Abstract
During erythroblast enucleation, membrane proteins distribute between extruded nuclei and reticulocytes. In hereditary spherocytosis (HS) and hereditary elliptocytosis (HE), deficiencies of membrane proteins, in addition to those encoded by the mutant gene, occur. Elliptocytes, resulting from protein 4.1R gene mutations, lack not only 4.1R but also glycophorin C, which links the cytoskeleton and bilayer. In HS resulting from ankyrin-1 mutations, band 3, Rh-associated antigen, and glycophorin A are deficient. The current study was undertaken to explore whether aberrant protein sorting, during enucleation, creates these membrane-spanning protein deficiencies. We found that although glycophorin C sorts to reticulocytes normally, it distributes to nuclei in 4.1R-deficient HE cells. Further, glycophorin A and Rh-associated antigen, which normally partition predominantly to reticulocytes, distribute to both nuclei and reticulocytes in an ankyrin-1–deficient murine model of HS. We conclude that aberrant protein sorting is one mechanistic basis for protein deficiencies in HE and HS.
Introduction
During erythroblast enucleation, plasma membrane and cytoskeletal proteins dynamically reorganize while the nucleus, surrounded by plasma membrane, separates from the nascent reticulocyte. A key aspect of this process is the partitioning of erythroblast proteins to extruded nuclei and/or nascent reticulocytes. Hence, protein sorting during enucleation plays a crucial role in determining the protein content of reticulocyte membranes and cytoskeleton. Koury et al1 have shown that cytoskeletal actin, spectrin, and protein 4.1 partition to reticulocytes, whereas we have discovered that one molecular mechanism regulating membrane-spanning protein sorting to reticulocytes is their degree of connectivity to the cytoskeleton.2
In hereditary spherocytosis (HS) and hereditary elliptocytosis (HE), as well as in murine models of these disorders, deficiencies of red cell membrane proteins, in addition to those encoded by the mutant gene, are well described. Elliptocytic erythrocytes, resulting from protein 4.1R gene mutations,3,4 lack not only protein 4.1R but also the membrane-spanning protein glycophorin C (GPC),5,6 a 4.1R binding partner with a key role in linking the cytoskeleton to the bilayer. In HS resulting from ankyrin-1 gene mutations,7-9 deficiencies of band 3, Rh-associated antigen (RhAG), and glycophorin A (GPA) have been documented.10,11 Similarly, in HS resulting from band 3 gene mutations, members of the band 3 macromolecular complex are decreased.12-14
Various mechanisms, either singly or in combination, could produce the protein deficiencies observed in HS and HE. Specifically, proteins might not be normally assembled on the erythroblast membrane, sorting during enucleation might be perturbed, or proteins might be intracellularly degraded or released in exosomes during reticulocyte maturation. The current study explores whether aberrant protein sorting during enucleation creates some of the specific protein deficiencies.
Methods
Antibodies
Rabbit antibodies specific for mouse GPC, band 3, and RhAG were generated in our laboratory.6 Anti-GPC was labeled with Alexa Fluor 555 (Invitrogen–Molecular Probes) according to the manufacturer's instructions. Other antibodies were obtained from commercial sources detailed in “Immunofluorescence microscopy.”
Mice
Immunofluorescence microscopy
Freshly harvested 4.1R-null and wild-type (WT) bone marrow cells were suspended in RPMI with 20% fetal calf serum (Invitrogen) and stained with Syto-17 (1μM; Invitrogen) and fluorescein isothiocyanate–conjugated TER 119 (0.25 μg/106 cells; eBioscience) or Alexa Fluor 555–labeled anti-GPC antibody (1 μg/106 cells) for 45 minutes at 37°C. After washing, the cells were imaged. nb/nb and WT bone marrow cells were fixed on Cell Tak (BD Biosciences)–coated coverslips with 3% paraformaldehyde for 5 minutes at room temperature. The cells were then blocked for 1 hour in 1% albumin-phosphate–buffered saline at room temperature and double stained overnight at 4°C with TER 119 (1:50; BD PharMingen) and either rabbit anti–mouse band 3, rabbit anti-GPC, or rabbit anti-RhAG (1:100). After washing with 0.1% albumin–phosphate–buffered saline, the cells were labeled with Alexa Fluor 594–conjugated goat anti–rabbit IgG (1:100; Invitrogen) and Alexa Fluor 488–conjugated donkey anti–rat IgG (1:500; Invitrogen) for 1 hour at room temperature. After washing, the slides were mounted with Vecta Shield (Vector Laboratories).
Results and discussion
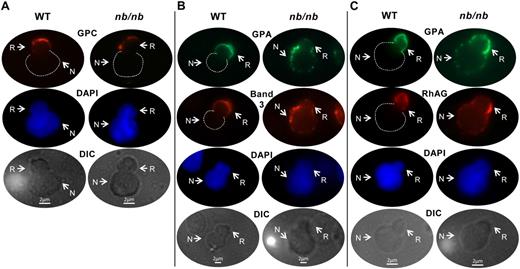
To explore whether aberrant protein sorting might be responsible for deficiencies of membrane proteins in HE, we examined sorting of GPC during enucleation of normal and protein 4.1R-null erythroblasts. Protein 4.1R knockout mice have fragmented red cells, which lack GPC, thus phenotypically mimicking human HE. By using immunofluorescent microscopy, we first analyzed GPC sorting in enucleating erythroblasts from WT bone marrow. We found that GPC partitioned almost exclusively to nascent reticulocytes, with little or no GPC observed in plasma membranes of extruding nuclei (Figure 1). Strikingly, in 4.1R-null erythroblasts, GPC distributed exclusively to nuclei (Figure 1). These data unequivocally establish that GPC deficiency in 4.1R-null erythrocytes is attributable, in large part, to markedly abnormal protein partitioning during enucleation. Hence, our findings provide a novel, molecular explanation for the underlying basis of specific membrane protein deficiencies observed in 4.1R-deficient HE.
Analysis of GPC sorting during enucleation of WT and 4.1R-null erythroblasts. Differential interference contrast (DIC) and immunofluorescent micrographs of wild-type (WT) and 4.1R-null enucleating erythroblasts, including nascent reticulocyte (R) and extruding nucleus (N), probed with fluorescein isothiocyanate–conjugated TER 119, specific for GPA (green), or Alexa Fluor 555–labeled rabbit anti–mouse GPC antibody (red). Nuclei were identified by Syto-17 staining (blue). The number of enucleating erythroblasts examined under each staining condition was 6 or more. The images were observed by the use of a Zeiss LSM META 510 Confocal microscope (Carl Zeiss Microimaging Inc) with an APOCHROMAT 63×/1.4 oil DIC objective and acquired by the use of Zeiss Laser Scanning Microscope LSM 510, Version 3.2 SP2 software with a Zeiss AxioCam HRm Rev. 2/3.3V camera. The images were processed with the use of Adobe Photoshop (Adobe Systems Inc).
Analysis of GPC sorting during enucleation of WT and 4.1R-null erythroblasts. Differential interference contrast (DIC) and immunofluorescent micrographs of wild-type (WT) and 4.1R-null enucleating erythroblasts, including nascent reticulocyte (R) and extruding nucleus (N), probed with fluorescein isothiocyanate–conjugated TER 119, specific for GPA (green), or Alexa Fluor 555–labeled rabbit anti–mouse GPC antibody (red). Nuclei were identified by Syto-17 staining (blue). The number of enucleating erythroblasts examined under each staining condition was 6 or more. The images were observed by the use of a Zeiss LSM META 510 Confocal microscope (Carl Zeiss Microimaging Inc) with an APOCHROMAT 63×/1.4 oil DIC objective and acquired by the use of Zeiss Laser Scanning Microscope LSM 510, Version 3.2 SP2 software with a Zeiss AxioCam HRm Rev. 2/3.3V camera. The images were processed with the use of Adobe Photoshop (Adobe Systems Inc).
To determine whether aberrant sorting was specific for 4.1R-associated proteins, we studied the behavior of GPA, a membrane-spanning molecule that is not part of the 4.1R complex. GPA was an ideal control because in a previous study2 we showed that GPA sorts almost exclusively to reticulocytes during normal erythroblast enucleation. In 4.1R-null cells, GPA partitioning was not perturbed; it sorted to nascent reticulocytes in both WT and 4.1R-null enucleating erythroblasts (Figure 1). Collectively, these findings provide clear evidence that cytoskeletal protein 4.1R is critical for normal sorting of GPC to reticulocytes. Moreover, they further strengthen the concept that cytoskeletal attachments are an important factor in regulating transmembrane protein sorting to reticulocytes.
We next asked whether aberrant sorting is also mechanistically responsible for creating deficiencies of specific membrane proteins in mature erythrocytes in HS. To address this, we used ankyrin-1–deficient nb/nb mice, a faithful model of human HS secondary to mutations in the gene encoding ankyrin-1. We first analyzed GPC, a membrane-spanning molecule that is not part of the ankyrin-1 complex. GPC partitioning was unperturbed in nb/nb cells; it sorted to nascent reticulocytes in both WT and nb/nb enucleating erythroblasts (Figure 2A). We then focused on the sorting patterns of band 3, RhAG, and GPA, because these 3 proteins are deficient in nb/nb erythrocytes and in human HS secondary to ankyrin-1 mutations. In WT enucleating erythroblasts, both band 3 and RhAG distributed predominantly to reticulocytes (Figure 2B-C), similar to GPA. In marked contrast, in ankyrin-1–deficient nb/nb enucleating erythroblasts, band 3, GPA, and RhAG sorted to both expelled nuclei and reticulocytes (Figure 2B-C). These data clearly demonstrate that one mechanism producing deficiencies of band 3, RhAG, and GPA in mature nb/nb red cells is their abnormal sorting during nuclear extrusion.
Analysis of band 3, RhAG, and GPA sorting during enucleation of WT and nb/nb erythroblasts. DIC and immunofluorescent micrographs of WT and nb/nb-enucleating erythroblasts, including nascent reticulocyte (R) and extruding nucleus (N); probed with rabbit anti–mouse GPC and Alexa Fluor 594–labeled goat anti–rabbit; IgG (red; A); probed with rat anti–mouse TER 119 and Alexa Fluor 488–conjugated donkey anti–rat IgG (green) or rabbit anti–mouse band 3 and Alexa Fluor 594–labeled goat anti–rabbit IgG (red; B); or probed with rat anti–mouse TER 119 and Alexa 488–conjugated donkey anti–rat IgG (green) or rabbit anti–mouse RhAG and Alexa Fluor 594–labeled goat anti–rabbit IgG (red; C). Nuclei were identified by 4′,6-diamidino-2-phenylindole staining (DAPI; blue). Dashed lines outline the spherical portion of extruding nuclei in the red and green images in which there is no fluorescent labeling of the nucleus. The number of enucleating erythroblasts examined under each staining condition was 6 or more. Of note, during extrusion the nucleus transiently deforms, and a portion of it is visualized within the nascent reticulocyte, as evidenced in these images.
Analysis of band 3, RhAG, and GPA sorting during enucleation of WT and nb/nb erythroblasts. DIC and immunofluorescent micrographs of WT and nb/nb-enucleating erythroblasts, including nascent reticulocyte (R) and extruding nucleus (N); probed with rabbit anti–mouse GPC and Alexa Fluor 594–labeled goat anti–rabbit; IgG (red; A); probed with rat anti–mouse TER 119 and Alexa Fluor 488–conjugated donkey anti–rat IgG (green) or rabbit anti–mouse band 3 and Alexa Fluor 594–labeled goat anti–rabbit IgG (red; B); or probed with rat anti–mouse TER 119 and Alexa 488–conjugated donkey anti–rat IgG (green) or rabbit anti–mouse RhAG and Alexa Fluor 594–labeled goat anti–rabbit IgG (red; C). Nuclei were identified by 4′,6-diamidino-2-phenylindole staining (DAPI; blue). Dashed lines outline the spherical portion of extruding nuclei in the red and green images in which there is no fluorescent labeling of the nucleus. The number of enucleating erythroblasts examined under each staining condition was 6 or more. Of note, during extrusion the nucleus transiently deforms, and a portion of it is visualized within the nascent reticulocyte, as evidenced in these images.
Although the deficiency of GPC in HE red cells secondary to 4.1R mutations and the deficiencies of band 3, GPA, and RhAG in HS red cells secondary to ankyrin-1 mutations are well documented, the concept that these deficiencies might be caused by mechanisms active at the time of enucleation had not been entertained. Our findings focus attention, for the first time, on this stage of erythropoiesis in HS and HE. Further, they raise the possibility that reticulocytes in HS and HE may differ from normal reticulocytes in their biophysical properties of membrane cohesion or membrane deformability because these 2 crucial properties are regulated by vertical linkages between specific membrane-spanning proteins embedded in the lipid bilayer and cytoskeletal proteins, such as the interactions of band 3, ankyrin-1, and spectrin or interactions of GPC, 4.1R, actin, and spectrin (recently reviewed in Mohandas and Gallagher15 ). If HS and HE reticulocytes are abnormal, then membrane loss of surface area may be exacerbated at this stage rather than occurring only in mature cells. Support for such a thesis can be inferred from an earlier study showing that surface area loss is a distinct feature of HS reticulocytes.16 Future studies will determine the relative contributions of sorting abnormalities and other mechanisms for the observed protein deficiencies in HS and HE.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Luanne Peters for generously providing the nb/nb mice.
This work was supported by National Institutes of Health grants DK26263, DK56267, DK32094, and HL31579 and by the Director, Office of Health and Environment Research Division, US Department of Energy, under contract DE-AC03-76SF00098.
National Institutes of Health
Authorship
Contribution: M.S., K.C., and J.V. performed research and analyzed data; N.M. analyzed data and edited the manuscript; and X.A. and J.A.C. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Joel Anne Chasis, Lawrence Berkeley National Laboratory, Bldg 84, 1 Cyclotron Rd, Berkeley, CA 94720; e-mail: jachasis@lbl.gov.
References
Author notes
X.A. and J.A.C. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal