Abstract
Abstract 3471
AML with the internal tandem duplication of the Fms-Like Tyrosine kinase-3 gene (FLT3-ITD) has a poor prognosis, with high relapse rates. Sorafenib is an oral multikinase inhibitor of tyrosine kinases including Flt-3 with activity in AML. We used this drug to treat patients relapsing after allogeneic HSCT and herein present the results.
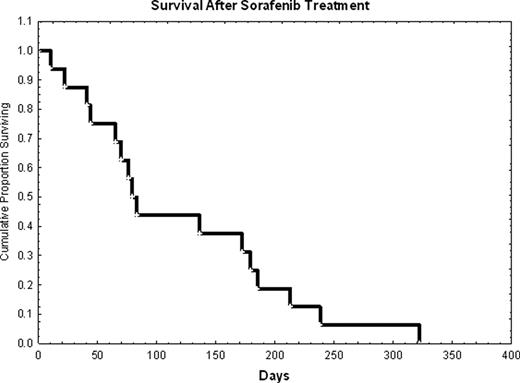
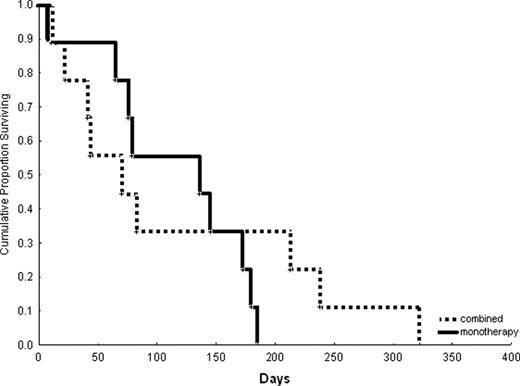
We retrospectively evaluated 16 patients with FLT3-ITD AML that received sorafenib for at least a 7-day course either alone (n=8; 50%) or in combination (n=8; 50%) to treat AML relapsing at a median of 3 months (range, 1–7) after HSCT. Median age was 34 years (range, 20–603). Cytogenetics was diploid in 69%, high-risk in 19% and unknown in 12%. Treatment history prior to HSCT included sorafenib in 38% of the cases, and 30% were status post a 2nd HSCT. Donors were related (50%) or unrelated (50%). Seven patients (44%) received and failed other salvage chemotherapy prior to starting sorafenib (median=2 treatments; range, 1–5). At start of sorafenib salvage treatment, median WBC was 22.5 K/mm3 (range, 0.6–119), and median bone marrow blast was 58% (range, 12–88%), while 75% of the patients had circulating blasts. Sorafenib doses were as follows: 400 mg (n=3) or 800 mg daily (n=4) in combination to idarubicin/Ara-C, or 800 mg daily with 5-azacitidine (n=1); single agent: 800 mg (n=6) or 1200 mg (n=2) daily. Median duration of sorafenib single agent therapy was 39.5 days (range, 10–100), and in combination it was 7 days (range, 7–32). Complete remission (CR) rate was zero; 2 patients achieved a PR. Median reduction in bone marrow (n=9) and peripheral blood (n=12) blasts was 0% (range, 0–46), and 50% (range, 0–88), respectively. Two patients were ‘bridged’ to a 2nd HSCT, which led to CR durations of 53 and 106 days. Median survival after sorafenib initiation was 81 days and it was similar with single agent versus combination therapy (Figure 1 and 2). All patients have died.
Treatment of overt AML relapse with sorafenib was unsuccessful as described here. It is possible that ‘pre-emptive’ treatment in the presence of minimal residual disease will be more effective, but this remains to be proven.
Survival of patients treated with sorafenib monotherapy versus in combination with other agents.
Survival of patients treated with sorafenib monotherapy versus in combination with other agents.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal