Abstract
Abstract 2351
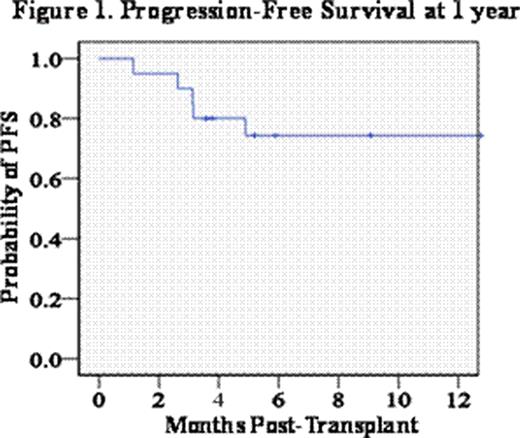
CBT can be curative for patients with high-risk hematologic malignancies. However, patients of older age, those with extensive prior therapy, or significant co-morbidities may not tolerate high-dose myeloablative conditioning. Reduced intensity (RI) or non-myeloablative (NMA) conditioning has been successfully used in CBT, especially in patients with lymphomas. However, patients with myeloid malignancies without extensive prior therapy have an increased risk of graft rejection following NMA CBT. Further, the addition of anti-thymocyte globulin (ATG) to enhance engraftment increases the risk of serious infections and Epstein-Barr virus post-transplant lymphoproliferative disease, and could increase the risk of relapse. Therefore, we investigated the efficacy and safety of a novel ATG-free RI conditioning prior to double unit CBT in patients with acute leukemias and myelodysplasia with the hypothesis that this regimen can induce a high incidence of sustained donor engraftment. Conditioning consisted of cyclophosphamide 50 mg/kg (day -6), fludarabine 30 mg/m2/day × 5 (days -6 to -2), thiotepa 5 mg/kg/day × 2 (days -5 and -4), and total body irradiation 200 cGy × 2 (days -2 and -1). All patients received cyclosporine-A and mycophenolate mofetil for graft-versus-host disease (GVHD) prophylaxis. Between 10/01/07-04/30/10, 20 patients were transplanted. The median age was 56 years (range 18–69). Thirteen (65%) had AML (9 CR1, 4 CR2), 4 (20%) had ALL (3 CR1, 1 CR3), and 3 (15%) had MDS (with one patient also having follicular lymphoma). The majority had high-risk disease. Indications for RI conditioning were the risk factors for transplant-related mortality (TRM) with high-dose conditioning of age ≥50 years, and/or extensive prior therapy, and/or significant co-morbidities. Thirteen patients had only 1 of these risk factors, whereas 7 had ≥2 risk factors. Units were predominantly 4–5/6 HLA-matched to the recipient (one 6/6, twenty-four 5/6, fifteen 4/6). The median infused cell doses of the larger units were 2.7 × 107 total nucleated cells/kg (range 1.46–5.56) and 0.95 × 105 CD34+ cells/kg (range 0.35–3.32), and 1.89 × 107/kg total nucleated cells/kg (range 1.42–2.47) and 0.59 × 105/kg CD34+ cells/kg (range 0.18–1.52) for the smaller units, respectively. The cumulative incidence of sustained donor engraftment at day 45 was 95% (95%CI: 81–100). The single patient with graft failure was 100% donor in the day 21 bone marrow, but died early post-transplant of multi-organ failure without count recovery. The median time to neutrophil recovery ≥0.5 × 109/l was 25 days (range 13–43). The median total donor chimerism in the day 21 bone marrow was 94% (both units combined, range 71–100), and sustained engraftment was accounted for by one unit in 18/19 engrafting patients. The incidence of grade II-IV acute GVHD at day 100 was 55% (95%CI: 32–78), and 46% (95%CI: 21–71) of patients have had late acute GVHD requiring ongoing therapy or chronic GVHD to date. The incidence of day 100 transplant-related mortality (TRM) was 20% (95%CI: 2–38). Notably, none of the 13 patients with only one risk factor died of transplant-related causes. By contrast, 5/7 (71%) patients with ≥2 risk factors died of TRM by day 100 (p=0.03, Table 1). Two additional patients died of relapse. With a median follow-up of 13 months (range 3–31), 1 year progression-free survival is 74% (95%CI: 55–94) (Figure 1). We demonstrate that this ATG-free RI conditioning is associated with a high incidence of sustained donor engraftment, and acceptable toxicities in older patients without other risk factors. While longer follow-up is needed, progression-free survival is encouraging provided multiple risk factors are not present. This conditioning combined with double unit grafts warrants further investigation, and may also be a promising alternative to high-dose conditioning in younger patients.
Day 100 TRM according to number of risk factors (age ≥50 years, extensive prior therapy, significant co-morbidities).
| Risk Factors . | Day 100 TRM . | P Value . |
|---|---|---|
| 1 (N = 13) | 0/13 (0%) | 0.03 |
| ≥2 (N = 7) | 5/7 (71%) |
| Risk Factors . | Day 100 TRM . | P Value . |
|---|---|---|
| 1 (N = 13) | 0/13 (0%) | 0.03 |
| ≥2 (N = 7) | 5/7 (71%) |
Giralt: Celgene: Honoraria, Speakers Bureau; Millenium: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal