Abstract
Finding an effective treatment for acute myeloid leukemia (AML) remains a challenge, and all cellular processes that are deregulated in AML cells should be considered in the design of targeted therapies. We show in our current study that the LKB1/AMPK/TSC tumor suppressor axis is functional in AML and can be activated by the biguanide molecule metformin, resulting in a specific inhibition of mammalian target of rapamycin (mTOR) catalytic activity. This induces a multisite dephosphorylation of the key translation regulator, 4E-BP1, which markedly inhibits the initiation step of mRNA translation. Consequently, metformin reduces the recruitment of mRNA molecules encoding oncogenic proteins to the polysomes, resulting in a strong antileukemic activity against primary AML cells while sparing normal hematopoiesis ex vivo and significantly reducing the growth of AML cells in nude mice. The induction of the LKB1/AMPK tumor-suppressor pathway thus represents a promising new strategy for AML therapy.
Introduction
In acute myeloid leukemia (AML), the oncogenic deregulation of mRNA translation markedly contributes to the malignant phenotype. However, the efficacy of clinically available therapies that target this process, such as rapamycin, remains moderate due to the induction of multiple resistance mechanisms,1 and new approach should be considered to bypass these processes.
The liver kinase B1 (LKB1) serine/threonine kinase is encoded by the tumor-suppressor gene, STK11, which harbors germ-line mutations in the inherited cancer predisposition, Peutz-Jeghers syndrome, and somatic mutations in sporadic cancers.2 The search for substrates of LKB1 that mediate its tumor-suppressor function led to the identification of the LKB1/AMPK/TSC adenosine monophosphate–activated protein kinase (AMPK) as a direct LKB1 substrate.3 AMPK is a heterotrimeric complex comprising a catalytic α- and 2 regulatory β- and γ-subunits, and LKB1 enhances AMPK activity through the phosphorylation of the α-subunit at T172.4 AMPK is allosterically activated by the accumulation of AMP molecules, due to metabolic stresses that inhibit adenosine triphosphate (ATP) production (eg, hypoxia, glucose deprivation) or stimulate ATP consumption5 and is also activated by drugs used to treat type 2 diabetes, including metformin.6 AMPK is therefore the main cellular energy sensor, acting as a central negative regulator of metabolic pathways, such as fatty acid oxidation and glucose consumption.7
The LKB1/AMPK pathway also regulates the protein synthesis rate through the control of the serine/threonine kinase mammalian target of rapamycin (mTOR),8 a process that is consistently deregulated in AML cells.9 When activated, AMPK stimulates tuberous sclerosis complex 1/2 (TSC1/2), which comprises the TSC1/hamartin and TSC2/tuberin proteins, through AMPK-mediated TSC2 phosphorylation at the T1227 and S1345 residues.10 This stimulates the GTPase-activating protein (GAP) function of TSC2 toward the small G-protein Rheb (Ras homolog enriched in brain), and increases the pool of guanosine diphosphate (GDP)-bound Rheb molecules. Although the molecular mechanism underlying mTOR activation by Rheb-guanosine triphosphate (GTP) is still under debate,11-13 it is well established that TSC2 activation switches off Rheb, resulting in the inhibition of mTOR activity.10
MTOR is a component of 2 exclusive complexes that are defined by their molecular composition and their substrate specificity, mTORC1 and mTORC2. Interestingly, mTORC1 is constitutively active in most cancers, including AML.14 This complex, defined by the interaction between raptor (regulatory-associated protein of mTOR) and mTOR, is generally thought to govern mRNA translation and cell growth in a rapamycin-sensitive way.15 Raptor acts as a scaffold for recruiting and activating mTOR substrates, including the 70-kDa S6 ribosomal protein kinase (P70S6K) and eukaryotic translation initiation factor 4E (eIF4E) binding protein 1 (4E-BP1).16 On the other hand, the rapamycin-insensitive mTORC2 complex, defined by the interaction between mTOR and rictor (rapamycin-insensitive companion of mTOR), controls the activity of the oncogenic kinase, Akt, but has not been linked, as yet, to the control of protein translation.17-19
Deregulated mTORC1 activity within cancer cells increases the synthesis of many oncogenic proteins regulated at the translation initiation level, through the phosphorylation of the physiologic translation repressor 4E-BP1 at multiple residues.20 The eIF4E protein is constitutively associated with the 7-methyl-GTP (7-mGTP) cap structure at the 5′ end of mRNA molecules and associates with the scaffold protein eIF4G to initiate the formation of translation-initiating complexes (eIF4F).21 This process is controlled by the translation repressor 4E-BP1, which inhibits the formation of eIF4F when hypophosphorylated by competitively inhibiting the recruitment of eIF4G to eIF4E and sequestering eIF4E in an inactive complex.22 The hierarchical phosphorylation of 4E-BP1, first on the T37 and T46 residues, and then finally on S65, decreases its affinity for eIF4E and facilitates the assembly of active eIF4E/eIF4G complexes that stimulate mRNA translation.23
Rapamycin and its derivates (eg, RAD001, CCI-779), referred to as rapalogs, are highly specific mTORC1 inhibitors that have been developed as anticancer drugs.14 However, the efficacy of those compounds has turned out to be quite modest in clinical trials, particularly in the case of AML,24,25 and even when used in combination with chemotherapy.26 There are several mechanisms that can explain the cellular resistance to rapalogs in AML.1 Most notably, we recently showed that rapamycin fails to block the expression of oncogenic proteins in AML, which is due to the persistence of highly phosphorylated 4E-BP1 molecules that maintain translation-initiating complexes in their active configuration in rapamycin-treated AML cells. However, we found that the specific inhibition of translation by the 4EGI-1 compound induces a massive apoptotic response in AML, and we therefore proposed the rapamycin-resistant translation process as a major target for novel AML therapies.9
In our current study therefore, our principal aim was to activate AMPK in AML, which we speculated would block translation in these cells through a strong negative control of mTOR activity. We show, from our experiments, that the LKB1/AMPK/TSC2 tumor-suppressor pathway is consistently functional in primary AML cells and, when specifically activated by metformin, blocks the catalytic activity of mTOR. Accordingly, metformin strongly represses translation in AML, through a complete inhibition of the phosphorylation of the key translation regulator 4E-BP1, resulting in a marked decrease in the expression of highly oncogenic proteins (eg, c-Myc, Cyclin D1, and Bcl-xL). Metformin thereby markedly decreases AML cell proliferation and survival, but barely affects the survival of normal hematopoietic progenitors in vitro. Moreover, metformin significantly reduces the growth of leukemic tumors in mice without evidence of any systemic toxicity.
Overall, our present findings indicate that the LKB1/AMPK pathway has an inducible tumor-suppressor activity in AML, and that this is mainly achieved through the repression of the mTOR-dependent oncogenic mRNA-translation process. The pharmacologic activation of AMPK thus represents a very promising new therapeutic perspective for AML patients.
Methods
Patients
Bone marrow samples were obtained from 25 patients with newly diagnosed AML (at the exclusion of AML 3, 6, and 7 phenotypes), all included in various chemotherapy trials initiated by the French Multicenter Group, Groupe Ouest Est des Leucémies et Autres Maladies du Sang (GOELAMS). Normal peripheral CD34+ cells were purified from 5 healthy allogenic donors. All biologic studies were approved by the GOELAMS Institutional Review Board, and signed informed consent was obtained in accordance with the Declaration of Helsinki.
Cell cultures and reagents
Blast cells were isolated from bone marrow aspirates from AML patients at diagnosis as previously described.27 The CD34+ cells from healthy donors were purified using MIDI-MACS immunoaffinity columns (Miltenyi Biotec). We used, when appropriate, 5, 10, or 15mM metformin (Sigma-Aldrich), 25μM LY294002 (Sigma-Aldrich), 100-1000 nmol/L Ku-0063794 (Chemdea), and 20μM compound-C (Calbiochem).
Immunoprecipitation and Western blotting
Whole-cell extracts, immunoprecipitations, and Western blots were performed as previously described.9 The images were captured using a charge-coupled device camera (LAS3000; FujiFilm). The signal intensity was quantified using Multigauge 2.0 software from Fujifilm.
Mammalian expression plasmids
We cloned the human Pim-2 sequence into a pcDNA3-FLAG plasmid (as described in the supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We used pACTAG-2 4E-BP1-HA constructs28 (4E-BP1WT, 4E-BP1E37E46, and 4E-BP1A37A46) and pcDNA3.1 FLAG-mTORWT, and -mTORSL1+IT plasmids.29
Adenoviral particle production and AML cell infection
Adenovirus-expressing mouse AMPKγ1-R69Q mutant (AMPKR69Q)30 production and AML cell-infection protocols are provided in the supplemental Methods.
RNA interference
siRNA transfection.
SiRNA transfections were done using AMAXA nucleofector, following the manufacturer's instruction (AMAXA Biosystems) as previously reported.9
shRNA-containing lentiviral particle infection.
The TSC2 and nontargeted shRNAs purchased from Open Biosystems are cloned in a pLKO.1 vector, and lentiviral particles were produced and used as described in the supplemental Methods.
Methyl guanosine cap affinity assay
mGTP pull-down experiments were performed as previously reported.9 Briefly, cell lysates were clarified and supernatants were incubated 2 hours with 7-mGTP–Sepharose beads (Amersham), then washed, and resuspended in boiling Laemmli sample buffer.
Polysome analysis
Polysome analyses were done as previously reported,9 in the MV4-11 leukemic cell line. Briefly, polysomes were separated through 10%-50% sucrose density gradients, and a polysome profile was generated by the continuous measure of 254 nm optic density. Twelve fractions (1 mL) were collected, and RNA was extracted on each fraction separately.
Genome-wide polysome profiling
First, 300 ng of total RNA extracted from sucrose gradients was reverse transcribed, following the Genechip Whole transcript (WT) Sense Target labeling assay kit (Affymetrix), as described in the supplemental Methods.
[3H]leucine and [3H]thymidine incorporation assays
Colony assays
The formation of erythroid (BFU-E) and granulo-macrophagic (CFU-GM) colony-forming units from normal CD34+ hematopoietic cells and CFU-L from primary AML cells, exposed or not to metformin, was assessed as previously reported.9
Flow cytometry
Apoptosis was determined by annexin V–phycoerythrin (PE) staining (Becton Dickinson), according to the manufacturer's instructions, as previously reported.9
AML xenografts in nude mice
OCI-AML3 cells were subcutaneously injected in nude mice as previously reported.32 Mice were treated with daily intraperitoneal metformin (n = 20) or vehicle (n = 20) injections. The tumor growth was measured 3 times a week. All experiments were conducted in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International.
Immunohistochemistry and TUNEL assay
Serial sections in paraffin blocks were stained with anti–phospho-4E-BP1 T37/46 and anti-Ki67 as previously reported.32 Apoptosis was assessed by the TUNEL technique, according to the manufacturer's instructions (Roche). The images were obtained using a microscope (DM-2000; Leica) equipped with a digital camera (DFC320; Leica) and IM50 4.0 software. Detailed protocol for both AML xenografts, immunohistochemistry, and TUNEL experiments are available in the supplemental Methods.
Statistical analysis
Data are expressed as mean values and standard error of the mean (SEM). Statistical significance of differences observed between experimental groups was determined using the Student t test. When appropriate, *** means that a statistical difference exists (P < .001) for comparison to the control condition.
Results
The LKB1/AMPK signaling pathway is consistently functional and controls mTOR activity in AML
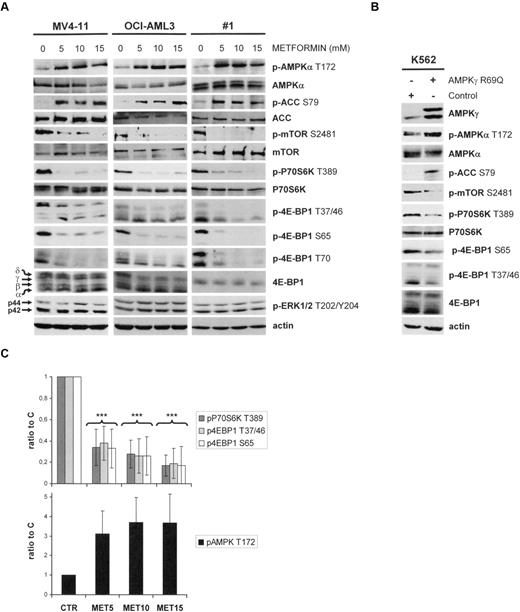
The AMPK agonist, metformin, consistently enhanced the phosphorylation of AMPKα at T172 in AML cells, a result that attests to the functionality of its upstream kinase, LKB1.5 Moreover, metformin induced the phosphorylation of the direct AMPK substrate, ACC at S79, which further reflects the stimulation of AMPK activity by this molecule6 (Figure 1A). Interestingly, we found that metformin inhibited the phosphorylation of mTOR at S2481 and of its substrates, P70S6K at T389 and 4E-BP1 at the T37/46, S65, and T70 residues. Conversely, the phosphorylation levels of p42/44 ERK (T202/Y204) was unaffected by metformin (Figure 1A).
The LKB1/AMPK signaling pathway in AML is functional and controls mTOR activity. (A) Western blot analysis of the phosphorylation levels for AMPKα T172, ACC S79, mTOR S2418, P70S6K T389, 4E-BP1 T37/46, 4E-BP1 S65, 4E-BP1 T70, and p42/44 ERK T202/Y204 in primary AML samples and in the MV4-11 and K562 leukemic cell lines cultured for 24 hours without or with 5, 10, or 15mM metformin. (B) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, mTOR S2481, P70S6K T389, 4E-BP1 T37/46, and 4E-BP1 S65 in the K562 leukemic cell line, expressing the AMPKR69Q activated mutant after adenoviral infection. AMPKR69Q is detected with an anti-AMPKγ antibody. (C) Quantification of Western blot signals. The ratios of phospho-P70S6K T389, phospho-4E-BP1 S65, phospho-4E-BP1 T37/46, or phospho-AMPK T172 to the actin signal intensity were calculated, and results were expressed relative to the control conditions (without metformin) in each experiment. Each histogram represents the mean of 25 independent experiments using primary AML samples, and vertical bars indicate the SEM.
The LKB1/AMPK signaling pathway in AML is functional and controls mTOR activity. (A) Western blot analysis of the phosphorylation levels for AMPKα T172, ACC S79, mTOR S2418, P70S6K T389, 4E-BP1 T37/46, 4E-BP1 S65, 4E-BP1 T70, and p42/44 ERK T202/Y204 in primary AML samples and in the MV4-11 and K562 leukemic cell lines cultured for 24 hours without or with 5, 10, or 15mM metformin. (B) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, mTOR S2481, P70S6K T389, 4E-BP1 T37/46, and 4E-BP1 S65 in the K562 leukemic cell line, expressing the AMPKR69Q activated mutant after adenoviral infection. AMPKR69Q is detected with an anti-AMPKγ antibody. (C) Quantification of Western blot signals. The ratios of phospho-P70S6K T389, phospho-4E-BP1 S65, phospho-4E-BP1 T37/46, or phospho-AMPK T172 to the actin signal intensity were calculated, and results were expressed relative to the control conditions (without metformin) in each experiment. Each histogram represents the mean of 25 independent experiments using primary AML samples, and vertical bars indicate the SEM.
We further expressed the AMPKR69Q dominant activated mutant33 in the K562 human leukemic cell line following adenoviral infection. Interestingly, its expression increased AMPKα T172 and ACC S79 phosphorylation and inhibited mTOR S2481, P70S6K T389, and 4E-BP1 S65 and T37/46 phosphorylation (Figure 1B), which confirmed the negative input resulting from LKB1/AMPK activation toward the mTOR signaling pathway.
These results were confirmed in 25 consecutive primary AML samples (their main characteristics are reported in supplementary Table 1), in which the decreased phosphorylation of P70S6K on T389 (mean: 60%-80%), and of 4E-BP1 on S65 (mean: 59%-79%) and on T37/46 (mean: 62%-76%), paralleled an increase in AMPKα phosphorylation at T172 (mean: 275%-383%) using 5-15mM metformin (Figure 1D; P < .001 for each). These results thus show that specific LKB1/AMPK activation strongly blocks mTOR signaling in AML, most notably resulting in a multisite dephosphorylation of the translation regulator 4E-BP1.
The biologic activity of metformin toward 4E-BP1 phosphorylation is strictly dependent upon the integrity of the AMPK/TSC2/mTOR axis
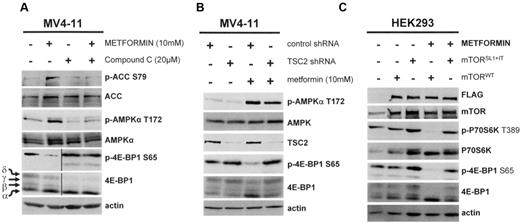
To confirm the specificity of metformin in AML, we blocked AMPK activity in the MV4-11 cell line using compound-C6. This AMPK inhibitor completely suppressed the metformin-induced increase in AMPKα T172 and ACC S79 phosphorylation, and blocked the decrease of 4E-BP1 S65 phosphorylation induced by this molecule, demonstrating that the biochemical activity of metformin in AML cells is, indeed, AMPK dependent (Figure 2A). Given that AMPK activates the TSC1/2 complex through TSC2 phosphorylation, thereby repressing mTOR activity,34 we next depleted TSC2 in the MV4-11 cell line by shRNA. Interestingly, 4E-BP1 S65 phosphorylation was unaffected by metformin in the TSC2 knockdown cells, in contrast to the control cells (Figure 2B).
The biologic activity of metformin is dependent upon the integrity of the AMPK/TSC2/mTOR axis. (A) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, and 4E-BP1 S65 in the MV4-11 leukemic cell line treated without or with 20 μM compound C for 30 minutes, then without or with 10mM metformin for 2 additional hours. (B) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, and 4E-BP1 S65 in TSC2 knock-down MV4-11 cells cultured for 24 hours without or with 10mM metformin. (C) Western blot analysis of the phosphorylation levels of P70S6K T389, and 4E-BP1 S65 in HEK293 cells transfected with mTORWT or mTORSL1+IT plasmids and then treated or not with 10mM metformin.
The biologic activity of metformin is dependent upon the integrity of the AMPK/TSC2/mTOR axis. (A) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, and 4E-BP1 S65 in the MV4-11 leukemic cell line treated without or with 20 μM compound C for 30 minutes, then without or with 10mM metformin for 2 additional hours. (B) Western blot analysis of the phosphorylation levels of AMPKα T172, ACC S79, and 4E-BP1 S65 in TSC2 knock-down MV4-11 cells cultured for 24 hours without or with 10mM metformin. (C) Western blot analysis of the phosphorylation levels of P70S6K T389, and 4E-BP1 S65 in HEK293 cells transfected with mTORWT or mTORSL1+IT plasmids and then treated or not with 10mM metformin.
We next assessed the role of mTOR in the metformin-induced inhibition of 4E-BP1 phosphorylation by comparing the effects of a constitutively activated mTOR catalytic domain mutant, mTORSL1+IT,29 with its wild-type counterpart (mTORWT) in HEK293 cells treated or not with metformin. In mTORWT-transfected cells, metformin fully inhibited P70S6K T389 and 4E-BP1 S65 phosphorylation, whereas the mTORSL1+IT-activated mutant abrogated the inhibitory effects of metformin against mTOR targets (Figure 2C). Taken together, our results clearly indicate that the biologic activity of metformin is mostly dependent upon the integrity of the AMPK/TSC2/mTOR axis.
The catalytic activity of mTOR controls 4E-BP1 phosphorylation in AML
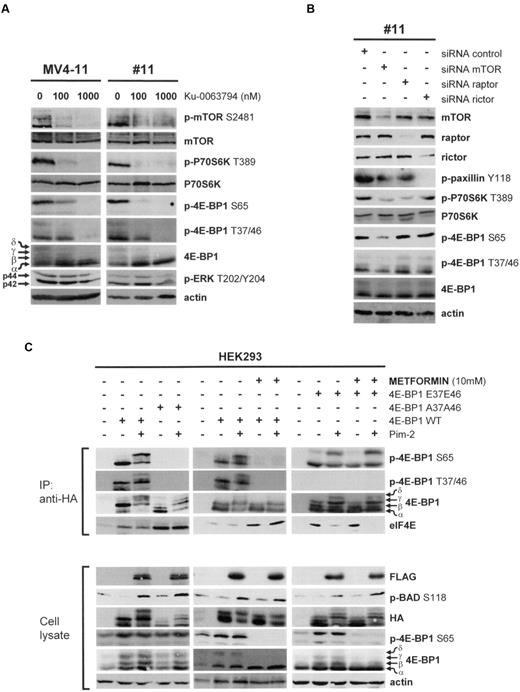
As mTOR appeared to be essential for metformin activity in vitro, we wished to dissect its role in 4E-BP1 phosphorylation in AML. As a first approach, we used the highly specific mTOR catalytic inhibitor Ku-0063794 (Chemdea).35 From 100 nmol/L, Ku-0063794 inhibited mTOR S2481, P70S6K T389, and 4E-BP1 T37/46 and S65 phosphorylation in AML cells (Figure 3A). These results strongly suggest that the catalytic activity of mTOR controls all 4E-BP1 phosphorylation events in AML, including those at its priming residues (T37/46).
The 4E-BP1 T37/46 priming residues are mTOR-dependent and limiting for translation initiation. (A) Primary AML cells and the MV4-11 cell line were cultured for 1 hour without or with 100 or 1000 nmol/L Ku-0063794. (B) Primary AML cells were transfected with mTOR, raptor, or rictor siRNAs. (C) HEK293 cells were transfected with 4E-BP1WT, 4E-BP1A37A46, 4E-BP1E37E46, and Pim-2 plasmids and then, eventually, treated for 24 hours with 10mM metformin, as indicated. At 48 hours after transfection, cells were lysed in 10% NP-40 lysis buffer and immunoprecipitations were performed using anti-HA antibodies. Immunoprecipitated proteins and whole-cell lysates were then subjected to Western blotting.
The 4E-BP1 T37/46 priming residues are mTOR-dependent and limiting for translation initiation. (A) Primary AML cells and the MV4-11 cell line were cultured for 1 hour without or with 100 or 1000 nmol/L Ku-0063794. (B) Primary AML cells were transfected with mTOR, raptor, or rictor siRNAs. (C) HEK293 cells were transfected with 4E-BP1WT, 4E-BP1A37A46, 4E-BP1E37E46, and Pim-2 plasmids and then, eventually, treated for 24 hours with 10mM metformin, as indicated. At 48 hours after transfection, cells were lysed in 10% NP-40 lysis buffer and immunoprecipitations were performed using anti-HA antibodies. Immunoprecipitated proteins and whole-cell lysates were then subjected to Western blotting.
To more fully address the question of the role of mTOR in AML, we decreased its expression by siRNA in primary AML cells and found that this knockdown paralleled a decrease in P70S6K T389, 4E-BP1 T37/46, and S65 phosphorylation (Figure 3B). Particularly, rictor knock down disrupted mTORC2 activity, as evidenced by the inhibition of Y118 phosphorylation of the mTORC2 substrate, paxillin,17 but had no impact upon 4E-BP1 T37/46 or S65 phosphorylation (Figure 3B). As reported, we also showed that raptor knock down did not decrease 4E-BP1 phosphorylation at the T37/46 and S65 residues (Figure 3B).9 The phosphorylation of 4E-BP1 is therefore mainly dependent on the catalytic activity of mTOR in AML.
The 4E-BP1 priming phosphorylation events (T37/46), which are limiting for translation initiation, are inhibited by metformin
The phosphorylation of 4E-BP1 is a multistep process in which T37/46 phosphorylation necessarily precedes that on S65.28 We have previously reported that the terminal phosphorylation of 4E-BP1 at S65 is raptor/mTORC1 independent, but requires the serine/threonine kinase Pim-2, which is constitutively overexpressed in AML cells, but not in nontransformed hematopoietic cells.9 HEK293 cells were transfected either with the 4E-BP1WT or the 4E-BP1A37A46 plasmids and cotransfected or not with a Pim-2 plasmid. The results obtained using immunoprecipitated 4E-BP1WT proteins show that increased Pim-2 activity, assessed by the phosphorylation of its direct substrate, Bad (S118), stimulates 4E-BP1 phosphorylation at S65 (Figure 3C). Interestingly, Pim-2 failed to stimulate S65 phosphorylation of the 4E-BP1A37A46 mutant protein, in which the T37/46 residues are mutated to alanine, thus mimicking a constitutive dephosphorylation. Moreover, the assembly of the translation-initiating complex (eIF4F) was markedly reduced in 4E-BP1A37A46-transfected cells, even when Pim-2 was overexpressed, as evidenced by an increase in the levels of eIF4E protein bound to the immunoprecipitated 4E-BP1A37A46, compared with 4E-BP1WT (Figure 3C). These results clearly indicate that the 4E-BP1 T37/46 residues are limiting for Pim-2-mediated phosphorylation at S65 and subsequent translation initiation.
We thereafter transfected HEK293 cells with Pim-2 and/or 4E-BP1WT and found that treatment with metformin totally mimicked the effects resulting from the expression of the 4E-BP1A37A46 mutant. This strongly suggests that the biochemical activity of metformin was achieved through the inhibition of 4E-BP1 phosphorylation at T37/46 (Figure 3C). Finally, we transfected the HEK293 cells with a 4E-BP1E37E46 mutant, in which the T37/46 residues are replaced by glutamic acid residues, mimicking a constitutive phosphorylation, without or with a Pim-2 plasmid. The expression of Pim-2 stimulated S65 phosphorylation on immunoprecipitated 4E-BP1E37E46 proteins and also enhanced the assembly of eIF4F, as attested by the observed decrease in the amounts of eIF4E bound to immunoprecipitated 4E-BP1E37E46 protein. Interestingly however, treatment with metformin, in this case, was insufficient to block the Pim-2-mediated increase in 4E-BP1 S65 phosphorylation and thus could not prevent the subsequent translation initiation (Figure 3C). These results together show that 4E-BP1 priming phosphorylation events (T37/46) are limiting for translation initiation and represent the molecular target of the biochemical activity of metformin against 4E-BP1.
Metformin inhibits translation and decreases the polysome recruitment of oncogenic mRNAs in AML cells
4E-BP1 phosphorylation is absolutely required for eIF4F assembly and efficient mRNA translation,22 and we speculated that metformin may interfere with this process in AML cells. We performed 7-mGTP pull-down experiments in primary AML cells and observed that treatment with metformin dissociates eIF4G from eIF4E, demonstrating that this molecule abrogates eIF4F assembly (Figure 4A).
Metformin inhibits translation and decreases the expression of oncogenic proteins in AML. (A) 7-mGTP affinity assays of primary AML samples cultured for 24 hours without or with 5, 10, or 15mM metformin (samples 14 and 19 are representative of 5 independent experiments). (B) Polysome analysis performed in the MV4-11 cell line cultured for 12 hours, without or with 10mM metformin. (C) Results of GeneChip (Affymetrix) analysis of RNA extracts from the sucrose gradients used to extract polysomes. The diagram represents mRNA whose polysome recruitment was decreased (green lines) or increased (red lines) in control (CTR1 and CTR2) and metformin (MET1 and MET2) replicates. (D) [3H]leucine pulse assays performed in primary AML samples treated for 2 hours without or with 5, 10, or 15mM metformin. Ratios to the control incubation without an inhibitor were then calculated, and the results are presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (E) Quantification of Western blot signals. Ratios of c-Myc, cyclin D1, or Bcl-xL to actin signal intensity were calculated, and results are expressed relative to the control conditions (without metformin) in each experiment. Each histogram represents the mean of 5 independent experiments using primary AML samples. The vertical bars indicate the SEM.
Metformin inhibits translation and decreases the expression of oncogenic proteins in AML. (A) 7-mGTP affinity assays of primary AML samples cultured for 24 hours without or with 5, 10, or 15mM metformin (samples 14 and 19 are representative of 5 independent experiments). (B) Polysome analysis performed in the MV4-11 cell line cultured for 12 hours, without or with 10mM metformin. (C) Results of GeneChip (Affymetrix) analysis of RNA extracts from the sucrose gradients used to extract polysomes. The diagram represents mRNA whose polysome recruitment was decreased (green lines) or increased (red lines) in control (CTR1 and CTR2) and metformin (MET1 and MET2) replicates. (D) [3H]leucine pulse assays performed in primary AML samples treated for 2 hours without or with 5, 10, or 15mM metformin. Ratios to the control incubation without an inhibitor were then calculated, and the results are presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (E) Quantification of Western blot signals. Ratios of c-Myc, cyclin D1, or Bcl-xL to actin signal intensity were calculated, and results are expressed relative to the control conditions (without metformin) in each experiment. Each histogram represents the mean of 5 independent experiments using primary AML samples. The vertical bars indicate the SEM.
We then performed polysome analysis using MV4-11 cells treated or not with metformin. Polysomes were separated through sucrose density gradients, and their profiles were generated by the continuous measurement of absorbance at 254 nm. Metformin caused an inhibition of translation initiation, as indicated by the shift from large to small polysomes and a concomitant increase in the free ribosome levels (Figure 4B). We concluded, from these results, that metformin strongly inhibits mRNA translation in AML cells.
We then separately pooled the RNA extracts from polysomal and nonpolysomal fractions and assessed the polysome recruitment of mRNA molecules using a Genechip Whole transcript Sense Target labeling assay kit (Affymetrix). The experimental procedure is depicted in supplemental Figure 1. The mRNA detected in the polysomal fraction corresponded to transcripts recruited to polysomes and therefore ongoing active translation. The quantitative variations of the mRNA detected in the polysomal fraction thus correlated to variations in the mRNA translation rate.
We performed this experiment in duplicate and, using an unsupervised analysis, found each replicate within the same cluster, indicating a high degree of interexperiment reproducibility (supplemental Figure 2). We analyzed the transcripts that underwent quantitative variations only in the polysomal fraction and found that 207 of these mRNAs were decreased, whereas 344 transcripts were increased in metformin-treated AML. Interestingly, most of the mRNAs that were found to be specifically decreased in the polysome fraction following metformin treatment have been reported to be involved in cancer pathways. Conversely, most of the up-regulated mRNAs belonged to metabolic pathways (Figure 4C left panel). Moreover, as shown in Figure 4C (right panel), metformin induced a decrease in the expression of the IL3 receptor and the CD52 proteins, whose transcripts were significantly reduced in the polysome-bound mRNA fraction in metformin-treated AML cells. We conclude, from these data, that LKB1/AMPK activation by metformin blocks the initiation step of translation and the recruitment to polysomes of oncogenic mRNA molecules in AML.
Metformin inhibits the synthesis of oncogenic proteins in AML cells
We further assessed the impact of metformin on protein synthesis in primary AML cells. The incorporation of [3H]leucine into neosynthesized proteins was reduced by 35%-50% under treatment with 5-15mM metformin (P < .001 for each; Figure 4D). Moreover, the expression of 3 highly oncogenic proteins, which we had previously reported to be regulated at the translation initiation level in AML cells (c-Myc, Cyclin D1, and Bcl-xL),9 was reduced by 55%-82% in 5-15mM metformin-treated primary AML cells, respectively (P < .001 for each; Figure 4E). These results show that AMPK activation by metformin results in a marked decrease of protein synthesis in primary AML cells, particularly for proteins involved in oncogenic processes.
Metformin induces the killing of primary AML cells but not normal hematopoietic progenitors
As we have previously reported the importance of the deregulated mRNA translation in AML biology,9 we wished to fully examine the antileukemic activity of metformin in primary AML samples. Metformin dramatically decreased AML cell proliferation, with an observed reduction in [3H]thymidine incorporation by 70%-90% under treatment with 5-15mM metformin, respectively (P < .001 for each; Figure 5A bottom panel). Accordingly, metformin treatment decreased the expression of the procycle proteins, CDK2 and SCFSKP2, and increased the expression of the cycle inhibitor, p21Cip1 (Figure 5A top panel). Moreover, metformin dramatically reduced the clonogenic growth of leukemic progenitors by 60%-78% at doses from 5 to 15mM metformin, respectively (P < .001; Figure 5B), whereas the clonogenic growth and differentiation of normal CD34+ hematopoietic progenitors was not significantly modified by metformin, in contrast to incubation with the broad-spectrum kinase inhibitor, LY294002 (Figure 5B).
LKB1/AMPK activation using metformin strongly decreases AML cell survival, but does not affect normal hematopoietic cells ex vivo. (A) Top panel, Western blot analysis of CDK2, SCFSKP2, and p21CIP1 in primary AML cells treated or not with metformin. Bottom panel, proliferation was assessed in primary AML cells cultured without or with 5, 10, or 15mM metformin and then pulsed for 6 hours with [3H]thymidine. Ratios to the controls without inhibitor were calculated, and the results are presented as a mean of 5 independent experiments. The vertical bars indicate the SEM (B) clonogenic assays of primary AML cells (CFU-L) and normal CD34+ hematopoietic progenitors (erythroid, BFU-E; and granulo-macrophagic, CFU-GM). Cells were cultured for 24 hours without or with 5, 10, or 15mM metformin, washed twice in PBS buffer, and then plated in methylcellulose medium. We used 25μM LY294002 as a control for CD34+ clonogenic growth inhibition.9,49 Each histogram represents the mean of 5 independent experiments, performed in duplicate. The results are expressed as a ratio to the control incubation without inhibitor and presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (C) Top panel, Western blot analysis of caspase-3 and Mcl-1 expression in primary AML cells cultured for 24 hours without or with 5, 10, or 15mM metformin (sample 4 is representative of 5 independent experiments). Bottom panel, Annexin V binding assays of cells cultured without or with 5, 10, or 15mM metformin for 24 hours. Results are expressed separately as the mean of 5 primary AML samples and 5 CD34+ samples, respectively. The vertical bars indicate the SEM.
LKB1/AMPK activation using metformin strongly decreases AML cell survival, but does not affect normal hematopoietic cells ex vivo. (A) Top panel, Western blot analysis of CDK2, SCFSKP2, and p21CIP1 in primary AML cells treated or not with metformin. Bottom panel, proliferation was assessed in primary AML cells cultured without or with 5, 10, or 15mM metformin and then pulsed for 6 hours with [3H]thymidine. Ratios to the controls without inhibitor were calculated, and the results are presented as a mean of 5 independent experiments. The vertical bars indicate the SEM (B) clonogenic assays of primary AML cells (CFU-L) and normal CD34+ hematopoietic progenitors (erythroid, BFU-E; and granulo-macrophagic, CFU-GM). Cells were cultured for 24 hours without or with 5, 10, or 15mM metformin, washed twice in PBS buffer, and then plated in methylcellulose medium. We used 25μM LY294002 as a control for CD34+ clonogenic growth inhibition.9,49 Each histogram represents the mean of 5 independent experiments, performed in duplicate. The results are expressed as a ratio to the control incubation without inhibitor and presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (C) Top panel, Western blot analysis of caspase-3 and Mcl-1 expression in primary AML cells cultured for 24 hours without or with 5, 10, or 15mM metformin (sample 4 is representative of 5 independent experiments). Bottom panel, Annexin V binding assays of cells cultured without or with 5, 10, or 15mM metformin for 24 hours. Results are expressed separately as the mean of 5 primary AML samples and 5 CD34+ samples, respectively. The vertical bars indicate the SEM.
Metformin also strongly induced blast cell apoptosis, as evidenced by an increase in Annexin V staining, from 43% to 60% in AML cells treated with 5-15mM of this drug, respectively, compared with a mean positive staining level of 22% under control conditions (P < .001 for each; Figure 5C bottom panel). This increased apoptosis was also revealed by the detection of caspase-3-activating cleavage and by a decrease in the expression of the antiapoptotic protein of the mitochondrial pathway, Mcl-1 (Figure 6D top panel). Significantly, metformin was found not to induce significant levels of apoptosis in normal CD34+ cells cultured under similar conditions (Figure 5C bottom panel). These data thus indicate that the activation of the LKB1/AMPK pathway by metformin results in marked tumor-suppressor activity in primary AML samples, with no evidence of toxicity against normal CD34+ hematopoietic progenitors.
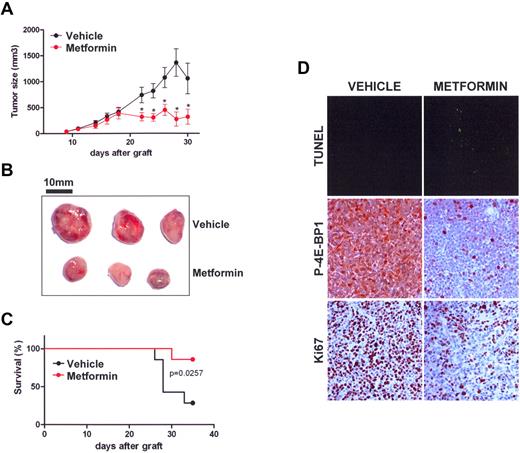
Metformin repress the growth of AML cells in vivo. (A) Tumor size in OCI-AML3 cells xenografted in nude mice treated with vehicle (black) or metformin (red). Mean of individual tumor sizes are plotted (n = 9 in each group). The P value was determined using the Student t test. (B) Representative photographs of xenografted tumors at day 22. Bars, 10 mm. (C) Kaplan-Meier survival curve of nude mice treated with vehicle (black) or metformin (red) after subcutaneous xenograft with OCI-AML3 cells (n = 9 in each group, 2000-mm3 cut-off). (D) Sections of tumors from mice injected with vehicle or metformin were stained by TUNEL or labeled with phospho-4E-BP1 T37/46 or Ki-67 antibodies. Representative photos of 3 experiments are shown.
Metformin repress the growth of AML cells in vivo. (A) Tumor size in OCI-AML3 cells xenografted in nude mice treated with vehicle (black) or metformin (red). Mean of individual tumor sizes are plotted (n = 9 in each group). The P value was determined using the Student t test. (B) Representative photographs of xenografted tumors at day 22. Bars, 10 mm. (C) Kaplan-Meier survival curve of nude mice treated with vehicle (black) or metformin (red) after subcutaneous xenograft with OCI-AML3 cells (n = 9 in each group, 2000-mm3 cut-off). (D) Sections of tumors from mice injected with vehicle or metformin were stained by TUNEL or labeled with phospho-4E-BP1 T37/46 or Ki-67 antibodies. Representative photos of 3 experiments are shown.
Metformin blocks the growth of human AML cells in mice
We developed a xenograft model for AML in nude mice using the OCI-AML3 leukemic cell line and treated these animals with daily intraperitoneal injections of metformin, which significantly reduced the growth of AML tumors (Figure 6A). We further demonstrated that this was a result of in vivo apoptosis induction by metformin, as shown by an increase in TUNEL staining in paraffin sections of AML tumors (Figure 6B). Interestingly, we observed a robust blockade of mTOR signaling by metformin within tumors, as shown by a near complete inhibition of 4E-BP1 phosphorylation (T37/46) in the metformin-treated group (Figure 6C).
Moreover, metformin significantly improved the survival of mice (Figure 6D) without demonstrating any apparent systemic toxicity, as assessed by routine blood tests for liver, kidney, and hematopoiesis functions (supplemental Figure 3). Similar results were obtained using the MV4-11 leukemic cell line (supplemental Figure 4A-B). These findings demonstrate that activation of the LKB1/AMPK axis by metformin also markedly represses the growth of AML cells in vivo.
Discussion
The treatment of AML remains a challenge for oncologists and the targeting of transformed versus nontransformed cells, which represents the cornerstone of anticancer therapy, must be reached by affecting any cellular process deregulated within cancer cells. Deregulation of protein translation is frequently observed in human cancers, leading to the accumulation of highly oncogenic proteins,21 and a recent report from our laboratory has underscored the importance of this process in AML biology.9 However, we also showed, in primary AML cells, that mRNA translation is resistant to mTORC1 inhibition by rapalogs,9 which contrasts with the current working model.24 In addition, several molecular mechanisms are implicated in this resistance phenotype.1
Protein translation thus appears to be a major candidate target for novel AML therapy, and novel molecular approaches to achieve this are thus urgently needed. We focused our interest, in our current study, on the LKB1/AMPK tumor-suppressor pathway, which physiologically represses protein synthesis when activated by energetic stress.10 The loss of LKB1 function is common in cancer, due to either genetic defects or other mechanisms, including epigenetic silencing.3 However, we consistently detected the expression of the LKB1 protein in human AML cell lines and in primary AML samples (supplemental Figure 5). We therefore used the indirect AMPK agonist, metformin, to treat AML cells.
Metformin activates AMPK by at least 2 LKB1-dependent mechanisms. First, metformin inhibits complex I of the mitochondrial respiratory chain, which results in the generation of reactive nitrogen species that activate PKCξ, which, in turn, phosphorylates LKB1 at S428. Phosphorylation of LKB1 at this residue is required for its translocation from the nucleus to the cytoplasm and subsequent AMPK activation.36 Metformin also increases intracellular AMP levels, which activates AMPK by inducing changes in the AMPKγ conformation that exposes the T172 residue of AMPKα, thus generating a good substrate for its upstream kinase, LKB1.37,38 Hence, the major increase in AMPKα T172 phosphorylation consistently detected in metformin-treated primary AML cells indicates the adequate activity of the serine/threonine kinase, LKB1.39 Accordingly, the activity of AMPK, detected by the increased phosphorylation of its direct substrate, ACC, at the S79 residue, is enhanced in AML cells treated with metformin.
It must be noted, however, that AMPK-independent effects of metformin upon glucose uptake, involving a decrease in the intracellular ATP concentration, have been described in nontransformed cells.40 Similarly, Ben Sahra and colleagues have suggested that an AMPK-independent mechanism may contribute to the decreased cell proliferation observed in metformin-treated prostate cancer cells.41 Nevertheless, our current data provide a robust argument for the specificity of metformin toward LKB1/AMPK activation in AML cells. First, the biochemical effects of metformin in AML cells are blocked by the AMPK inhibitor, compound-C. Second, the biologic activity of metformin is mimicked by the direct AMPK agonist, A-769662,42 (supplemental Figure 6A) and by the activated AMPKR69Q mutant, in both cases resulting in an increased level of AMPKα T172 and ACC S79 phosphorylation and decreased mTOR activity. Interestingly, the A-769662 compound also induced the apoptosis of AML cells (supplemental Figure 6B). These results show that the LKB1/AMPK tumor-suppressor pathway is constitutively functional and may be specifically activated by metformin in AML cells.
A major role of the LKB1/AMPK axis involves the control of the mTOR-dependent translation process, and this requires the integrity of the TSC1/2 inhibitory complex.10 Gwinn and collaborators have recently suggested that AMPK-mediated raptor phosphorylation is an important metabolic check point in TSC2-deficient murine embryonic fibroblasts and induces raptor 14.3.3 sequestration and mTORC1 inactivation.43 However, we have reported that 4E-BP1 phosphorylation in AML cells is totally independent of raptor expression.9 Kalender and coworkers also recently showed that in nontransformed cells, metformin blocks mTOR activity in a TSC2- and AMPK-independent manner, and they implicated Rag GTPases in this process.30 Although this may also occur in AML cells, we show here that TSC2 protein expression was consistently detected in AML cells (supplemental Figure 5), and that depletion of TSC2 proteins by shRNA partially protected AML cells from metformin-induced cell death (supplemental Figure 8), underscoring the contribution of the TSC1/2 complex in the biologic activity of metformin in AML.
Our results thus indicate that the AMPK/TSC2/mTOR axis functions adequately in AML, allowing us to further test the effects of metformin against mTOR activity in 25 consecutive primary AML samples. We showed, from these analyses, that metformin strongly inhibits mTOR catalytic activity and does not produce off-target effects against mTOR-independent signaling pathways.
Although we established that the LKB1/AMPK/TSC2 tumor-suppressor pathway is functional in AML, the observed metformin-induced 4E-BP1 dephosphorylation raised the question of the molecular mechanisms underlying this process, with respect to our previous results, indicating that 4E-BP1 phosphorylation resists mTORC1 inhibition in AML.9 We first demonstrated that the 4E-BP1 T37/46 priming phosphorylation events were directly under the control of the catalytic activity of mTOR in AML, regardless of raptor/mTORC1 or rictor/mTORC2 expression, raising the possibility that a third mTOR-containing complex exists, as suggested by other groups,35,44 or that uncomplexed mTOR molecules may directly control 4E-BP1 phosphorylation in AML cells. We then demonstrated that the mTOR-dependent phosphorylation of 4E-BP1 at T37/46 residues was limiting for subsequent S65 phosphorylation, for example, by Pim-2.9 Our interpretation is that the inhibition of mTOR catalytic activity following LKB1/AMPK activation prevented 4E-BP1 “priming” phosphorylation at T37/46, thus increasing the pool of unphosphorylated 4E-BP1 molecules, which could no more be phosphorylated by Pim-2 or, possibly, by other kinases, at S65, resulting in a blockade of translation.
As a result of 4E-BP1 dephosphorylation, metformin markedly inhibited protein translation in AML, which is in contrast to rapamycin, and explains, in part, the superior antileukemic activity of metformin. Accordingly, metformin decreased protein synthesis at a global level and particularly that of oncogenic proteins regulated at the translation initiation level. Based on our genome-wide analysis of the mRNA recruitment to polysomes, we speculate that the antileukemic activity of metformin primarily occurs through a decrease in the translation of oncogenic mRNA molecules.
In primary AML cells, metformin, indeed, demonstrates a remarkable antileukemic activity in vitro, reducing AML cell proliferation, decreasing the clonogenic growth of AML progenitors, and inducing the apoptosis of primary AML cells. Significantly, these effects occur with a minimal degree of toxicity against normal immature CD34+ hematopoietic progenitors. This favorable therapeutic index was confirmed in vivo, as treatment with metformin reduced the size of AML tumors without apparent systemic toxicity and enhanced the survival of tumor-bearing immunocompromised mice.
AMPK activation thus results in a profound inhibition of oncogenic protein synthesis, a process that we previously reported to play a very significant role in AML cell survival.9 In addition, some metabolic pathways controlled by AMPK could have importance in AML biology. The overexpression of lipogenic enzymes, such as the AMPK substrate, ACC, is a common characteristic of cancers and the specific knock down of ACC inhibits cell proliferation and induces caspase-dependent apoptosis in prostate and breast cancer cell lines.45,46 We have shown previously that in AML, metformin inactivates ACC through its phosphorylation at S79, which may contribute to the antitumor activity of this molecule. Moreover, cancer cells generally tend to rely heavily on glycolysis, rather than on mitochondrial oxidative metabolism, a phenomenon referred as the “Warburg effect,”47 and treatment of AML with metformin may also take advantage of this difference with normal tissue, as seen for 2-deoxy-D-glucose in solid cancer cell lines.48
At least 6 clinical studies of metformin as a cancer therapy are currently ongoing, mainly as a neoadjuvant therapy in breast and prostate cancer, but also in advanced tumors in combination with CCI-779. These trials further support the feasibility of using metformin in cancer patients, although the optimal concentrations of this molecule required for achieving antitumor activity have yet to be determined.
Overall, our current results demonstrate a potent tumor-suppressor role for the LKB1/AMPK pathway in AML, which is markedly activated by metformin and which mainly occurs through the repression of the mTOR-dependent oncogenic mRNA translation. We thus provide a strong rationale for the development of AMPK agonists as novel AML therapeutic agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all participating investigators from the GOELAMS. This work was supported by grants from the Ligue Nationale Contre le Cancer (LNCC comité de Paris, laboratoire associé), the Institut National du Cancer (INCa), the Fondation de France (comité leucémies) and the Association Laurette Fugain. We thank Dr N. Azar (Pitié-Salpetrière Hospital, Paris, France) for providing us with normal CD34+ cells. 4E-BP1 and mTOR plasmids were kindly given by Drs N. Sonenberg and E. Petroulakis (McGill University, Vancouver, BC, Canada) and Dr T. Maeda (University of Tokyo, Japan), respectively. We thank Drs F. Letourneur, S. Jacques, and N. Cagnard for their help in mRNA chips experiments. We thank Drs P.O. Vidalain and Y. Jacob (Institut Pasteur, Paris, France) for kindly providing us with the pCiNeo3x-Flag-GW vector.
Authorship
Contribution: A.S.G. performed research, analyzed data, and wrote the manuscript; N.C., T.T.M., L.W., M.L., C.A., O.B., V.B., and S.P. performed research and analyzed data; M.F., B.V., O.H., and I.C.M. analyzed data; N.I. and F.D. contributed AML patient samples and analyzed clinical data; C.L. and P.M. analyzed data and wrote the manuscript; and D.B. and J.T. designed research, performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Didier Bouscary and Jerome Tamburini, Institut Cochin, Inserm U1016 and UMR8104, 22 rue Mechain, 75014 Paris, France; e-mail: didier.bouscary@cch.aphp.fr and jerome.tamburini@inserm.fr.
References
Author notes
N.C. and T.T.M. contributed equally to this work.
D.B. and J.T. contributed equally to this work.

![Figure 4. Metformin inhibits translation and decreases the expression of oncogenic proteins in AML. (A) 7-mGTP affinity assays of primary AML samples cultured for 24 hours without or with 5, 10, or 15mM metformin (samples 14 and 19 are representative of 5 independent experiments). (B) Polysome analysis performed in the MV4-11 cell line cultured for 12 hours, without or with 10mM metformin. (C) Results of GeneChip (Affymetrix) analysis of RNA extracts from the sucrose gradients used to extract polysomes. The diagram represents mRNA whose polysome recruitment was decreased (green lines) or increased (red lines) in control (CTR1 and CTR2) and metformin (MET1 and MET2) replicates. (D) [3H]leucine pulse assays performed in primary AML samples treated for 2 hours without or with 5, 10, or 15mM metformin. Ratios to the control incubation without an inhibitor were then calculated, and the results are presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (E) Quantification of Western blot signals. Ratios of c-Myc, cyclin D1, or Bcl-xL to actin signal intensity were calculated, and results are expressed relative to the control conditions (without metformin) in each experiment. Each histogram represents the mean of 5 independent experiments using primary AML samples. The vertical bars indicate the SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-02-269837/7/m_zh89991059730004.jpeg?Expires=1765910276&Signature=QlTQY6DGLJBXxUAaXJLwzDFG1WyPUOJDX805SB~syvqs8ExKGVr6v2aVDl46TqGa9iMUW67oifM5lZuN0ep7XavEGVru0vKwWs-kPYZ3sCyYAg2gXVjKdBqeW7eZVDIl1Jp-ZaPA~ab5Wi9vT-Gp0cAoGW6GKo5QsUU5IAMAHa03AAaj38pk-bSawNYqis1kyQVa4ZOQJwouMIe1wfcak6QxrQIGI-eNwAy~Gr2-zDluVdpHds74u~CORuWSWNLNxuk1raFtCSWdIzGxB1NSBbhZWWXepFksOeZ70UBAddeLtYa0XfAuOqPTXj~~ajmXvH79U6d3Lx4BhKruGz0b4Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. LKB1/AMPK activation using metformin strongly decreases AML cell survival, but does not affect normal hematopoietic cells ex vivo. (A) Top panel, Western blot analysis of CDK2, SCFSKP2, and p21CIP1 in primary AML cells treated or not with metformin. Bottom panel, proliferation was assessed in primary AML cells cultured without or with 5, 10, or 15mM metformin and then pulsed for 6 hours with [3H]thymidine. Ratios to the controls without inhibitor were calculated, and the results are presented as a mean of 5 independent experiments. The vertical bars indicate the SEM (B) clonogenic assays of primary AML cells (CFU-L) and normal CD34+ hematopoietic progenitors (erythroid, BFU-E; and granulo-macrophagic, CFU-GM). Cells were cultured for 24 hours without or with 5, 10, or 15mM metformin, washed twice in PBS buffer, and then plated in methylcellulose medium. We used 25μM LY294002 as a control for CD34+ clonogenic growth inhibition.9,49 Each histogram represents the mean of 5 independent experiments, performed in duplicate. The results are expressed as a ratio to the control incubation without inhibitor and presented as a mean of 5 independent experiments. Vertical bars indicate the SEM. (C) Top panel, Western blot analysis of caspase-3 and Mcl-1 expression in primary AML cells cultured for 24 hours without or with 5, 10, or 15mM metformin (sample 4 is representative of 5 independent experiments). Bottom panel, Annexin V binding assays of cells cultured without or with 5, 10, or 15mM metformin for 24 hours. Results are expressed separately as the mean of 5 primary AML samples and 5 CD34+ samples, respectively. The vertical bars indicate the SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/20/10.1182_blood-2010-02-269837/7/m_zh89991059730005.jpeg?Expires=1765910276&Signature=BUhngeZO-B4Cj~7EGYUTyjahl9tqVZdJqnvJ0CfZjkr7N8L2JaSwnjqjLyx4tsdSQ8lPvreNsmdPavW-pXIxUy2Vpfk4d00aFfWSXhj7eUaN8Jmx1dKsp2q-aUL8ititXK1s5ZFCm4YFWVIrx7XgRLxm1XvwE3Ka8CS5JY~8Jd-Y3gPOAtXB~LeKW7oOaHotxhEkocfVWuWYeW~5m9C7DgQ5bwp4xXYkpxM45yt0r~tgRW0WbeOxlMrAYHErjSo~CuZY7fVULTF1QIersdEmH4TFzWCwTyDNToKx9g6nbSZQGh1rkAoNEEnwIeR5AbTgV6mBsd7Ymantv-8O8CHuCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)