 3,061 (878–4,446) | 579 (165–1,976) | .004 |
| Troponin T, ng/mL | 0.03 (0.01–0.08) | 0.01 (0.01–0.02) | .001 |
| Alkaline phosphatase, U/L | 88 (74–182) | 88 (69–127) | .21 |
| b2M, mg/L | 3.05 (2.47–4.25) | 2.51 (2.03–3.38) | .004 |
| Urine total protein, g/d | 6.1 (0.56–8.99) | 3.5 (0.23–7.25) | .08 |
Improved supportive care and refined patient selection has improved the safety margin for patients undergoing SCT; short-term mortality showed a more than 40% decrease after 2005. Recognition of this decrease in mortality is important for physicians caring for these patients as they weigh the pros and cons of SCT vs novel agent-based treatment in management of amyloidosis.
Gertz:Celgene: Honoraria; Millenium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lacy:Celgene: Research Funding. Dispenzieri:Celgene: Honoraria, Research Funding; Binding Site: Honoraria. Kumar:Celgene: Consultancy, Research Funding; Millennium: Research Funding; Merck: Consultancy, Research Funding; Novartis: Research Funding; Genzyme: Consultancy, Research Funding; Cephalon: Research Funding.
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal