Abstract
A long-acting factor VIII (FVIII) as a replacement therapy for hemophilia A would significantly improve treatment options for patients with hemophilia A. To develop a FVIII with an extended circulating half-life, but without a reduction in activity, we have engineered 23 FVIII variants with introduced surface-exposed cysteines to which a polyethylene glycol (PEG) polymer was specifically conjugated. Screening of variant expression level, PEGylation yield, and functional assay identified several conjugates retaining full in vitro coagulation activity and von Willebrand factor (VWF) binding.PEGylated FVIII variants exhibited improved pharmacokinetics in hemophilic mice and rabbits. In addition, pharmacokinetic studies in VWF knockout mice indicated that larger molecular weight PEG may substitute for VWF in protecting PEGylated FVIII from clearance in vivo. In bleeding models of hemophilic mice, PEGylated FVIII not only exhibited prolonged efficacy that is consistent with the improved pharmacokinetics but also showed efficacy in stopping acute bleeds comparable with that of unmodified rFVIII. In summary site-specifically PEGylated FVIII has the potential to be a long-acting prophylactic treatment while being fully efficacious for on-demand treatment for patients with hemophilia A.
Introduction
Hemophilia A is caused by deficiencies in coagulation factor VIII (FVIII) and is the most common hereditary coagulation disorder, with an estimated incidence of 1 per 5000 males. FVIII circulates as a heterodimer composed of a heavy chain of approximately 200 kDa and a light chain of 80 kDa. The heavy chain contains the structurally related A1 and A2 domains, as well as a unique B domain, and the light chain comprises the A3, C1, and C2 domains1 (Figure 1A). The current treatment for hemophilia A involves intravenous injection of recombinant or plasma-derived human FVIII. Injections of FVIII are either given on demand in response to a bleeding event or as a prophylactic therapy that is administered 2 to 4 times a week. Although numerous studies have shown that prophylactic therapy decreases the complications of hemophilia A, the need for frequent intravenous injections creates barriers to patient compliance and affects patient quality of life.2
The requirement for frequent injections is primarily due to the short circulating FVIII half-life of 12 to 14 hours in patients.3,4 FVIII clearance from circulation has been partly attributed to specific binding to the low-density lipoprotein receptor-related protein (LRP1), a hepatic clearance receptor with broad ligand specificity.5-7 The low-density lipoprotein receptor was also shown to play a role in FVIII clearance, potentially by cooperating with LRP1.8 Both interactions are facilitated by binding to cell-surface heparan sulfate proteoglycans (HSPGs). FVIII plasma half-life in mice can be prolonged when LRP1 or both LRP1 and HSPGs are blocked.9 LRP1 binding sites on FVIII have been localized to residues 484 to 509 in A2,6 residues 1811 to 1818 in A3,7 and an epitope in the C2 domain.5 The asialoglycoprotein receptor, which is abundantly expressed in the liver, could also be involved in the catabolism of FVIII, and the binding to asialoglycoprotein receptor is primarily mediated by the B domain of FVIII.10 In addition, the stability of FVIII in circulation relies on its interaction with von Willebrand factor (VWF). The half-life of FVIII is reduced to less than 3 hours in patients with type 3 von Willebrand disease (VWD).11 Studies in animal models indicate that the decreased FVIII half-life seen in VWD can be partially compensated by blockade of the LRP1 receptor12 ; thus, prolongation of FVIII half-life by VWF may be partially due to preventing the interaction with LRP1. VWF has also been shown to protect FVIII from proteolysis.13
PEGylation has been shown to increase the half-life and reduce immunogenicity of protein therapeutics. Regulatory approval has been granted for 8 PEGylated proteins.14,15 PEGylation is the covalent attachment of long-chained chemically activated polyethylene glycol (PEG) molecules to proteins. The most common method used for PEGylation is the attachment of the PEG moiety to lysine residues or N-terminal amines that are present in the native protein, but this often leads to a loss of potency because of steric interference of the binding interaction between the protein and its target.16 This strategy is even more problematic for FVIII because it is required to interact with multiple partners such as VWF, coagulation factor X (FX), and activated factor IX (FIXa) for full activity. Reagents targeting conjugation to amine groups can randomly react to ϵ-amine group of lysines, α-amine group of N-terminal amino acids, and δ-amine group of histidines.17 Full-length FVIII has 158 lysines, 2 N-termini, and 75 histidines. Thus, not controlling the site of PEGylation on FVIII when using the amine chemistry has led to loss of coagulation activity and impairment of binding to VWF.18 An additional drawback of random PEGylation is the Gaussian distribution of multi-PEGylated, mono-PEGylated, and un-PEGylated FVIII. The heterogeneity in product profile would significantly complicate the reliable synthesis of the therapeutic protein required for consistent effectiveness.
With site-directed PEGylation, however, the PEG is targeted only to the designated site(s). The potential loss of activity could probably be avoided or minimized because PEGylation occurs in a controlled manner, and a homogeneous product is possible. With only a single site for attachment, conjugation can be saturated to maximize yield. An option for site-specific PEGylation is cysteine-specific conjugation with PEG-maleimide. FVIII has 4 cysteines in the B domain and 19 cysteines in the other domains. Of the 19 cysteines in B domain–deleted (BDD) FVIII, 16 form disulfides and the other 3 are free cysteines. The structural model of BDD-FVIII suggests that all 3 free cysteines are buried19-22 and would not be accessible for reaction with a PEG polymer.
By introducing cysteine mutations on the surface of BDD-FVIII through site-directed mutagenesis, we have successfully achieved site-specific PEGylation of BDD-FVIII. In the current study, different molecular weight PEGs have been conjugated to sites spanning all 5 domains (A1, A2, A3, C1, and C2) of BDD-FVIII. The PEGylated FVIII variants have been analyzed to determine the efficiency and specificity of the PEGylation reaction and to examine the effects of PEGylation on FVIII activity. Finally, the circulating half-lives of PEGylated FVIII variants have also been evaluated in a number of animal species.
Methods
Site-directed mutagenesis of FVIII
To generate substrates for site-directed PEGylation of FVIII, a cysteine codon was introduced into the BDD-FVIII SQN23 (GeneBank accession, NM_000132.31; protein sequence ABV90867) at the desired site using Quick-Change II site-directed mutagenesis kit (Stratagene). The amino acid numbering of the introduced cysteine is based on the numbering of mature full-length FVIII amino acid sequence. After mutagenesis, appropriate fragments, containing the sequence verified mutation, were transferred into the FVIII backbone in the mammalian expression vector described previously.24
Expression of FVIII Cys variants
The FVIII variants were transfected into HKB11 cells (ATCC CRL-12568)25 with 293 Fectin Transfection Reagent (Invitrogen). To establish stable cell lines, at 3 days after transfection, the transfected cells were placed under selection with 50 μg/mL Hyg B in a growth medium supplemented with 5% fetal bovine serum. The Hyg B–resistant colonies were screened for FVIII expression by Coatest (Chromogenix). The FVIII-expressing stable cells were then adapted to a serum-free medium for large-scale expression.
Small-scale screening for PEGylation efficiency
Supernatant (5-20 mL) from HKB11 cells at 3 days after transfection was concentrated with the use of an Amicon-centra Ultra device MWCO 30K. The proteins were incubated with approximately 30 μL of anti-FVIII antibody C7F7 immobilized on resin overnight at 4°C, washed, eluted, dialyzed, and reduced with Tris-(2-carboxyethyl)-phosphine (TCEP). After removing the reductant, the reduced FVIII variants were conjugated with 5 kDa of PEG-maleimide (NOF America Corporation). The PEG-conjugated FVIII variants were analyzed along with un-PEGylated FVIII variants by Western blotting, and the conjugation efficiency was estimated according to the shift of conjugated FVIII.
Purification and PEGylation of FVIII Cys muteins
Cell culture supernatant containing the FVIII variant was concentrated by ultrafiltration and then applied to an immunoaffinity column conjugated with an anti–light chain antibody, C7F7. FVIII eluates were further purified by ion-exchange column chromatography. The purified FVIII Cys variants were reduced with TCEP and then incubated with PEG of sizes ranging from 5 to 60 kDa (NOF America Corporation) for conjugation at 4°C overnight. PEGylated FVIII was separated from un-PEGylated FVIII on a cation exchange column (GE HealthCare).
SDS-PAGE and immunoblotting analyses of FVIII Cys variants
FVIII was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie Blue for protein or barium iodide for PEG. For immunoblotting, FVIII samples separated by SDS-PAGE were blotted onto nitrocellulose membranes. The membranes were blocked with 5% milk before incubation with primary anti-FVIII antibodies (GM A3 for FVIII light chain and R8B12 for FVIII heavy chain, both from Green Mountain Antibodies), and then incubated with secondary antibodies and processed with Pico Kits (Pierce Biotechnology).
Measurement of FVIII activity
FVIII activity was measured by a 2-stage chromogenic assay with the use of Coatest (Chromogenix). The FVIII reference standards were calibrated against the World Health Organization seventh FVIII International Standard. One-stage activated partial thromboplastin time assays were performed with the use of kits from Trinity Bioscience (Amax/Alexon). For FVIII activity in the presence of monoclonal antibodies (mAbs) against FVIII (R8B12, mAb 413 [a kind gift from Dr Evgueni L. Saenko's laboratory, University of Maryland, Baltimore] and ESH4 [American Diagnostica]), FVIII (at 0.003 IU/mL) was incubated with the antibodies (diluted as indicated) at 37°C before being analyzed by Coatest. For Coatest analysis of pharmacokinetic (PK) samples, rFVIII and PEG-FVIII variants in VWF knockout (KO) mouse and rabbit plasma were first captured with a mAb R8B12.
MALDI-MS analysis
The FVIII Cys variants, before and after PEGylation, were digested with thrombin (Sigma-Aldrich) and analyzed by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). The Voyager DE MALDI mass spectrometer (Applied Biosystems) was run in positive, linear mode, scanning 20 to 150 kDa. A laser power of approximately 2300 kV was applied, to minimize cleavage of the linked PEG. Samples were calibrated against bovine serum albumin.
Real-time biospecific interaction analysis of the binding of FVIII to VWF
VWF-FVIII binding constants were determined with the use of surface plasmon resonance (Biacore 3000) by flowing the samples over a VWF immobilized chip. Approximately 250 resonance units (RU) of VWF (Haemtech) was immobilized on sensor chip CM5 by an amine-coupling method. Full-length rFVIII, BDD-FVIII, and PEG-FVIII variants were tested on the VWF-immobilized chip at 5 different protein concentrations from 0.45 to 16nM. A280 total protein values were used for BDD-FVIII and PEG-FVIII variants, based on molecular extinction coefficient. The reference subtracted binding curves were double referenced by subtracting the blank buffer curve. All of the binding curves of each sample were fit into 1:1 Langmuir binding model to determine simultaneous kon and koff for the VWF-FVIII interaction and analyzed by Biacore Control software Version 4.1.
Animals
FVIII KO (exon 16 KO; The Jackson Laboratory B6;129S4-F8tm1Kaz/J) hemophilia A (HemA) mice26 were obtained from Haig Kazazian at the University of Pennsylvania. VWF KO mice, B6.129S2-VWFtm1Wgr/J27 were obtained from Denisa Wagner at Harvard Medical School. New Zealand white rabbits were purchased from Western Oregon Rabbit Company. All study protocols were approved by the Bayer Healthcare Institutional Animal Care and Use Committee and were conducted in accordance with the US Department of Agriculture Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Pharmacokinetics
HemA mice or VWF KO mice were dosed with 100 to 250 IU/kg BDD-FVIII or PEG-FVIII variants through the tail vein. At each specified time point, 5 mice from each treatment group were humanely sacrificed by CO2, followed immediately by blood collection from the vena cava. New Zealand White rabbits were administered 100 IU/kg full-length rFVIII (Kogenate FS; Bayer) or PEG-FVIII variants through the lateral ear vein. After dosing, blood samples were collected serially through the medial artery into collection tubes containing 3.8% sodium citrate at predesignated time points. FVIII activities in HemA mouse plasma were determined by Coatest. FVIII activity in VWF KO mouse plasma and rabbit plasma were measured by Coatest after a human FVIII-specific mAb R8B12 capture. The plasma FVIII activity versus time was plotted and fitted by a noncompartmental model and linear trapezoidal (Linear/Log Interpolation) method in WinNonLin 5.0.1 (Pharsight) to derive major PK parameters.
Bleeding models in HemA mice
For tail clip studies, HemA mice were anesthetized with isoflurane, and the tails were prewarmed at 37°C in 0.9% saline for 10 minutes. Animals were then injected with 100 μL/mouse full-length rFVIII (Kogenate FS; Bayer) or PEG-FVIII variants through the right jugular vein. Five minutes later, the tail was cut at 4 mm from the tip and immediately placed into a new tube containing 10 mL of saline (prewarmed at 37°C). The blood collected over 40 minutes was quantified by weighing tubes before and after the blood collection. The tail vein transection studies were performed as described previously,28 except that HemA mice were dosed with full-length rFVIII or PEG-FVIII variants by tail vein injection at 24 to 48 hours before the transection of 1 lateral tail vein.
Statistical analyses
Mann-Whitney test, Kruskal-Wallis test with Dunn post test, F test, survival curves, and log-rank test were performed with GraphPad Prism 5 (GraphPad Software Inc). A 2-tailed P value less than .05 was considered statistically significant.
Results
Rationale for site selection
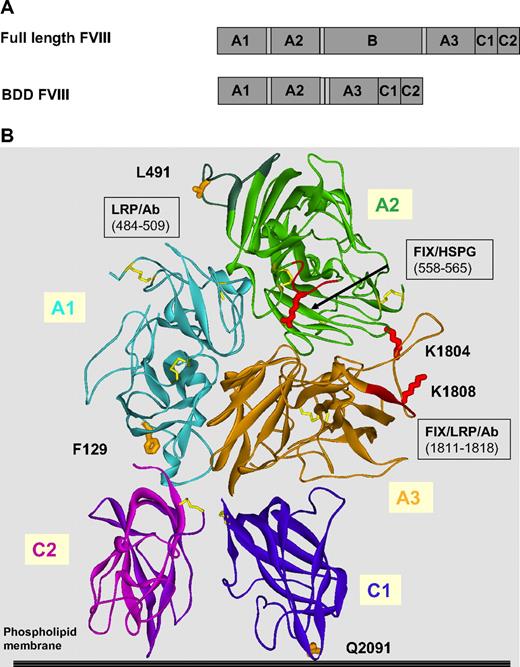
All of the cysteines in BDD-FVIII are either involved in disulfide linkages or buried within the interior of the molecule19-22 ; thus, they are inaccessible to modification by a large PEG polymer. This opens up the possibility of site-specific PEGylation of FVIII through the introduction of cysteines onto the surface of FVIII. On the basis of the structure of FVIII, single cysteine point mutations were made in the A2 and A3 domains at reported LRP1 binding sites on FVIII (Table 1; Figure 1B). In an attempt to systematically cover the surface of FVIII, numerous other cysteine mutations, spaced by 2 to 3 nm, were introduced into FVIII. Finally, based on improved PK of single mutants, FVIII variants with double cysteine mutations were also constructed. The positions of a subset of representative cysteine mutations on the 3-dimensional model of FVIII21,22 are shown in Figure 1B.
Small scale screening of FVIII cysteine variants for PEGylation
| Cys mutation . | Transient expression, IU/mL . | PEGylation efficiency* . | ||
|---|---|---|---|---|
| Position . | Domain . | H-PEG . | L-PEG . | |
| Y81C | A1 | 0.36 | ++ | − |
| F129C | A1 | 0.25 | ++ | − |
| K377C | A2 | 0.11 | +++ | − |
| H378C | A2 | 0.15 | +++ | − |
| K422C | A2 | 0.28 | − | − |
| Q468C | A2 | 0.69 | + | − |
| L491C | A2 | 0.60 | +++ | − |
| L504C | A2 | 0.38 | + | − |
| K556C | A2 | 0.09 | ++ | − |
| K570C | A2 | <0.05 | − | − |
| D1795C | A3 | 0.27 | − | + |
| Q1796C | A3 | 0.29 | − | + |
| R1803C | A3 | 0.11 | − | ++ |
| K1804C | A3 | 0.18 | − | +++ |
| K1808C | A3 | 0.54 | − | ++ |
| N1810C | A3 | 0.21 | − | ++ |
| T1812C | A3 | 0.16 | − | − |
| K1813C | A3 | 0.35 | − | − |
| N1864C | A3 | 0.15 | − | ++ |
| T1911C | A3 | 0.28 | − | +++ |
| N2118C | C1 | 0.13 | − | + |
| Q2091C | C1 | 0.20 | − | ++ |
| Q2284C | C2 | 0.17 | − | + |
| L491C/K1808C | A2/A3 | 0.11 | +++ | ++ |
| L491C/K1804C | A2/A3 | 0.13 | +++ | +++ |
| BDD FVIII | NA | 0.30 | − | − |
| Cys mutation . | Transient expression, IU/mL . | PEGylation efficiency* . | ||
|---|---|---|---|---|
| Position . | Domain . | H-PEG . | L-PEG . | |
| Y81C | A1 | 0.36 | ++ | − |
| F129C | A1 | 0.25 | ++ | − |
| K377C | A2 | 0.11 | +++ | − |
| H378C | A2 | 0.15 | +++ | − |
| K422C | A2 | 0.28 | − | − |
| Q468C | A2 | 0.69 | + | − |
| L491C | A2 | 0.60 | +++ | − |
| L504C | A2 | 0.38 | + | − |
| K556C | A2 | 0.09 | ++ | − |
| K570C | A2 | <0.05 | − | − |
| D1795C | A3 | 0.27 | − | + |
| Q1796C | A3 | 0.29 | − | + |
| R1803C | A3 | 0.11 | − | ++ |
| K1804C | A3 | 0.18 | − | +++ |
| K1808C | A3 | 0.54 | − | ++ |
| N1810C | A3 | 0.21 | − | ++ |
| T1812C | A3 | 0.16 | − | − |
| K1813C | A3 | 0.35 | − | − |
| N1864C | A3 | 0.15 | − | ++ |
| T1911C | A3 | 0.28 | − | +++ |
| N2118C | C1 | 0.13 | − | + |
| Q2091C | C1 | 0.20 | − | ++ |
| Q2284C | C2 | 0.17 | − | + |
| L491C/K1808C | A2/A3 | 0.11 | +++ | ++ |
| L491C/K1804C | A2/A3 | 0.13 | +++ | +++ |
| BDD FVIII | NA | 0.30 | − | − |
The activity of FVIII Cys variants transiently expressed in HKB11 cells was measured by a chromogenic assay.
H-PEG indicates heavy-chain PEGylation; and L-PEG, light-chain PEGylation.
The FVIII variants were conjugated with 5-kDa PEG, and the PEGylation efficiency was estimated by comparing the intensity of the PEGylated band with unPEGylated band on a SDS-PAGE and Western blot. The +++ indicates approximately greater than 80% PEGylation yield, ++, approximately 30% to 70% yield; + approximately 10% to 30% yield; −, approximately less than 10% yield.
Structure of BDD-FVIII and locations of cysteine mutations. (A) The domain structure of full-length FVIII and BDD-FVIII. (B) The structure of BDD-FVIII as reported (Protein Data Bank ID, 3CDZ)21 and the individual domains (A1, A2, A3, C1, and C2) are color coded. Positions for selected cysteine mutations and site-directed PEGylation (F129, L491, K1804, K1808, and Q2091) are highlighted. Positions in FVIII that interact with FIX, FX, LRP, HSPG are indicated.
Structure of BDD-FVIII and locations of cysteine mutations. (A) The domain structure of full-length FVIII and BDD-FVIII. (B) The structure of BDD-FVIII as reported (Protein Data Bank ID, 3CDZ)21 and the individual domains (A1, A2, A3, C1, and C2) are color coded. Positions for selected cysteine mutations and site-directed PEGylation (F129, L491, K1804, K1808, and Q2091) are highlighted. Positions in FVIII that interact with FIX, FX, LRP, HSPG are indicated.
Activity and PEGylation efficiency of FVIII Cys variants
FVIII variants carrying the engineered cysteines (FVIII Cys variants) were produced by transfection of HKB11 cell line, and FVIII activity was detected in conditioned media by the chromogenic assay. Almost all of the FVIII variants exhibited FVIII activity, although the activity level varied depending on mutation sites (Table 1). FVIII Cys variants were purified by affinity chromatography with the use of immobilized antibody C7F7, which is specific for an epitope on the a3 domain of FVIII. A mild reduction protocol was developed to selectively remove the tissue culture medium–derived cysteine or other reductants capped on the introduced free cysteine, without irreversibly reducing the existing disulfide bonds. The mild TCEP reduction did not affect FVIII activity (data not shown). The reduced FVIII Cys variant was then conjugated with the PEG-maleimide reagent.
To rapidly screen a large number of FVIII Cys variants for PEGylation efficiency, a small-scale PEGylation process, based on affinity capture of FVIII from conditioned media, was developed with 5 kDa PEG. As shown in Table 1, the PEGylation efficiency is site dependent and varies, ranging from less than 10% (FVIII variants K422C, K570C, T1812C, and T1813C) to greater than 80% (FVIII variants K337C, H378C, L491C, K1804C, and T1911C).
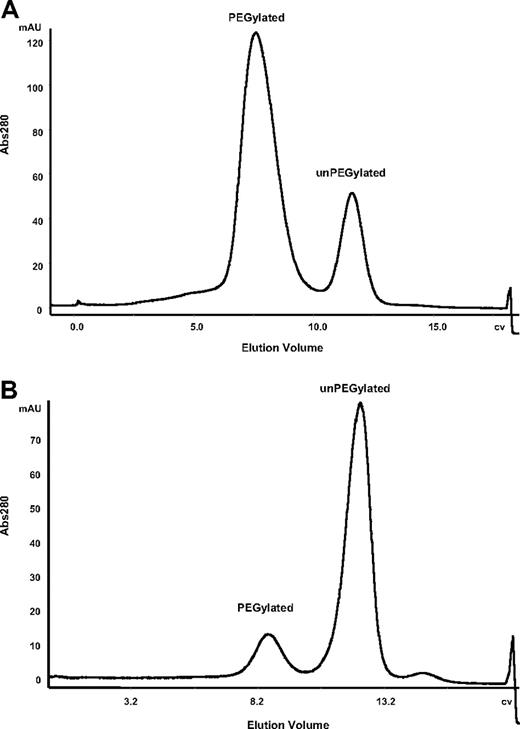
After PEGylation, the PEGylated FVIII was separated from free PEG and un-PEGylated FVIII by cation exchange chromatography. PEG shields ionic charge on FVIII and results in the PEGylated molecule eluting at a lower salt concentration than the unPEGylated protein. The PEGylation efficiency in the small-scale screening (Table 1) was confirmed in the larger scale PEGylation and subsequent cation exchange chromatography. An elution profile of FVIII K1804C and K1808C conjugated to a 60-kDa PEG is shown in Figure 2. On the basis of the A280 profiles, it was estimated that FVIII K1804C was more efficiently PEGylated (∼ 70%) than FVIII K1808C (∼ 15%) despite the proximity of these 2 amino acids in the linear sequence.
PEGylation efficiency varies depending on the site of conjugation. Chromatographic profile for PEGylated and un-PEGylated FVIII separated on a SP cation exchange column. FVIII K1804C (A) and FVIII K1808C (B) were conjugated with a 60-kDa PEG. CV indicates column volume.
PEGylation efficiency varies depending on the site of conjugation. Chromatographic profile for PEGylated and un-PEGylated FVIII separated on a SP cation exchange column. FVIII K1804C (A) and FVIII K1808C (B) were conjugated with a 60-kDa PEG. CV indicates column volume.
Site specificity in PEGylation of FVIII Cys variants
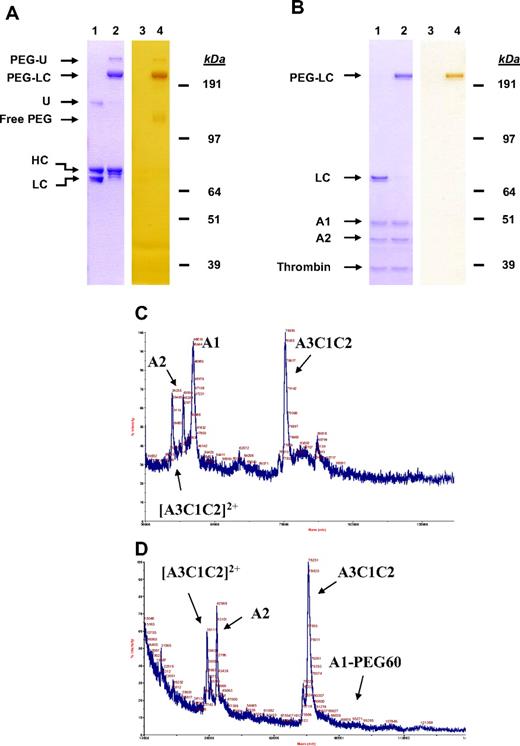
The conjugation of PEG is specific to the introduced Cys as exemplified by FVIII K1804C in the light chain (Figure 3). After the conjugation of FVIII K1804C with a 60-kDa PEG, the light chain was shifted from approximately 80 kDa to above 190 kDa apparent molecular weight on a Coomassie Blue–stained SDS-PAGE (Figure 3A lanes 1-2). Staining of PEG molecule with barium iodide confirmed that the up-shifted band was a PEGylated molecule (Figure 3A lanes 3-4). Similar analysis for other FVIII Cys muteins also confirmed that PEGylation was specifically targeted to the respective Cys-containing chain (data not shown).
Cysteine-directed PEGylation of FVIII is highly specific. (A) FVIII K1804C before (lanes 1,3) and after (lanes 2,4) conjugation with a 60-kDa PEG was separated on SDS-PAGE and stained with Coomassie Blue for protein (lanes 1,2) or with barium iodide for PEG (lanes 3 and 4). (B) FVIII K1804C before (lanes 1,3) and after (lanes 2,4) conjugation with a 60-kDa PEG was digested with thrombin, separated on SDS-PAGE, and stained with Coomassie Blue for protein (lanes 1,2) or with barium iodide for PEG (lanes 3,4). (C-D) FVIII F129C before (C) and after (D) conjugation with a 60-kDa PEG was digested with thrombin and analyzed by MALDI-MS. Samples were spotted onto a MALDI target (0.3 μL) with the same volume of a MALDI matrix composed of sinappinic acid (Sigma-Aldrich) in 0.1% trifluoroacetic acid in water (70%) and acetonitrile (30%). HC indicates heavy chain; and LC, light chain.
Cysteine-directed PEGylation of FVIII is highly specific. (A) FVIII K1804C before (lanes 1,3) and after (lanes 2,4) conjugation with a 60-kDa PEG was separated on SDS-PAGE and stained with Coomassie Blue for protein (lanes 1,2) or with barium iodide for PEG (lanes 3 and 4). (B) FVIII K1804C before (lanes 1,3) and after (lanes 2,4) conjugation with a 60-kDa PEG was digested with thrombin, separated on SDS-PAGE, and stained with Coomassie Blue for protein (lanes 1,2) or with barium iodide for PEG (lanes 3,4). (C-D) FVIII F129C before (C) and after (D) conjugation with a 60-kDa PEG was digested with thrombin and analyzed by MALDI-MS. Samples were spotted onto a MALDI target (0.3 μL) with the same volume of a MALDI matrix composed of sinappinic acid (Sigma-Aldrich) in 0.1% trifluoroacetic acid in water (70%) and acetonitrile (30%). HC indicates heavy chain; and LC, light chain.
To more accurately identify the site of PEGylation, purified PEG-FVIII was subjected to MALDI-MS analysis after thrombin digestion. MALDI-MS analysis of FVIII F129C before PEG conjugation detected A1 (46 kDa), A2 (43 kDa), and A3C1C2 (78 kDa) fragments at the predicted masses (Figure 3B). However, after conjugation of FVIII F129C with a 60-kDa PEG, the A2 and A3C1C2 fragments were still present, whereas the A1 fragment was no longer evident at 46 kDa, consistent with PEGylation in the A1 domain (Figure 3C). A new peak representing A1-PEG60 was not observed at the predicted 110-kDa position because the PEGylated molecule is less efficiently ionized during MS analysis (Figure 3C). Further characterization by peptide mapping and MS has confirmed that PEG was conjugated at F129C (J.S. et al, unpublished data, November 17, 2009).
PEGylated FVIII is fully active in vitro
Most FVIII Cys variants, including L491C, K1804C, and K1808C, exhibited no effect on FVIII activity by the addition of PEG up to 60 kDa, although the PEGylation sites are in relative proximity to FIX or FX binding sites (Figure 1). The specific activity, as measured by the 2-stage chromogenic assay of these 60-kDa PEG-FVIII variants after cation exchange chromatography, was similar to that before PEGylation (Table 2). Full retention of activity was also seen when the 1-stage activated partial thromboplastin time assay was used; however, the activity was dependent on the activator used for the assay (L. Tang, manuscript in preparation). However, FVIII Q2091C showed 59% reduction in specific activity after conjugation with a 60-kDa PEG. The residue Q2091 is close to the phospholipid-binding surface, and PEGylation may impede phospholipid binding. In addition, the conjugation of FVIII F129C/K1804C with one 60-kDa PEG at each site also resulted in a 43% loss of FVIII activity.
Specific activity of FVIII variants before and after conjugation with 60-kDa PEG
| Sample ID . | Domain(s) of PEGylation . | Specific activity, IU/mg . | |
|---|---|---|---|
| Before PEGylation . | After PEGylation . | ||
| BDD FVIII | N/A | 8855 | N/A |
| F129C | A1 | 10521 | 9863 |
| L491C | A2 | 9428 | 8804 |
| K1804C | A3 | 9357 | 9717 |
| K1808C | A3 | 9754 | 11560 |
| Q2091 | C1 | 10546 | 4343 |
| L491C/K1804C | A2 + A3 | 8184 | 9499 |
| F129C/K1804C | A1 + A3 | 10695 | 6085 |
| Sample ID . | Domain(s) of PEGylation . | Specific activity, IU/mg . | |
|---|---|---|---|
| Before PEGylation . | After PEGylation . | ||
| BDD FVIII | N/A | 8855 | N/A |
| F129C | A1 | 10521 | 9863 |
| L491C | A2 | 9428 | 8804 |
| K1804C | A3 | 9357 | 9717 |
| K1808C | A3 | 9754 | 11560 |
| Q2091 | C1 | 10546 | 4343 |
| L491C/K1804C | A2 + A3 | 8184 | 9499 |
| F129C/K1804C | A1 + A3 | 10695 | 6085 |
The activity of non-PEGylated and PEGylated FVIII were measured by a chromogenic assay, and the protein concentration was determined by A280 absorbance based on extinction coefficient.
N/A indicates not applicable.
PEGylated FVIII retains affinity to VWF
The capability of PEGylated FVIII variants to bind VWF was assessed with Biacore analysis. Among the 60-kDa PEG-FVIII variants tested, most showed a Kd around 0.5nM (Table 3), which is comparable with the reported Kd of 0.2 to 0.5nM for full-length rFVIII and BDD-FVIII.29-32 Although the association rate of PEG-FVIII was decreased approximately 2- to 3-fold in comparison to rFVIII or BDD-FVIII, the dissociation rate was essentially unchanged. The maximum VWF binding capacity as represented by RU max also showed no significant difference between PEG-FVIII and rFVIII or BDD-FVIII, with RU values presented in Table 3 being within assay variation. The decrease in association rate was attributed to the PEGylation rather than the Cys mutation by additionally analyzing the non-PEGylated mutants (data not shown). The di-PEGylated FVIII L491C/K1804C appeared to behave similarly to other mono-PEGylated FVIII in VWF binding. However, the di-PEGylated FVIII F129C/K1804C showed a more significant decrease in binding of VWF.
BIAcore analysis of VWF binding of FVIII variants PEGylated with 60-kDa PEG
| Sample . | ka, 1/ms . | kd, 1/s . | Rmax, RU . | Kd, nM . |
|---|---|---|---|---|
| Full-length FVIII | 3.01 × 10−6 | 9.10 × 10−4 | 42.2 | 0.30 |
| BDD-FVIII | 5.23 × 10−6 | 8.22 × 10−4 | 39.1 | 0.16 |
| K1804C | 1.57 × 10−6 | 6.34 × 10−4 | 32.8 | 0.4 |
| F129C | 1.48 × 10−6 | 8.25 × 10−4 | 27.7 | 0.56 |
| L491C/K1804C | 1.19 × 10−6 | 6.84 × 10−4 | 26.5 | 0.57 |
| F129C/K1804C | 7.99 × 10−5 | 8.93 × 10−4 | 23.2 | 1.12 |
| Sample . | ka, 1/ms . | kd, 1/s . | Rmax, RU . | Kd, nM . |
|---|---|---|---|---|
| Full-length FVIII | 3.01 × 10−6 | 9.10 × 10−4 | 42.2 | 0.30 |
| BDD-FVIII | 5.23 × 10−6 | 8.22 × 10−4 | 39.1 | 0.16 |
| K1804C | 1.57 × 10−6 | 6.34 × 10−4 | 32.8 | 0.4 |
| F129C | 1.48 × 10−6 | 8.25 × 10−4 | 27.7 | 0.56 |
| L491C/K1804C | 1.19 × 10−6 | 6.84 × 10−4 | 26.5 | 0.57 |
| F129C/K1804C | 7.99 × 10−5 | 8.93 × 10−4 | 23.2 | 1.12 |
Approximately 250 RU of vWF (Haemtech) was immobilized on either flow cell (Fc) 2 or 4 of Sensor chip CM5 at a 10 μg/mL protein concentration in 10mM sodium acetate, pH 5.5, buffer by an amine coupling method. Flow cell 1 or 3 was used as a reference cell and had been activated and deactivated by amine coupling reagents (EDC/NHS and Ethanolamine). Full-length FVIII, BDD-FVIII, and the PEG-FVIII muteins were tested on the VWF immobilized chip at 5 different protein concentrations with at least 2 concentrations run in duplicate (running buffer, 0.45-16nM in 50mM Tris, 150mM NaCl, 0.05% Surfactant P20, pH 7.2). All samples were allowed to associate with immobilized VWF for 5 minutes at 50 μL/minute flow rate and dissociate in running buffer for 3 minutes. The sensor chip was regenerated with 200mM CaCl2.2H20 in assay running buffer. The reference subtracted (Fc 2-1 or Fc 4-3) binding curves were double referenced by subtracting blank buffer curve.
PEGylated FVIII remains active in the presence of monoclonal antibodies directed against the PEGylated epitope
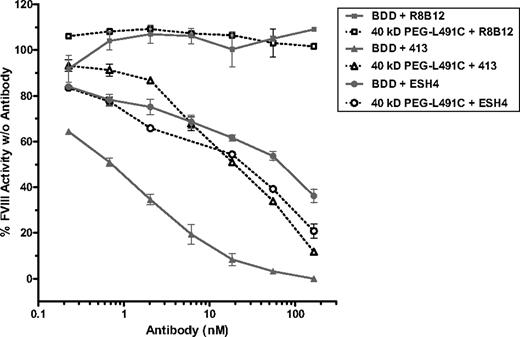
FVIII inhibitory antibodies most frequently target FVIII residues 484 to 508 of the A2 domain, residues 1778 to 1823 of the A3 domain, residues 2248 to 2312 and 2315 to 2330 of the C2 domains (see reviews of Ananyeva et al33 and Shima34 ). Some of the cysteine mutations are located in these regions, and the addition of PEG may interfere with the binding by inhibitor antibodies. To test this hypothesis, we determined the activity of PEG-FVIII variants in the presence of FVIII inhibitory antibodies. Nonsaturating amounts of BDD-FVIII or 40-kDa PEG-L491C were incubated with increasing amounts of mAb 413, an antibody that binds to residues 484 to 508 and inhibits FVIIIa function.35,36 The controls are a noninhibitory antibody, R8B12, which binds to the A2 domain (497-510 and 584-593),37 and an inhibitory antibody, ESH4, which targets the C2 domain (2303-2332) and inhibits its binding to platelets.38 By a chromogenic assay, BDD-FVIII and 40-kDa PEG L491C showed comparable activity in the presence of R8B12 (Figure 4). Both proteins were similarly inhibited by ESH4 antibody that targets epitope(s) outside of the PEGylation region (C2 vs A2 domain), whereas 40-kDa PEG L491C retained 40% to 60% more activity than BDD-FVIII in the presence of mAb 413 (Figure 4).
PEGylated FVIII sustained more activity relative to BDD-FVIII in the presence of specific inhibitory monoclonal anti-FVIII antibodies. Percentage of residual FVIII activity was determined by chromogenic assay in the presence versus absence of anti-FVIII antibodies. Unsaturated amount (0.003 IU/mL) of BDD-FVIII or 40-kDa PEG-L491C was incubated with increasing concentration of noninhibitory anti-FVIII mAb R8B12 specific for residues 497 to 510 and 584 to 593, or inhibitory mAb 413 against epitope at 484 to 509, or inhibitory anti-FVIII mAb ESH4 targeting C-terminus of FVIII (2303-2332). Results presented are means and ranges of duplicate experiments.
PEGylated FVIII sustained more activity relative to BDD-FVIII in the presence of specific inhibitory monoclonal anti-FVIII antibodies. Percentage of residual FVIII activity was determined by chromogenic assay in the presence versus absence of anti-FVIII antibodies. Unsaturated amount (0.003 IU/mL) of BDD-FVIII or 40-kDa PEG-L491C was incubated with increasing concentration of noninhibitory anti-FVIII mAb R8B12 specific for residues 497 to 510 and 584 to 593, or inhibitory mAb 413 against epitope at 484 to 509, or inhibitory anti-FVIII mAb ESH4 targeting C-terminus of FVIII (2303-2332). Results presented are means and ranges of duplicate experiments.
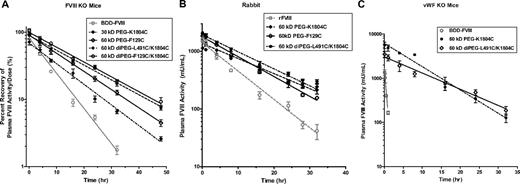
PEGylated FVIII shows improved pharmacokinetics
The PK of the PEGylated FVIII variants was evaluated in HemA mice and rabbits (Figure 5A-B). In HemA mice, most PEG-FVIII variants tested have greater than 90% recovery of FVIII activity in plasma 5 minutes after dosing, except for 30-kDa PEG-K1804C for which only 72% of the FVIII dose was recovered (Figure 5A). The terminal half-life (t1/2) of FVIII is extended from 5.9 hours in BDD-FVIII to 9.8 hours in 30-kDa PEG-K1804C, 11.1 hours in 60-kDa PEG-F129C, and 13.6 hours in both 60-kDa diPEG-F129C/K1804C and 60-kDa diPEG-L491C/K1804C. These latter 2 FVIII molecules were conjugated with 60-kDa PEG molecules on each of their 2 mutation sites. The clearance is progressively reduced from 5.9 mL/h/kg (BDD-FVIII) to 5.0 mL/h/kg in 30-kDa PEG-K1804C, 3.8 mL/h/kg in 60-kDa PEG-F129C, and 2.5 to 2.8 mL/h/kg in 60-kDa diPEG-F129C/K1804C and 60-kDa diPEG-L491C/K1804C.
Improved PK of PEGylated FVIII in mice and rabbits. (A) HemA mice received 100 to 200 IU/kg BDD-FVIII, 30-kDa PEG-K1804C, 60-kDa PEG-F129C, 60-kDa diPEG-L491C/K1804C, or 60-kDa diPEG-F129C/K1804C. Citrated blood was collected at 5 minutes and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are the percentage of recovery of FVIII activity in plasma after normalization with the input dose as mean ± SEM from 5 mice in each treatment group at each time point. (B) New Zealand white rabbits were dosed with 100 IU/kg full-length rFVIII, 60-kDa PEG-K1804C, 60-kDa PEG-F129C, or 60-kDa diPEG-L491C/K1804C. Citrated blood was collected at 5 minutes and 1, 4, 8, 16, 24, and 32 hours after injection. Plasma FVIII activities were determined by Coatest after R8B12 mAb capture. Results presented are mean ± SEM from 5 rabbits/treatment at each time point. (C) VWF KO mice were dosed with 250 IU/kg BDD-FVIII, 60-kDa PEG-K1804C, or 60-kDa diPEG-L491C/K1804C. Citrated blood was collected at 5, 15, and 30 minutes and 1, 2, 4, 6, and 8 hours from BDD-FVIII–treated mice or at 5 minutes and 4, 8, 16, 24, 32, and 48 hours from PEG-FVIII–treated mice. Plasma FVIII activities were determined by Coatest after R8B12 mAb capture. Results presented are mean ± SEM from 5 mice/treatment at each time point. The decay curves were fit with a noncompartmental model in WinNonLin.
Improved PK of PEGylated FVIII in mice and rabbits. (A) HemA mice received 100 to 200 IU/kg BDD-FVIII, 30-kDa PEG-K1804C, 60-kDa PEG-F129C, 60-kDa diPEG-L491C/K1804C, or 60-kDa diPEG-F129C/K1804C. Citrated blood was collected at 5 minutes and 4, 8, 16, 24, 32, and 48 hours after tail vein injection. Plasma FVIII activities were measured by Coatest. Results presented are the percentage of recovery of FVIII activity in plasma after normalization with the input dose as mean ± SEM from 5 mice in each treatment group at each time point. (B) New Zealand white rabbits were dosed with 100 IU/kg full-length rFVIII, 60-kDa PEG-K1804C, 60-kDa PEG-F129C, or 60-kDa diPEG-L491C/K1804C. Citrated blood was collected at 5 minutes and 1, 4, 8, 16, 24, and 32 hours after injection. Plasma FVIII activities were determined by Coatest after R8B12 mAb capture. Results presented are mean ± SEM from 5 rabbits/treatment at each time point. (C) VWF KO mice were dosed with 250 IU/kg BDD-FVIII, 60-kDa PEG-K1804C, or 60-kDa diPEG-L491C/K1804C. Citrated blood was collected at 5, 15, and 30 minutes and 1, 2, 4, 6, and 8 hours from BDD-FVIII–treated mice or at 5 minutes and 4, 8, 16, 24, 32, and 48 hours from PEG-FVIII–treated mice. Plasma FVIII activities were determined by Coatest after R8B12 mAb capture. Results presented are mean ± SEM from 5 mice/treatment at each time point. The decay curves were fit with a noncompartmental model in WinNonLin.
PEGylated FVIII variants also exhibited different degrees of PK improvement in rabbits, depending on PEGylation site. As shown (Figure 5B), 60-kDa PEG-K1804C and 60-kDa PEG-F129C resulted in a significant increase in t1/2 to 12 (± 1.3) hours and 9.8 (± 0.5) hours, respectively, in comparison with 6.7 (± 0.5) hours for full-length rFVIII. The mean residence time of the PEG-K1804C (17.5 ± 1.3 hours) and PEG-F129C (12.9 ± 0.3 hours) was also significantly longer than that of rFVIII (8.3 ± 0.5 hours). However, di-PEGylation did not lead to a corresponding significant effect on half-life extension compared with single PEGylation. The t1/2 of 60-kDa diPEG-L491C/K1804C (11.0 ± 0.4 hours) was similar to that of single PEGylated FVIII variants.
In addition, PEGylation significantly stabilizes FVIII in the absence of VWF, as shown in VWF KO mice. In contrast to BDD-FVIII, which cleared rapidly without the protection from VWF, resulting in a t1/2 as short as 18 minutes, the t1/2 of 60-kDa PEG-K1804C and 60-kDa diPEG-L491C/K1804C was 5.7 hours and 8.2 hours, respectively (Figure 5C). Thus, PEGylation restored the circulating t1/2 of FVIII comparable with that in HemA mice, and the magnitude of increase in t1/2 of PEG-FVIII also correlated to the increase in PEG mass.
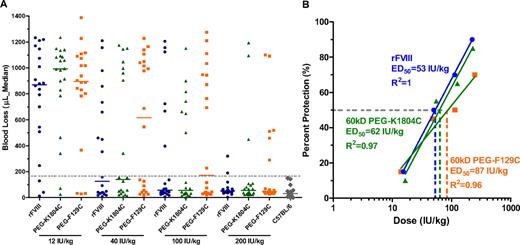
PEGylation preserves FVIII activity in treating acute tail bleeding in HemA mice
To determine whether PEGylated FVIII has comparable acute efficacy as rFVIII in vivo, tail clip–induced blood loss was determined in HemA mice 5 minutes after treatment with escalating doses of full-length rFVIII, 60-kDa PEG-K1804C, and 60-kDa PEG-F129C (Figure 6A). Animals were considered protected if blood loss was 155 μL or less, which is the mean plus 3 SDs from a population of normal C57Bl/6 mice after the tail clip. From the dose-response curve in protection against blood loss (Figure 6B), the dose of each treatment that achieves protection from acute bleeding in 50% of treated animals (ED50) was determined. The ED50 for 60-kDa PEG-K1804C is 62 IU/kg, which is comparable with that of rFVIII (53 IU/kg), whereas the dose response curve for 60-kDa PEG-F129C with ED50 of 87 IU/kg was significantly different from that of rFVIII (P = .017, F test)
PEGylation preserves FVIII activity in treating acute bleeding by tail clip in HemA mice. (A) Blood loss in HemA mice after tail clip at 5 minutes after treatment with 12, 40, 100, or 200 IU/kg full-length rFVIII, 60-kDa PEG-K1804C, or 60-kDa PEG-F129C (20 mice/dose/treatment). Bar represents the median blood loss. Dotted line represents the normal level of blood loss (155 μL; mean + 3 SDs) found in C57Bl/6 mice. (B) Plots of dose response in percentage of protection for data shown in panel A. Mice with blood loss of 155 μL or less are considered protected. The ED50 for rFVIII (●), 60-kDa PEG-K1804C (▴), and 60-kDa PEG-F129C (■) is calculated from the respective dose response curve and labeled on the graph.
PEGylation preserves FVIII activity in treating acute bleeding by tail clip in HemA mice. (A) Blood loss in HemA mice after tail clip at 5 minutes after treatment with 12, 40, 100, or 200 IU/kg full-length rFVIII, 60-kDa PEG-K1804C, or 60-kDa PEG-F129C (20 mice/dose/treatment). Bar represents the median blood loss. Dotted line represents the normal level of blood loss (155 μL; mean + 3 SDs) found in C57Bl/6 mice. (B) Plots of dose response in percentage of protection for data shown in panel A. Mice with blood loss of 155 μL or less are considered protected. The ED50 for rFVIII (●), 60-kDa PEG-K1804C (▴), and 60-kDa PEG-F129C (■) is calculated from the respective dose response curve and labeled on the graph.
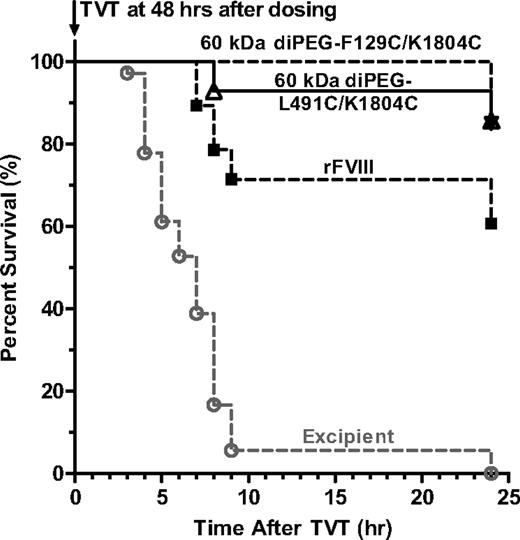
PEGylation extends the prophylactic efficacy of FVIII in protection from venous bleeding in HemA mice
To determine whether extended PK of PEGylated FVIII results in a prolonged prophylactic efficacy in vivo, we used a tail vein transection model in HemA mice.28 Mice were treated with 40 IU/kg full-length rFVIII, 60-kDa diPEG-F129C/K1804C, or 60-kDa diPEG-L491C/K1804C at 48 hours before the transection of 1 lateral tail vein, and moribundity was observed over 24 hours after the injury (Figure 7). All FVIII treatment groups are significantly different from the excipient group (P < .001, log-rank test). Relative to the 60% survival in HemA mice treated with rFVIII, 60-kDa diPEG-F129C/K1804C and 60-kDa diPEG-L491C/K1804C conferred a significantly higher survival of 85% (P = .048) and 86% (P = .03), respectively. Similar analysis for other PEG-FVIII variants has identified that 60-kDa PEG-K1804C achieves prophylactic protection twice as long as the same dose of rFVIII (T.L., D. Lillicrap, X.Z., A. Labelle, S. Powell, Y.C., H.T., Z. Cui, B.M., J.E.M., G.F.P., H.J., manuscript submitted, August 20, 2009).
PEGylation extends the prophylactic efficacy of FVIII in the tail vein transection bleeding model of HemA mice. HemA mice received tail vein injection of 40 IU/kg full-length rFVIII (n = 28), 60-kDa diPEG-L491C/K1804C (n = 28), 60-kDa diPEG-F129C/K1804C (n = 20), or excipient (n = 36). Forty-eight hours later, a lateral tail vein was transected, and the moribundity was monitored hourly for the first 11 hours then at 24 hours. Two-tailed P values are generated by log-rank tests comparing the respective survival curves. TVT indicates tail vein transection.
PEGylation extends the prophylactic efficacy of FVIII in the tail vein transection bleeding model of HemA mice. HemA mice received tail vein injection of 40 IU/kg full-length rFVIII (n = 28), 60-kDa diPEG-L491C/K1804C (n = 28), 60-kDa diPEG-F129C/K1804C (n = 20), or excipient (n = 36). Forty-eight hours later, a lateral tail vein was transected, and the moribundity was monitored hourly for the first 11 hours then at 24 hours. Two-tailed P values are generated by log-rank tests comparing the respective survival curves. TVT indicates tail vein transection.
Discussion
PEGylation is widely used for improving the PK profiles of therapeutic proteins. Several marketed drugs that have been in use for more than a decade have proven the applicability and safety of PEGylation.39 PEG conjugation increases the circulation time of drugs by interfering with renal clearance by glomerular filtration, protecting against enzymatic digestion, blocking interaction with clearance receptors, and reducing the generation of neutralizing antibodies.16
Most of the PEGylated drugs on the market use a nonspecific PEGylation strategy, primarily targeting the amine groups on lysine and histidine residues and the N-terminus. PEGylation of FVIII with a similar strategy has been shown to reduce coagulation activity and impair binding to VWF.18 FVIII is a large molecule that requires interaction with multiple macromolecules and cells to maintain its function. Given these requirements, it is not surprising that nonspecific addition of a large polymer reduces functional activity. Several PEGylated drugs, such as PEGylated interferon α-2a and PEGylated growth hormone, have been shown to have attenuated activity in vitro because of steric interference of PEG on the binding of the therapeutic protein to its target. The loss of activity is compensated by the prolonged circulation time, which results in an increased exposure and overall improvement of the pharmacologic profile in vivo.16 In the case of coagulation factors, efficacy is correlated with the level of activity of a given factor present in the blood at the time of injury, and an increased exposure does not compensate for loss of activity. Therefore, any modification that prolongs the half-life of FVIII must also maintain its full activity.
Site-directed PEGylation offers the opportunity to target conjugation of the PEG moiety and to potentially avoid the effect on biologic efficacy.17,40,41 In this study, single cysteine residues were introduced on the surface of BDD-FVIII, and these residues were conjugated with maleimide PEG. Most of the engineered cysteine mutations had no effect on the in vitro coagulation activity of FVIII, although some of the cysteine mutations are at, or near, the binding sites of VWF, FIXa, and FX (Table 1; Figure 1B). The PEGylation has been shown to be highly site specific, occurring in the targeted domain (Table 1; Figure 3). PEGylation efficiency is also determined by the site of conjugation (Table 1), and a difference of a few amino acids, such as K1804C and K1808C, can significantly affect the PEGylation (Figure 2). The differential PEGylation efficiency at these 2 positions is consistent with the BDD structural model,20-22 showing that K1808C, with an accessible surface area of 10.5 nm is less accessible than the K1804C site, which has an accessible surface area of 13.89 nm. Furthermore, the K1808C site is within 0.6 nm of a glycosylation site at asparagine 1810, whereas K1804C is predicted to be 1.8 nm from the glycosylation site; thus, PEGylation may be sterically blocked.
Although some PEGylated FVIII variants lost activity (data not shown), others preserved coagulation efficacy in vitro, suggesting that the PEGylation site is critical for retaining the activity. PEG conjugation at position 2091, which is near the phospholipid-binding interface in the C1 domain, resulted in a 2-fold decrease in specific activity. It appeared that the size of PEG did not affect the activity of PEG FVIII variants tested, and FVIII variants conjugated with a PEG up to 60 kDa still retained full specific activity (Table 2), including variants with conjugation sites near the FIX binding site (such as K1808C and K1804C; Figure 1B). A possible explanation for this observation is that the main effect of PEGylation is steric hindrance, which leads to a modest reduction in association rate, and not a conformational change that significantly affects the interaction with other macromolecules.15 Consistent with this explanation, variants in which PEG was conjugated near epitopes for inhibitory antibodies (such as L491C) displayed full activity, whereas antibody binding to the same region significantly diminished activity.
Interestingly, it appears that conjugation of a PEG molecule at these sites makes a FVIII variant more resistant to some inhibitory antibodies, as evidenced by studies with mAb (Figure 4) directed against PEGylated epitopes. This observation suggests a potential benefit of using PEGylated FVIII in patients with inhibitory antibodies, which remains to be verified clinically. However, it should be pointed out that this protection is specific to the binding site of the antibody and it is unlikely that a molecule can be designed with PEG attachments that would disrupt the large variety of inhibitors present in patients. Furthermore, the addition of a nonnative cysteine may also affect the immunogenicity profile of our variants.
PEGylation of a number of FVIII Cys variants, including those located on the light chain, did not significantly reduce VWF binding (Table 3). The sustained interaction between PEGylated FVIII and VWF in vivo may account for a limited (2-fold) half-life extension of PEG-FVIII variants, irrespective of the increase in PEG size (Figure 5A-B). Considering that 95% of FVIII in circulation is in complex with VWF,42 it is conceivable that FVIII can also be eliminated as a complex by the clearance pathway of its chaperone VWF. Thus, although PEGylation may significantly improve the survival of free FVIII, which is approximately 5% in circulation, it may have limited potential to improve the t1/2 of most FVIII that is in complex with VWF. Consistent with this notion, the absence of VWF alters the limit on the half-life extension of PEGylated FVIII, as shown in VWF KO mice (Figure 5C). The half-lives of 60-kDa PEG-K1804C and 60-kDa diPEG-L491C/K1804C in VWF KO mice were extended 19- and 27-fold relative to BDD-FVIII, respectively. Whether PEG on FVIII variants can effectively attenuate the clearance of FVIII-VWF complex is an area that merits further investigation. The results in VWF-deficient mice also indicate that PEG can partially compensate for lack of VWF and raise the potential of using PEGylated FVIII in treating some forms of VWD.
The PEGylated FVIII variants not only displayed full in vitro coagulation activity and improved PK, but they also showed prolonged efficacy in HemA mice, which is consistent with the increased half-life (Figure 7). Moreover, the PEGylated FVIII variants showed an acute efficacy comparable with unmodified FVIII in stopping bleeding in a HemA mouse tail clip (Figure 6). The data clearly indicate that the site-directed PEGylated FVIII variants have potential not only as a prophylactic therapy but also for treating bleeds in on-demand and surgical conditions.
In conclusion, a series of site-directed PEGylated FVIII variants were developed by conjugation of maleimide PEG to the surface of FVIII by engineered cysteines, and the PEGylation was shown to be highly specific. The site of PEGylation on FVIII is critical to PEGylation efficiency, preservation of the coagulation activity, and the improvement of PK. Depending on the site of PEGylation, the FVIII variants retained coagulation activity and VWF binding capability in vitro. In animal studies, the PEGylated FVIII variants showed improved PK, prolonged efficacy consistent with the improved PK, and acute efficacy comparable with unmodified FVIII. Finally, the high PEGylation yield and consistent conjugation at a single site makes manufacture of site-directed PEGylated FVIII readily scalable. These FVIII conjugates could potentially be used for both prophylaxis and on-demand treatment of hemophilia A.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susmitha Sompalli for VWF Biacore data; Marian Seto for help with structural figures; Xue Wu for plasmid vector construction; Lal Ratnapala, Henna Atwal, and Jiajie Niu for technical assistance of small scale PEGylation screening; Amanda Martinez, Julie Nguyen, and other colleagues in Cell Culture Development and Isolation departments at Bayer Berkeley site for FVIII material preparation; Naji Khabbaz and other colleagues in Analytics Development and Support at Bayer Berkeley site for Chromogenic and other assay support; and Thomas Barnett, Liang Tang, and Pam Esmon for helpful discussions.
Authorship
Contribution: B.M., C.P., and H.J. designed and conducted the research, analyzed the data, and wrote the manuscript; H.T., J.S., Y.C., T.L., X.Z., J.N., and J.C. conducted the research; J.S. conducted the research and wrote the manuscript; M.A.F. and G.F.P. designed the research and analyzed the data; J.E.M. designed the research, analyzed the data, and wrote the manuscript; and J.-M.G. and B.S. designed and conducted the PK research.
Conflict-of-interest disclosure: All authors worked for Bayer HealthCare during the time of this study.
Correspondence: John E. Murphy, Biologics Research, Bayer HealthCare LLC, 2600 Hilltop Dr, Richmond, CA 94806; e-mail: johne.murphy.b@bayer.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal