Abstract
Recent in vitro studies provide evidence for autoantibody-induced suppression of megakaryocytopoiesis and show a reduction in megakaryocyte production and maturation in the presence of immune thrombocytopenia (ITP) plasma. Here, we present CD34+ cells from healthy umbilical cord blood mononuclear cells cultured in medium containing thrombopoietin, stem cell factor, interleukin-3, and 10% plasma from either ITP patients or healthy subjects. The quantity, quality, and apoptosis of megakaryocytes were measured. We observed that most ITP plasma boosted megakaryocyte quantity but impaired quality, resulting in significantly less polyploidy cells (N ≥ 4) and platelet release. In these megakaryocytes, we found a lower percentage of cell apoptosis, a lower expression of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and a higher expression of Bcl-xL. Furthermore, there was a decrease of sTRAIL in ITP plasma and in cell culture supernatants of this group compared with the control group. Our findings suggest that decreased apoptosis of megakaryocytes also contributes to in vitro dysmegakaryocytopoiesis and reduced platelet production. The abnormal expression of sTRAIL in plasma and TRAIL and Bcl-xL in megakaryocytes may play a role in the pathogenesis of impaired megakaryocyte apoptosis in ITP.
Introduction
Immune thrombocytopenia (ITP) is an immune-mediated bleeding disorder in which platelets are opsonized by autoantibodies and destroyed by phagocytic cells in the reticuloendothelial system.1-4 Although autoantibodies produced by autoreactive B cells against self-antigens, specifically immunoglobulin G (IgG) antibodies against glycoprotein IIb (GPIIb)/IIIa and/or GPIb/IX, are considered to play a crucial role, T-cell-mediated immune abnormalities, such as Th1 bias,5,6 the decreased number or defective function of regulatory T cells,7,8 and the platelet destruction by cytotoxic T cells9,10 have been considered equally important in the pathogenesis of ITP. As a result of the accelerated destruction, platelet production is thought to compensatorily increase. However, platelet turnover studies in the 1980s reported that two-thirds of ITP patients showed decreased or normal platelet production, suggesting that impaired platelet production may also contribute to thrombocytopenia.11
Recent in vitro studies, showing reduced megakaryocyte production and maturation in the presence of autoantibodies against platelet glycoproteins in ITP plasma, have provided evidence for autoantibody-induced suppression of megakaryocytopoiesis.12,13 However, reduced platelets cannot be ascribed to decreased megakaryocyte production in ITP patients with normal or increased bone marrow megakaryocytes.
It has been known that platelets are formed from mature megakaryocytes and arise from the development of long and thin cytoplasmic extensions called proplatelets.14-16 Several reports indicate a close relationship among apoptosis, megakaryocyte maturation, and platelet release. Zauli et al17 found that the onset of megakaryocyte apoptosis coincides with the maximum in the high ploidy megakaryocyte fraction and the number of platelets released in the culture. De Botton et al18 elegantly provided evidence that proplatelet formation was a consequence of local caspase activation during megakaryocyte differentiation. Therefore, we suppose that abnormal megakaryocyte apoptosis may be responsible for persistent thrombocytopenia in ITP.
In the present study, we observed that most ITP plasma inhibited megakaryocyte apoptosis, boosted megakaryocyte mass, and depressed platelet production in vitro, suggesting a possible role of megakaryocyte apoptosis in the pathogenesis of ITP.
Methods
Patients and controls
Whole blood was collected from 49 patients (28 females and 21 males) with chronic ITP, all of whom did not undergo any form of therapy for at least 3 weeks before blood sampling (Table 1). The diagnosis in all patients, based on the criteria for chronic ITP as previously described,19 exhibited isolated thrombocytopenia for more than 12 months, normal or increased bone marrow megakaryocytes, normal spleen size, and no secondary immune or nonimmune abnormality that could account for the thrombocytopenia. Patients with systemic lupus erythematosus and/or antiphospholipid syndrome were excluded from this study, as were pregnant patients or those with concomitant human immunodeficiency or hepatitis C virus infection. Median age (range) and median platelet count (range) at the time of enrollment were 33 years (16-70 years) and 19 × 109/L, (2-68 × 109/L), respectively. Control blood was obtained from 22 healthy blood donors (13 females and 9 males; age range, 23-65 years; median, 36 years) with no history of blood transfusions or pregnancies. Platelet counts ranged from 159 to 287 × 109/L, with the median count of 208 × 109/L. Enrollment took place between March 2007 and August 2009 at the Department of Hematology, Qilu Hospital, Shandong University. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the Medical Ethical Committee of Qilu Hospital, Shandong University.
Patients with chronic ITP
| Patient no.* . | Age, y . | Sex . | Platelet count, ×109/L . | Antiplatelet antibodies† . | Major previous drugs . | Bleeding symptoms . | |
|---|---|---|---|---|---|---|---|
| Anti-GPIIb/IIIa . | Anti-GPIb/IX . | ||||||
| 1 | 19 | F | 23 | − | + | None | EC |
| 2 | 32 | M | 68 | + | − | Pred | EC, EP |
| 3 | 40 | F | 11 | − | − | Dex VCR | PT |
| 4 | 36 | F | 21 | − | + | Pred VCR Splenectomy | PT, EC |
| 5 | 39 | M | 13 | − | − | None | None |
| 6 | 42 | M | 55 | − | − | Pred CSA | PT |
| 7 | 27 | F | 25 | + | − | Dex Danazol | EP |
| 8 | 34 | M | 10 | − | − | None | GUH |
| 9 | 27 | F | 5 | + | + | None | PT, EP |
| 10 | 16 | F | 20 | + | − | Pred VCR Splenectomy | GH |
| 11 | 17 | M | 38 | − | − | None | PT, EC |
| 12 | 22 | F | 2 | − | + | None | EC |
| 13 | 36 | F | 30 | + | + | None | EP |
| 14 | 50 | F | 49 | + | − | Dex | PT |
| 15 | 50 | F | 30 | + | + | Pred | PT, EP |
| 16 | 55 | F | 3 | + | + | Pred VCR | EP |
| 17 | 49 | M | 8.77 | + | − | None | None |
| 18 | 42 | F | 49 | + | − | None | EP, EC |
| 19 | 16 | M | 19 | + | − | Pred | EP |
| 20 | 41 | F | 33 | − | + | Pred | PT, GH |
| 21 | 16 | M | 37 | − | + | Pred VCR | PT |
| 22 | 46 | F | 8 | − | − | None | PT, EC |
| 23 | 20 | M | 50 | − | − | None | PT, EC |
| 24 | 17 | M | 11 | − | − | Pred | EP |
| 25 | 33 | M | 9 | − | − | Pred VCR | PT, GUH |
| 26 | 27 | F | 18 | − | − | None | None |
| 27 | 21 | M | 53 | − | − | IVIG | None |
| 28 | 32 | F | 19 | − | + | Pred VCR | PT |
| 29 | 27 | M | 9 | − | + | None | EP |
| 30 | 38 | M | 41 | + | + | Dex | PT, GUH |
| 31 | 23 | F | 13 | + | − | Pred CSA | EC, EP |
| 32 | 25 | M | 62 | − | − | Dex | PT, EC |
| 33 | 34 | F | 15 | + | + | None | EC |
| 34 | 39 | F | 23 | − | + | None | None |
| 35 | 17 | M | 38.1 | − | − | Pred Danazol | PT, EP |
| 36 | 63 | F | 7.61 | − | − | Pred | EP |
| 37 | 42 | F | 10 | − | − | None | EP, EC |
| 38 | 37 | F | 17 | + | + | None | EC |
| 39 | 24 | M | 55 | − | − | Pred | PT |
| 40 | 26 | F | 42 | + | − | None | PT, EC |
| 41 | 70 | M | 10 | + | − | None | PT, EC |
| 42 | 29 | M | 2 | + | − | None | EC, EP |
| 43 | 25 | F | 14 | − | + | Pred | EP |
| 44 | 19 | F | 2 | − | − | None | EP |
| 45 | 61 | F | 6 | − | − | None | PT |
| 46 | 29 | M | 4 | − | − | Pred | GUH |
| 47 | 47 | F | 66 | − | − | Dex | PT, GH |
| 48 | 53 | M | 31 | + | + | None | PT |
| 49 | 36 | F | 50 | + | − | Pred | PT, EP |
| Patient no.* . | Age, y . | Sex . | Platelet count, ×109/L . | Antiplatelet antibodies† . | Major previous drugs . | Bleeding symptoms . | |
|---|---|---|---|---|---|---|---|
| Anti-GPIIb/IIIa . | Anti-GPIb/IX . | ||||||
| 1 | 19 | F | 23 | − | + | None | EC |
| 2 | 32 | M | 68 | + | − | Pred | EC, EP |
| 3 | 40 | F | 11 | − | − | Dex VCR | PT |
| 4 | 36 | F | 21 | − | + | Pred VCR Splenectomy | PT, EC |
| 5 | 39 | M | 13 | − | − | None | None |
| 6 | 42 | M | 55 | − | − | Pred CSA | PT |
| 7 | 27 | F | 25 | + | − | Dex Danazol | EP |
| 8 | 34 | M | 10 | − | − | None | GUH |
| 9 | 27 | F | 5 | + | + | None | PT, EP |
| 10 | 16 | F | 20 | + | − | Pred VCR Splenectomy | GH |
| 11 | 17 | M | 38 | − | − | None | PT, EC |
| 12 | 22 | F | 2 | − | + | None | EC |
| 13 | 36 | F | 30 | + | + | None | EP |
| 14 | 50 | F | 49 | + | − | Dex | PT |
| 15 | 50 | F | 30 | + | + | Pred | PT, EP |
| 16 | 55 | F | 3 | + | + | Pred VCR | EP |
| 17 | 49 | M | 8.77 | + | − | None | None |
| 18 | 42 | F | 49 | + | − | None | EP, EC |
| 19 | 16 | M | 19 | + | − | Pred | EP |
| 20 | 41 | F | 33 | − | + | Pred | PT, GH |
| 21 | 16 | M | 37 | − | + | Pred VCR | PT |
| 22 | 46 | F | 8 | − | − | None | PT, EC |
| 23 | 20 | M | 50 | − | − | None | PT, EC |
| 24 | 17 | M | 11 | − | − | Pred | EP |
| 25 | 33 | M | 9 | − | − | Pred VCR | PT, GUH |
| 26 | 27 | F | 18 | − | − | None | None |
| 27 | 21 | M | 53 | − | − | IVIG | None |
| 28 | 32 | F | 19 | − | + | Pred VCR | PT |
| 29 | 27 | M | 9 | − | + | None | EP |
| 30 | 38 | M | 41 | + | + | Dex | PT, GUH |
| 31 | 23 | F | 13 | + | − | Pred CSA | EC, EP |
| 32 | 25 | M | 62 | − | − | Dex | PT, EC |
| 33 | 34 | F | 15 | + | + | None | EC |
| 34 | 39 | F | 23 | − | + | None | None |
| 35 | 17 | M | 38.1 | − | − | Pred Danazol | PT, EP |
| 36 | 63 | F | 7.61 | − | − | Pred | EP |
| 37 | 42 | F | 10 | − | − | None | EP, EC |
| 38 | 37 | F | 17 | + | + | None | EC |
| 39 | 24 | M | 55 | − | − | Pred | PT |
| 40 | 26 | F | 42 | + | − | None | PT, EC |
| 41 | 70 | M | 10 | + | − | None | PT, EC |
| 42 | 29 | M | 2 | + | − | None | EC, EP |
| 43 | 25 | F | 14 | − | + | Pred | EP |
| 44 | 19 | F | 2 | − | − | None | EP |
| 45 | 61 | F | 6 | − | − | None | PT |
| 46 | 29 | M | 4 | − | − | Pred | GUH |
| 47 | 47 | F | 66 | − | − | Dex | PT, GH |
| 48 | 53 | M | 31 | + | + | None | PT |
| 49 | 36 | F | 50 | + | − | Pred | PT, EP |
EC indicates ecchymoses; Pred, prednisone; EP, epistaxis; Dex, dexamethasone; VCR, vincristine; PT, petechiae; CSA, cyclosporine; GUH, genitourinary hemorrhage; GH, gingival hemorrhage; and IVIG, intravenous γ-globulin.
Plasma of patients 1 to 26 increased megakaryocyte production (group A); plasma of patients 27 to 40 suppressed megakaryocyte production (group B); and plasma of patients 41 to 49 did not affect megakaryocyte production (group C).
Antiplatelet antibodies were assayed by modified monoclonal antibody-specific immobilization of platelet antigens.
Plasma preparation
Plasma samples were obtained from ethylenediaminetetraacetic acid anticoagulated blood by centrifugation at 3000g for 30 minutes at 20°C to remove platelets and stored at −80°C before use.
IgG purification
IgG antibody was purified from plasma by affinity chromatography using 4HiTrap Protein A HP column, which was operated with AKTA explorer 100 chromatography system (GE Healthcare) according to the manufacturer's instructions. The IgG concentration was adjusted to 1.2 mg/mL. The final IgG preparations were dialyzed overnight with culture medium.
Adsorption of autoantibodies from ITP plasma
ITP patient plasma (0.5 mL) was mixed with the washed control platelets (1 × 109/mL plasma) and incubated at 4°C for 1 hour. After centrifugation at 3000g for 5 minutes, the supernatant plasma was again adsorbed with fresh platelets at 4°C for 1 to 1.5 hours. The adsorbed plasma was then analyzed by modified monoclonal antibody specific immobilization of platelet antigens for the presence of autoantibodies as previously described in detail by Hou et al.20
Megakaryocyte culture
CD34+ cells were purified from healthy umbilical cord blood mononuclear cells (MNCs) obtained from Ficoll-Hypaque gradient centrifugation (Pharmacia Biotech) using a magnetic cell separation method (MACS; Miltenyi Biotec). The purity was subsequently checked and always exceeded 92%.
Colony-forming unit megakaryocytes (CFU-MKs) were quantitated using a commercially available kit (MegaCult-C; Stem Cell Technologies) according to the manufacturer's instructions. A total of 5 × 103 CD34+ cells were planted in each chamber slide, supplemented with thrombopoietin (TPO, 50 ng/mL), interleukin-3 (IL-3; 10 ng/mL), and IL-6 (10 ng/mL; StemCell Technologies) in 1.5-mL volume containing 10% plasma from ITP patients or healthy controls for 12 days of incubation at 37°C in a humidified atmosphere of 5% CO2. After dehydration, fixation, and immunocytochemical staining with mouse anti–human GPIIb/IIIa antibody and biotin-conjugated goat anti–mouse IgG, megakaryocyte colonies were defined as groups of 3 or more GPIIb/IIIa-positive cells.
CD34+ cells were cultured at 2 × 105/well in 24-well plates in the presence of 100 μL plasma from patients or controls and 900 μL serum-free medium (Stem Cell Technologies) supplemented with 100 ng recombinant human thrombopoietin (Sansheng Pharmacy), 100 ng stem cell factor (Diao Pharmacy), and 10 ng recombinant human interleukin-3 (Biosouth Research Laboratories). Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 for 8 to 15 days.
Flow cytometric analysis
Thereafter, cells were labeled with phycoerythrin-cyano dyes 5(PEcy5)–conjugated CD41a monoclonal antibody (mAb; BD Biosciences), PEcy5-conjugated IgG1 (BD Biosciences) used as an isotype control, according to the manufacturer's instructions, and then analyzed with a BD FACScan flow cytometer (BD Biosciences). CD41a+ cells were gated and recognized as megakaryocytes, and the number of megakaryocytes was determined: cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data).
To measure ploidy distribution, the megakaryocytes were identified after being labeled with PEcy5-conjugated CD41a mAb and incubated with 500 μL propidium iodide (PI, BD Biosciences PharMingen) containing RNase. CD41a+ cells were gated and ploidy distribution was assessed by the intensity of the PI fluorescence.
Detection of platelets produced in culture was performed as previously described.21 In brief, cultured cells were centrifuged at 350g for 15 minutes and incubated with PEcy5-conjugated CD41a mAb. After incubation, each sample was diluted to 300 μL and collected at a median rate for 50 seconds by flow cytometry. An analytical gate was determined according to scatter properties of normal blood platelets treated similarly using a log scale for forward scatter and side scatter, which excluded large contaminating cells (megakaryocytes) and small debris or microparticles.
Megakaryocyte apoptosis was measured using the annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Jingmei Biotech) according to the manufacturer's instructions. Cells were labeled with PEcy5-conjugated CD41a mAb, incubated with FITC-conjugated annexin V and PI, and subsequently analyzed by flow cytometry. CD41a+ cells were gated, and apoptotic megakaryocytes were annexin V+ PI− cells within that population.
To analyze the expression of certain proteins (cyclin B1/cyclin D3, Bcl-2/Bcl-xL) in megakaryocytes, PEcy5-conjugated CD41a mAb-labeled cells were incubated with FITC-conjugated mouse anti–human cyclin B1/FITC-conjugated mouse IgG1 (BD Bioscience), FITC-conjugated mouse anti–human cyclin D3/FITC-conjugated mouse IgG1κ (BD Biosciences), phycoerythrin (PE)–labeled mouse anti–human Bcl-2/PE-labeled mouse IgG1 (BD Biosciences), or FITC-labeled mouse anti–human Bcl-xL/FITC-labeled mouse IgG3 (Southern Biotechnology). The labeled cells were resuspended after washing and analyzed within 2 hours using flow cytometer.
To observe the expression of certain external apoptosis pathway proteins (Fas/FasL, tumor necrosis factor-related apoptosis-inducing ligand [TRAIL]/TRAIL-R2, caspase-3/caspase-8) in megakaryocytes, PEcy5-conjugated CD41a mAb-labeled cells were incubated with FITC-conjugated mouse anti–human CD95/FITC-conjugated mouse IgG1κ (BD Biosciences), PE-conjugated mouse anti–human CD178/ PE-conjugated mouse IgG1(BD Biosciences), PE-conjugated mouse anti–human TRAIL/PE-conjugated mouse IgG1 (BD Biosciences), or PE-conjugated mouse anti–human DR5(TRAIL-R2)/PE-conjugated mouse IgG1κ (eBioscience). And we used the CaspGLOW fluorescein active caspase-3/caspase-8 staining kit (BioVision) to analyze the active caspase-3/caspase-8 expression in PEcy5-conjugated CD41a mAb-labeled megakaryocyte cells according to the manufacturer's instructions. The labeled cells were resuspended after washing and analyzed within 2 hours using a flow cytometer.
We also measured the expression of TRAIL on the surface of produced platelets. Cultured cells were centrifuged at 350g for 15 minutes and incubated with PEcy5-conjugated CD41a mAb and PE-conjugated mouse anti–human TRAIL. After incubation, each sample was diluted to 300 μL, and collected at median rate for 50 seconds by flow cytometry as described in the previous paragraph.
ELISA for TPO, IL-11, sFas, and sTRAIL in plasma
The levels of plasma TPO and IL-11 in controls and patients were measured by sandwich enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer's instructions. In addition, we measured the plasma levels of sFas (Bender Medsystems) and sTRAIL (Diaclone) in controls and ITP patients by sandwich ELISA kits.
Statistical analysis
All data are presented as mean plus or minus SD. Differences among 4 groups were assessed using analysis of variance, and differences among 4 groups at different measuring time were assessed using repeated-measures of analysis of variance. Because some of the data had heterogeneity of variance, comparison between these numbers was analyzed by Kruskal-Wallis test. P value less than .05 was considered statistically significant. Statistical analysis was performed with SPSS Version 13.0 statistical software programs.
Results
Effects of ITP plasma on megakaryocyte production, maturation, and platelet release
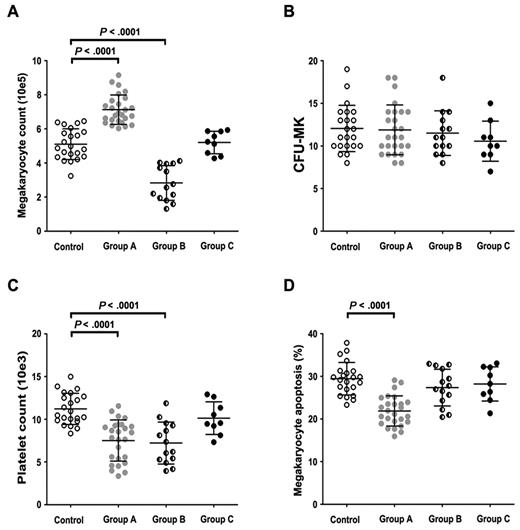
As shown in Figure 1A, ITP patients can be divided into 3 groups according to the effect that their plasma had on megakaryocyte production after 15 days of coculture. Compared with healthy controls (mean ± SD, 5.10 ± 0.90 × 105), the number of megakaryocytes in group A (26 ITP patients) was significantly increased (7.13 ± 0.86 × 105, P < .0001), whereas that in group B (14 ITP patients) was markedly decreased (2.83 ± 1.02 × 105; P < .0001). However, the plasma of 9 ITP patients in group C did not significantly affect the number of megakaryocytes (5.20 ± 0.66 × 105; P = .776).
Effects of whole plasma on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) ITP plasma of group A significantly increased the yield of megakaryocytes on day 15 compared with control cultures (7.13 ± 0.86 × 105 vs 5.10 ± 0.90 × 105, P < .0001). ITP plasma of group B significantly reduced the yield of megakaryocytes on day 15 compared with control cultures (2.83 ± 1.02 × 105 vs 5.10 ± 0.90 × 105, P < .0001). Significant difference in megakaryocyte counts was not seen between group C and control cultures (5.20 ± 0.66 × 105 vs 5.10 ± 0.90 × 105, P = .776). (B) In the presence of whole plasma, in vitro CFU-MK formation in groups A, B, C, and controls were 11.89 ± 2.93, 11.50 ± 2.62, 10.56 ± 2.35, and 12.05 ± 2.72, respectively. There was no remarkable differences among the 4 groups (P = .553). (C) In the presence of whole plasma, platelet release in group A was significantly lower than controls (7.51 ± 2.41 × 103 vs 11.21 ± 1.82 × 103, P < .0001). Platelet release in group B was significantly lower than controls (7.21 ± 2.45 × 103 vs 11.21 ± 1.82 × 103, P < .0001). There was no remarkable differences between group C and controls (10.12 ± 1.91 × 103 vs 11.21 ± 1.82 × 103, P = .214). (D) In the presence of whole plasma, group A cultures showed decreased apoptotic megakaryocytes (21.88% ± 3.53%) compared with group B (27.36% ± 4.31%), group C (28.21% ± 4.02%) and control cultures (29.43% ± 3.80%, P < .0001). Data are mean ± SD.
Effects of whole plasma on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) ITP plasma of group A significantly increased the yield of megakaryocytes on day 15 compared with control cultures (7.13 ± 0.86 × 105 vs 5.10 ± 0.90 × 105, P < .0001). ITP plasma of group B significantly reduced the yield of megakaryocytes on day 15 compared with control cultures (2.83 ± 1.02 × 105 vs 5.10 ± 0.90 × 105, P < .0001). Significant difference in megakaryocyte counts was not seen between group C and control cultures (5.20 ± 0.66 × 105 vs 5.10 ± 0.90 × 105, P = .776). (B) In the presence of whole plasma, in vitro CFU-MK formation in groups A, B, C, and controls were 11.89 ± 2.93, 11.50 ± 2.62, 10.56 ± 2.35, and 12.05 ± 2.72, respectively. There was no remarkable differences among the 4 groups (P = .553). (C) In the presence of whole plasma, platelet release in group A was significantly lower than controls (7.51 ± 2.41 × 103 vs 11.21 ± 1.82 × 103, P < .0001). Platelet release in group B was significantly lower than controls (7.21 ± 2.45 × 103 vs 11.21 ± 1.82 × 103, P < .0001). There was no remarkable differences between group C and controls (10.12 ± 1.91 × 103 vs 11.21 ± 1.82 × 103, P = .214). (D) In the presence of whole plasma, group A cultures showed decreased apoptotic megakaryocytes (21.88% ± 3.53%) compared with group B (27.36% ± 4.31%), group C (28.21% ± 4.02%) and control cultures (29.43% ± 3.80%, P < .0001). Data are mean ± SD.
CFU-MK formation values in group A, B, C, and controls were 11.89 ± 2.93, 11.50 ± 2.62, 10.56 ± 2.35, and 12.05 ± 2.72, respectively. There were no remarkable differences among the 4 groups (P = .553), suggesting that increased megakaryocyte counts are not mediated by accelerated proliferation of megakaryocyte progenitors (Figure 1B).
To rule out the possibility that TPO and IL-11, the known positive regulators of megakaryopoiesis,22-24 might be elevated in ITP plasma from group A compared with controls and other groups, we assayed the plasma levels of TPO and IL-11 by ELISA. Consistent with previous reports,25-27 TPO levels in plasma did not differ significantly among ITP patients in group A (71.14 ± 12.15 pg/mL), group B (68.24 ± 17.25 pg/mL), group C (66.36 ± 15.21 pg/mL), and controls (57.08 ± 24.04 pg/mL). Plasma IL-11 concentration in group A (126.74 ± 44.23 pg/mL) was remarkably higher than that in controls (31.19 ± 9.20 pg/mL; P < .0001), but there was no significant difference among groups A (126.74 ± 44.23 pg/mL), B (130.03 ± 40.16 pg/mL), and C (134.15 ± 38.03 pg/mL).
The percentages of megakaryocyte polyploidy (N ≥ 4) in groups A, B, C, and controls were 16.35% ± 4.90%, 16.11% ± 5.66%, 24.66% ± 2.49%, and 24.57% ± 2.83%, respectively. The platelet release values in groups A, B, C, and control cultures were 7.51 ± 2.41 × 103, 7.21 ± 2.45 × 103, 10.12 ± 1.91 × 103, and 11.21 ± 1.82 × 103, respectively (Figure 1C). The megakaryocyte polyploidy and platelet counts in groups A and B were significantly lower than those in controls (P < .0001).
Inhibited apoptosis and overexpressed Bcl-xL in megakaryocytes cultured with group A ITP plasma
As shown in Figure 1D, reduced megakaryocyte apoptosis was observed in group A (21.88% ± 3.53%) compared with that of controls (29.43% ± 3.80%; P < .0001) on day 15. Megakaryocyte apoptosis did not differ significantly among group B (27.36% ± 4.31%), group C (28.21% ± 4.02%), and controls. Meanwhile, megakaryocytes in each group cultures all showed a decreased expression of Bcl-xL during the culture process with a significantly higher level in group A cultures. At days 8 and 15, Bcl-xL expression in group A (82.29% ± 5.02%, 72.57% ± 5.28%) was significantly higher than in controls (51.02% ± 4.77%, 47.34% ± 5.87%; P < .0001), whereas the expression of Bcl-2 was relatively stable and showed no significant difference in megakaryocytes among the 4 groups.
Effects of patient IgG on megakaryocyte yield, maturation, apoptosis, and platelet release
To evaluate the role of autoantibody on the quantity and quality of megakaryocytes, we studied the effects of patient and control IgG on megakaryocyte production, ploidy distribution, platelet release, and megakaryocyte apoptosis.
A significant reduction in megakaryocyte production, ploidy distribution, and platelet release was seen in cultures containing IgG from both group A (3.15 ± 0.93 × 105, 14.12% ± 6.09%, 5.95 ± 2.27 × 103) and group B (3.02 ± 1.01 × 105, 15.68% ± 5.98%, 6.15 ± 2.37 × 103) plasma compared with cultures containing IgG from group C (4.57 ± 0.78 × 105, 23.14% ± 2.27%, 9.85 ± 1.61) × 103) and control plasma (4.90 ± 0.48 × 105, 23.98% ± 2.23%, 10.97 ± 1.92 × 103; Figure 2A,C). On the other hand, no significant difference was seen in megakaryocyte apoptosis and CFU-MK among cultures containing IgG from groups A, B, C, and control plasma, indicating that megakaryocyte apoptosis and CFU-MK are not affected by autoantibodies in ITP plasma (Figure 2B,D).
Effects of IgG on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) In the presence of patient IgG from both groups A and B, most cultures showed reduced megakaryocyte yield compared with cultures containing control IgG (3.15 ± 0.93 × 105, 3.02 ± 1.01 × 105 vs 4.90 ± 0.48 × 105, P < .0001). There was no remarkable differences between group C and controls (P = .811). (B) In the presence of IgG from ITP patients or healthy controls, significant difference in CFU-MK was not seen among groups A, B, C, and controls (P = .568). (C) In the presence of IgG, platelet release in groups A and B were significantly lower than controls (5.95 ± 2.27 × 103, 6.15 ± 2.37 × 103 vs 10.97 ± 1.92 × 103, P < .0001). There were no remarkable differences between group C and controls (P = .184). (D) In the presence of IgG from ITP patients or healthy controls, significant differences in megakaryocyte apoptosis were not seen among groups A, B, C, and controls (P = .353). Data are mean ± SD.
Effects of IgG on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) In the presence of patient IgG from both groups A and B, most cultures showed reduced megakaryocyte yield compared with cultures containing control IgG (3.15 ± 0.93 × 105, 3.02 ± 1.01 × 105 vs 4.90 ± 0.48 × 105, P < .0001). There was no remarkable differences between group C and controls (P = .811). (B) In the presence of IgG from ITP patients or healthy controls, significant difference in CFU-MK was not seen among groups A, B, C, and controls (P = .568). (C) In the presence of IgG, platelet release in groups A and B were significantly lower than controls (5.95 ± 2.27 × 103, 6.15 ± 2.37 × 103 vs 10.97 ± 1.92 × 103, P < .0001). There were no remarkable differences between group C and controls (P = .184). (D) In the presence of IgG from ITP patients or healthy controls, significant differences in megakaryocyte apoptosis were not seen among groups A, B, C, and controls (P = .353). Data are mean ± SD.
After adsorption of autoantibody from patient plasma, megakaryocyte number, polyploidy percentage, and platelet release rose to control level, and even more megakaryocytes were present in cultures with adsorbed group A plasma (7.85 ± 1.30 × 105) than those cultured with control plasma (5.29 ± 0.74 × 105; Figure 3A,C). There was no apparent effect of adsorbed plasma on CFU-MK among the 4 groups (Figure 3B). However, after adsorption, megakaryocyte apoptosis in group A (22.44% ± 3.56%) was still lower than that in controls (30.24% ± 3.96%; P < .0001; Figure 3D) and almost showed no remarkable differences between autoantibody-adsorbed and unadsorbed plasma. These results indicate that there must be factors, other than autoantibody in ITP plasma, which might influence in vitro megakaryocyte apoptosis and platelet release.
Effects of antibody-adsorbed plasma on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) After adsorption of autoantibody from patient plasma, megakaryocyte number rose to control level, and even more megakaryocytes were present in cultures with adsorbed group A plasma than those cultured with control plasma (7.85 ± 1.30 × 105, 5.29 ± 0.74 × 105, P < .0001). (B) After antibody adsorption, significant difference in CFU-MK was not seen among groups A, B, C, and controls (P = .816). (C) After adsorption, significant difference in platelet release was not seen among groups A, B, C, and controls (P = .061). (D) After adsorption, megakaryocyte apoptosis in group A was still lower than that in controls (22.44% ± 3.56% vs 30.24% ± 3.96%, P < .0001). All data were presented as mean ± SD.
Effects of antibody-adsorbed plasma on in vitro megakaryocyte production, CFU-MK, platelet release, and megakaryocyte apoptosis. (A) After adsorption of autoantibody from patient plasma, megakaryocyte number rose to control level, and even more megakaryocytes were present in cultures with adsorbed group A plasma than those cultured with control plasma (7.85 ± 1.30 × 105, 5.29 ± 0.74 × 105, P < .0001). (B) After antibody adsorption, significant difference in CFU-MK was not seen among groups A, B, C, and controls (P = .816). (C) After adsorption, significant difference in platelet release was not seen among groups A, B, C, and controls (P = .061). (D) After adsorption, megakaryocyte apoptosis in group A was still lower than that in controls (22.44% ± 3.56% vs 30.24% ± 3.96%, P < .0001). All data were presented as mean ± SD.
Abnormal expression of cyclin B1/D3 in megakaryocytes cultured with patient IgG
Several lines of evidence show that cyclin B1 and cyclin D3 are 2 very important proteins participating in megakaryocytic endomitosis and high ploidy formation. To investigate whether the inhibition of in vitro megakaryocyte polyploidization by ITP IgG was mediated by abnormal expression of these cyclins, we examined the effect of IgG on the expression of cyclin D3 and cyclin B1 in megakaryocytes. Cultured with control IgG, megakaryocytes expressed cyclin B1 with a reduction from 44.3% at day 5 to 21.6% at day 9 and a limited expression of cyclin D3 (3.5%) at day 9. Whereas when cultured with patient IgG (from groups A and B), megakaryocytes expressed cyclin B1 at a persistently low level (20.5%) and almost did not express cyclin D3 during the whole culture period (Figure 4A). These results indicate that the abnormal expression of cyclin B1 and cyclin D3 might contribute to megakaryocyte dysmaturity when cultured with ITP patient IgG.
Abnormal expression of cyclin B1/D3, TRAIL, caspase-8, and caspase-3 in megakaryocytes. (A) Cultured with control IgG, megakaryocytes expressed cyclin B1 with a reduction from 44.3% at day 5 to 21.6% at day 9 and a limited expression of cyclin D3 (3.5%) at day 9. Although cultured with patient IgG (from groups A and B), megakaryocytes expressed cyclin B1 at a persistently low level (20.5%) and almost did not express cyclin D3 during the whole culture time. (B) Cultured with whole plasma, TRAIL expression on the surface of megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, TRAIL expression in group A was remarkably lower than that in controls (P < .0001), but there was no significant difference among groups B, C, and control group. (C) Cultured with whole plasma, caspase-8 expression in megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, caspase-8 expression in group A was remarkably lower than that in controls (P = .015 and P = .004), but there was no significant difference between groups B, C, and controls. (D) Cultured with whole plasma, caspase-3 expression in megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, caspase-3 expression in group A was remarkably lower than that in controls (P = .023 and P < .0001), but there was no significant difference among groups B, C, and controls.
Abnormal expression of cyclin B1/D3, TRAIL, caspase-8, and caspase-3 in megakaryocytes. (A) Cultured with control IgG, megakaryocytes expressed cyclin B1 with a reduction from 44.3% at day 5 to 21.6% at day 9 and a limited expression of cyclin D3 (3.5%) at day 9. Although cultured with patient IgG (from groups A and B), megakaryocytes expressed cyclin B1 at a persistently low level (20.5%) and almost did not express cyclin D3 during the whole culture time. (B) Cultured with whole plasma, TRAIL expression on the surface of megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, TRAIL expression in group A was remarkably lower than that in controls (P < .0001), but there was no significant difference among groups B, C, and control group. (C) Cultured with whole plasma, caspase-8 expression in megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, caspase-8 expression in group A was remarkably lower than that in controls (P = .015 and P = .004), but there was no significant difference between groups B, C, and controls. (D) Cultured with whole plasma, caspase-3 expression in megakaryocytes did not differ significantly among ITP patients in groups A, B, C, and controls at day 6. At days 10 and 14, caspase-3 expression in group A was remarkably lower than that in controls (P = .023 and P < .0001), but there was no significant difference among groups B, C, and controls.
Abnormal expression of TRAIL, caspase-8, and caspase-3 in megakaryocytes cultured with group A patient plasma
To investigate the mechanism of impaired megakaryocyte apoptosis as well as whether inhibition of in vitro megakaryocyte apoptosis by ITP plasma was mediated by abnormal expression of certain proteins in the apoptosis pathway, we examined the expression of crucial key factors (Fas/FasL, TRAIL/TRAIL-R2, caspase-3/caspase-8) in megakaryocytes at days 6, 10, and 14 of the culturing process. As a result, at day 6, TRAIL, caspase-3, and caspase-8 expression in megakaryocytes did not differ significantly among the 4 groups, whereas at days 10 and 14, the expression of TRAIL, caspase-3, and caspase-8 in group A was remarkably lower than that of controls, but there was no significant difference among groups B, C, and the controls (Figure 4B-D). On the other hand, the decreased expression of TRAIL, caspase-3, and caspase-8 in group A was consistent with inhibited megakaryocyte apoptosis and decreased platelet production at days 10 and 14 of the culture process. However, the expression of Fas and FasL was low and showed no significant difference on the surface of megakaryocytes among the 4 group cultures at any detecting points. The expression of TRAIL-R2 on megakaryocytes and the expression of TRAIL on produced platelets were relatively stable and showed no significant difference among the 4 groups at any detecting points.
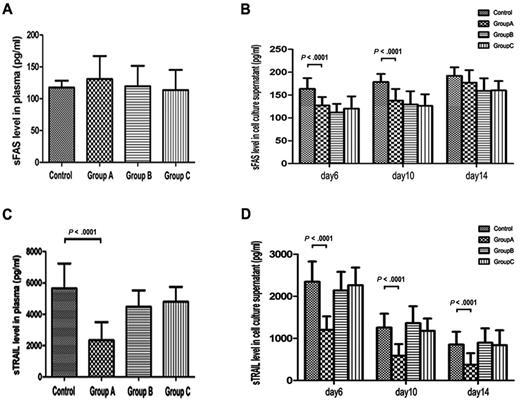
Decreased sTRAIL expression in plasma and cell culture supernatants of group A
We measured sFas and sTRAIL in plasma and cell culture supernatants to determine whether these 2 factors contributed to abnormal megakaryocyte apoptosis. Figure 5A shows the plasma sFas levels of different groups. The plasma levels of sFas in groups A, B, C, and controls were 130.83 ± 36.05 pg/mL, 119.55 ± 31.86 pg/mL, 113.59 ± 31.54 pg/mL, and 117.40 ± 10.71 pg/mL, respectively, and showed no significant difference, whereas the sFas levels in cell culture supernatant of group A (127.38 ± 18.28 and 137.72 ± 25.67 pg/mL) were significantly lower than those in control supernatants (163.75 ± 23.21 and 178.68 ± 17.40 pg/mL) at days 6 and 10, respectively (P < .0001). However, no difference was found at day 14. There were no remarkable differences among groups A, B, and C at any detecting points (Figure 5B). In addition, plasma sTRAIL concentration in group A (2343.24 ± 1155.81 pg/mL) was remarkably lower than that of controls (5653.37 ± 1583.32 pg/mL; P < .0001; Figure 5C). And at days 6, 10, and 14, sTRAIL concentrations in cell culture supernatants of group A (1200.67 ± 321.49, 585.59 ± 277.98, and 373.65 ± 272.51 pg/mL) were remarkably lower than those in controls (2347.18 ± 479.40, 1257.13 ± 329.24, and 852.32 ± 307.32 pg/mL; P < .0001; Figure 5D). sTRAIL levels in plasma and cell culture supernatants showed no remarkable differences among groups B, C, and controls at any detecting points.
sFas and sTRAIL levels in plasma and cell culture supernatants of ITP patients and healthy controls. (A) Plasma sFas did not differ significantly among groups A, B, C, and controls (P = .126). (B) sFas levels in cell culture supernatants of group A were significantly lower than those in control supernatants at day 6 and 10 (P < .0001). At day 14, sFas levels in cell culture supernatants did not differ significantly between ITP patients in group A and controls, and there were no remarkable differences among groups A, B, and C at any detecting time. (C) Plasma sTRAIL concentration was deceased in group A ITP patients compared with healthy controls (P < .0001). There were no remarkable differences among groups B, C, and controls. (D) At days 6, 10, and 14, sTRAIL concentration in cell culture supernatants of group A were remarkably lower than those in controls (P < .0001). There were no remarkable differences among groups B, C, and controls at any detecting time.
sFas and sTRAIL levels in plasma and cell culture supernatants of ITP patients and healthy controls. (A) Plasma sFas did not differ significantly among groups A, B, C, and controls (P = .126). (B) sFas levels in cell culture supernatants of group A were significantly lower than those in control supernatants at day 6 and 10 (P < .0001). At day 14, sFas levels in cell culture supernatants did not differ significantly between ITP patients in group A and controls, and there were no remarkable differences among groups A, B, and C at any detecting time. (C) Plasma sTRAIL concentration was deceased in group A ITP patients compared with healthy controls (P < .0001). There were no remarkable differences among groups B, C, and controls. (D) At days 6, 10, and 14, sTRAIL concentration in cell culture supernatants of group A were remarkably lower than those in controls (P < .0001). There were no remarkable differences among groups B, C, and controls at any detecting time.
Discussion
In the early 1980s, studies of autologous platelet survival showed that, in the majority of ITP patients, platelet turnover was either reduced or normal. It would be expected that platelet destruction was not the sole pathogenic mechanism, and impaired platelet production might also contribute to persistent thrombocytopenia. Chang et al12 reported that production of megakaryocytes was significantly reduced in the presence of ITP plasma with detectable antiplatelet GPIb autoantibodies. McMillan et al13 recently documented that ITP plasma with detectable anti-GPIIb/IIIa, anti-GPIb, or both not only reduced the yield of megakaryocytes but also suppressed megakaryocyte maturation.
Our present results in patients with chronic ITP are similar, in some way, to previous studies mentioned in the previous paragraph. We showed that some (group B) ITP plasma suppressed megakaryocytopoiesis and autoantibody from some ITP plasma exerted suppression on megakaryocyte production, maturation, and platelet release. Autoantibody adsorption further confirmed that the autoantibody may be partly responsible for the reduced yields of megakaryocytes and platelets. Several possible mechanisms may contribute to the suppression of megakaryocyte production by autoantibodies. First, megakaryocytes express GPIIb/IIIa or GPIb/IX on their surfaces during maturation as well as platelets, autoantibodies binding to megakaryocytes, and platelets could mediate megakaryocyte and platelets destroyed by phagocytic cells.28 Second, autoantibody-induced activation of complement may play a role in megakaryocyte apoptosis.29 Unfortunately, in our study, a significant difference in megakaryocyte apoptosis was not detected between cultures containing antibody-adsorbed and unadsorbed plasma. It is unlikely that autoantibody affects megakaryocyte apoptosis in this way. In addition, para-apoptosis was found in 83% of megakaryocytes and 64% of megakaryoblasts from ITP marrows.30 Houwerzijl et al also reported that morphology compatible with para-apoptosis could be induced in cultured megakaryocytes with ITP plasma.31 Whether para-apoptosis mediated megakaryocyte destruction was induced by autoantibody requires further investigation. Moreover, as with 2 important cyclins in megakaryocyte development and high ploidy formation,32 the absence of cyclin D3 during the whole culture and low expression of cyclin B1 at early stage may have been the mechanism by which patient IgG inhibited megakaryocyte polyploidization. As a result, IgG in ITP plasma destroyed megakaryocytes and affected megakaryocyte polyploidy, leading to less megakaryocyte ploidy and platelet release. However, even when cultured with autoantibody-adsorbed plasma, more megakaryocytes were not reasonably accompanied with more polyploidy cells and platelet production, indicating that autoantibody was not the sole factor in impairing megakaryocyte maturation and platelet production.
In the present work, we were surprised to find that the group with the most ITP plasma (group A) boosted megakaryocyte counts while impairing megakaryocyte maturation and its ability to produce platelets. It was compatible with early morphologic studies of ITP bone marrow showing normal or increased number of megakaryocytes.33,34 This was somewhat inconsistent with the findings of Chang et al12 and McMillan et al.13 Possible reasons for these differences could be the following: (1) Most of the patients enrolled in the Chang et al study12 were diagnosed as acute ITP, whereas the patients in our group were chronic. (2) In McMillan et al paper,13 they mainly investigated patients whose plasma inhibited megakaryocyte production and did not analyze other patients with increased megakaryocytes. (3) In vitro megakaryocyte culturing time was 8 or 10 days in their studies, but we prolonged our study to 15 days, to preferably investigate the role of megakaryocyte apoptosis in chronic ITP. (4) We used cord blood-derived megakaryocytes in our research. Compared with megakaryocytes derived from peripheral blood or adult bone marrow, megakaryocytes derived from cord blood yielded the higher numbers of megakaryocytes, but these cells were smaller with less endomitosis, showing reduced polyploidization and platelet number. Therefore, different sources of megakaryocytes may influence the results in the study. Because increased megakaryocyte counts were not a result of an accelerated proliferation of megakaryocyte progenitors or elevated levels of TPO and IL-11, the 2 positive regulators of megakaryocytopoiesis in our experiment, there must be some other reasons leading to increased megakaryocyte mass with impaired maturation and platelet production.
Several reports have indicated a close relationship among megakaryocyte maturation, apoptosis, and platelet release. Local caspase activation can lead to proplatelet formation and thus platelet release.16,18,35 Houwerzijl et al31 have reported that the inhibited megakaryocyte apoptosis may contribute to thrombocytopenia and augment dysfunctional megakaryocytes. We found reduced megakaryocyte apoptosis in the boosted megakaryocyte group, indicating that the reduced megakaryocyte apoptosis could be associated with a boosted megakaryocyte mass, but an impaired megakaryocyte maturation and reduced platelet production. It would be interesting and informative to further investigate the causative factors leading to impaired megakaryocyte apoptosis in ITP patients.
The role of the apoptosis inducing ligands of the TNF family and their death receptors has been explored for years, especially the role of Fas/FasL pathway. Shenoy et al36 found that 25% pediatric patients with chronic hematologic autoimmunity had profound defects in lymphocyte death via the Fas pathway. Although it has been shown that the Fas/FasL system may accelerate megakaryocytic apoptosis and platelet release,35 Fas and FasL are mainly expressed on T and B lymphocytes, inducing cellular apoptosis disorder associated with cellular and humoral immune reaction. Yoshimura et al37 reported that there were 2 groups of ITP patients, one with elevated levels of sFas/sFasL in their plasma and the other with normal levels, suggesting that the pathogenesis of some ITP patients might include an alteration of the Fas/FasL pathway. In our study, the sFas levels in cell culture supernatant of group A were significantly lower than those in control supernatants at days 6 and 10, which was consistent with the data from Yoshimura et al.37 However, no difference was found at day 14, the surface expressions of Fas and FasL on megakaryocytes were low in both ITP patients and controls, and sFas levels in plasma showed no difference between ITP patients and controls. These findings indicate that Fas/FasL interaction is important in lymphocyte apoptosis but plays little role in decreased megakaryocyte apoptosis in ITP patients. The sFas in plasma seems not to be the factor contributing to decreased platelet production in ITP.
Several studies indicate that TRAIL is involved in the pathogenesis of some autoimmune diseases, including systemic lupus erythematosus, Sjogren syndrome, and autoimmune thyroid diseases.38-40 Previous studies41-43 show that TRAIL can promote the maturation and apoptosis of megakaryocytes. Human megakaryocytes cultured in vitro express TRAIL and TRAIL-R2 on their surfaces. Similarly, platelets also express TRAIL, which may be obtained from megakaryocytes. We found that TRAIL expression on megakaryocytes and sTRAIL concentrations in plasma and cell culture supernatants of group A patients were prominently decreased compared with the other 3 groups. Therefore, decreased sTRAIL might be a contributing factor to impaired megakaryocyte apoptosis. As we expected, the expression of both caspase-8 and caspase-3 decreased in group A megakaryocytes. We inferred that decreased TRAIL expression may have led to less caspase-8 and caspase-3 activation, thus triggering less megakaryocyte apoptosis. In addition, although TRAIL expression on the surface of platelets was almost equal, platelets produced in group A were much lower than controls. In a word, the total TRAIL expression on the platelet surface of group A might be in a much lower level, which may enhance the role of TRAIL in impaired megakaryocyte apoptosis through the TRAIL/TRAIL-R2 pathway. Recently, Sedger et al44 have reported that FasL and TRAIL double deficient mice develop extreme lymphoproliferative disease and fatal autoimmune thrombocytopenia, which is a little different from the results of our study. However, severe lymphoproliferative disease is the result of maximal resistance of lymphocytes to activation-induced cell death, and dysregulated lymphocyte homeostasis resulted in the production of antiplatelet IgM and IgG causing thrombocytopenia. As a result, spontaneous thrombocytopenia in these mice is secondary to lymphoproliferative disease. This is somewhat different from the mechanism of primary immune thrombocytopenia.
Recent studies showed that Bcl-xL is up-regulated during megakaryocyte differentiation but is absent from senescent megakaryocytes.45,46 Overexpression of Bcl-xL could decrease platelet formation.47 Therefore, Bcl-xL, as an antiapoptosis factor, should play a role in megakaryocyte differentiation and apoptosis, as well as in platelet formation. Zhang et al48 discovered that Bcl-xL is down-regulated early during in vitro differentiation of megakaryocytes from essential thrombocythemia patients, leading to overproduction of megakaryocytes and platelets. In the current research, we found that megakaryocytes in group A had higher Bcl-xL expression compared with other groups on day 8, before the peak day of high ploidy (days 10-12; data not shown). Thus, we conclude that overexpression of Bcl-xL in immature megakaryocytes might suppress megakaryocyte maturation and platelet production by inhibiting apoptosis.
Studies have demonstrated that CD8+ T cell-induced lysis of platelets in chronic ITP may be involved in the pathogenesis of this disorder.9 A new murine model of severe ITP induced by both antibody and CD8+ T cells has further confirmed the T cell-mediated immune disorder in ITP.10 Li et al49 discovered that activated CD8+ T cells in bone marrow of patients with chronic ITP might suppress megakaryocyte apoptosis and lead to impaired platelet production in vitro. sFas, derived from activated CD8+ T cells, might act as a paracrine regulator of the megakaryocytic apoptosis. In the present study, we found ITP plasma inhibited megakaryocyte apoptosis, and we could not rule out the function of CD8+ T cells or their secretary factors in ITP plasma on in vitro megakaryocyte culture.
Taken together, the observation that suppressed megakaryocyte apoptosis by some ITP plasma led to boosted megakaryocyte mass and reduced platelet production in vitro, may interpret the increased bone marrow megakaryocytes with decreased peripheral platelets in vivo. The abnormal expression of sTRAIL in plasma, TRAIL and Bcl-xL in megakaryocytes may play a role in the pathogenesis of impaired megakaryocyte apoptosis in ITP. Further studies on promoting megakaryocyte apoptosis and blocking antiapoptotic proteins should be performed to further clarify the role of megakaryocyte apoptosis in the pathogenesis of ITP and thereby provide a new stratagem for ITP treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Bojun Shen and Huaishui Hou of Shandong Umbilical Blood Center for technical assistance and advice and Michelle Roh from University of California San Diego for critical reading of the manuscript.
This work was supported by the Tai Shan Scholar Foundation, National Natural Science Foundation of China (grants 81070396, 81070411, 81070407, 81070408, 30570779, 30600259, 30770922, 30800491, 30801258, and 30971278), the National 973 Basic Research Program of China (grant 2011CB503906), Foundation for the Author of National Excellent Doctoral Dissertation of PR China (grant 200561), Program for New Century Excellent Talents in University (grant NCET-07-0514), Key Project of Chinese Ministry of Education (grant 109097), Key Clinical Research Project of Public Health Ministry of China 2010-2012, Natural Science Foundation of Shandong Province (grant ZR2009CM001), Clinical Medicine Center Foundation of Shandong Province, Leading Medical Professionals Foundation of Shandong Province, Outstanding Young Scientist Research Award Foundation of Shandong Province (grant 2008BSO3009), and Independent Innovation Foundation of Shandong University (grant 2009TS053) State Program of National Natural Science Foundation of China for Innovative Research Group (81021001).
Authorship
Contribution: L.Y. performed research, analyzed data, and wrote the manuscript; L.W. performed research and wrote the manuscript; C.-h.Z. performed research and analyzed data; X.-j.Z. and Y.H. analyzed data; J.P. analyzed data and wrote the manuscript; and M.H. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ming Hou, Hematology Oncology Centre, Qilu Hospital, Shandong University, 107 West Wenhua Rd, Jinan, Shandong 250012, China; e-mail: houming@medmail.com.cn; or Jun Peng, Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education and Chinese Ministry of Health, Jinan, 250012, China; e-mail:junpeng88@sina.com.cn.
References
Author notes
L.Y. and L.W. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal