Abstract
Neurofibromatosis type 1 (NF1) is the most common genetic disorder with a predisposition to malignancy and affects 1 in 3500 persons worldwide. NF1 is caused by a mutation in the NF1 tumor suppressor gene that encodes the protein neurofibromin. Patients with NF1 have cutaneous, diffuse, and plexiform neurofibromas, tumors comprised primarily of Schwann cells, blood vessels, fibroblasts, and mast cells. Studies from human and murine models that closely recapitulate human plexiform neurofibroma formation indicate that tumorigenesis necessitates NF1 loss of heterozygosity in the Schwann cell. In addition, our most recent studies with bone marrow transplantation and pharmacologic experiments implicate haploinsufficiency of Nf1 (Nf1+/−) and c-kit signaling in the hematopoietic system as required and sufficient for tumor progression. Here, we review recent studies implicating the hematopoietic system in plexiform neurofibroma genesis, delineate the physiology of stem cell factor–dependent hematopoietic cells and their contribution to the neurofibroma microenvironment, and highlight the application of this research toward the first successful, targeted medical treatment of a patient with a nonresectable and debilitating neurofibroma. Finally, we emphasize the importance of the tumor microenvironment hypothesis, asserting that tumorigenic cells in the neurofibroma do not arise and grow in isolation.
Introduction
Although several reports characterized neurofibromatosis type 1 (NF1) or NF1-like syndromes as early as the 18th century,1-3 Friedrich von Recklinghausen did not publish his seminal, detailed case reports until 1882.4 Von Recklinghausen observed that neurofibromas contain elements of both neuronal and fibroblastic tissue. In 1911, H. Greggio reported his observation that mast cells infiltrate the neurofibroma.5,6 Vincent Riccardi later postulated a pivotal role for mast cells and melanocytes in NF1 pruritus, pigmentation defects, and, potentially, neurofibroma formation.7
Today, NF1 is recognized as a common and fully penetrant genetic disease that shows variable expressivity.6-9 The disease is transmitted in an autosomal dominant fashion as a mutation in NF1, a tumor suppressor gene encoding the protein neurofibromin. Neurofibromin functions as a p21ras (Ras) guanosine triphosphatase (GTP)–activating protein (GAP), accelerating the hydrolysis of Ras-GTP thousands of fold and functioning at least in part to negatively regulate multiple Ras-dependent cellular signaling pathways.6,10-16
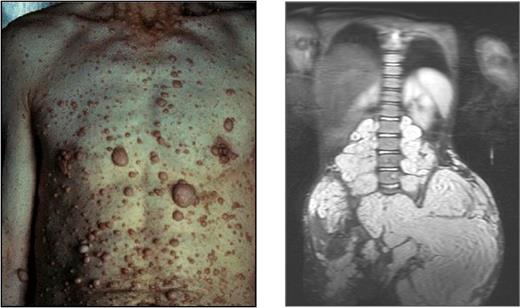
Mutations of NF1 predispose patients to variable neuronal, hematopoietic, and skeletal disorders, including myeloid leukemia, kyphoscoliosis, long bone pseudoarthrosis, and cutaneous, subcutaneous, and diffuse plexiform neurofibromas.6,17 Neurofibromas are pathognomonic for the disease. Cutaneous neurofibromas arise from small peripheral nerves during adolescence or adulthood and are observed in greater than 95% of patients with NF1 (Figure 1 left).18 Plexiform neurofibromas, by contrast, appear in approximately 15% to 40% of patients with NF1 and arise during early development from cranial nerves and proximal large peripheral nerve sheaths (Figure 1 right). These interdigitating tumors are composed of Schwann cells, fibroblasts, degranulating mast cells, and vascular cells.6,19 Although cutaneous neurofibromas have limited growth and a very low propensity for transformation, plexiform neurofibromas can constitute an early and lifelong source of disfigurement, disability, mortality, and potential transformation to a metastatic malignant peripheral nerve sheath tumor.
Examples of cutaneous and plexiform neurofibromas. Cutaneous neurofibromas growing on the chest and abdomen and an magnetic resonance image of a large plexiform neurofibroma compressing the spinal column. Photographs courtesy of the Children's Tumor Foundation; www.ctf.org.
Examples of cutaneous and plexiform neurofibromas. Cutaneous neurofibromas growing on the chest and abdomen and an magnetic resonance image of a large plexiform neurofibroma compressing the spinal column. Photographs courtesy of the Children's Tumor Foundation; www.ctf.org.
As indicated by human tissue analyses and animal models, pleomorphic NF1 manifestations result from widespread NF1 heterozygosity and variable Nf1 loss of heterozygosity (LOH) in multiple cell lineages. Malignant and nonmalignant pathologies in NF1 can originate in neural crest–derived tissue: LOH in Schwann cells permits neurofibroma formation, LOH in chromaffin cells initiates pheochromocytomas, and LOH in melanocytes produce pigmented lesions such as café-au-lait macules and Lisch nodules.20-22 NF1 morbidities also arise from other germ layers: LOH in skin-derived precursors leads to cutaneous neurofibroma formation, LOH in myeloid cells induces myelomonocytic leukemia, and LOH in glial cells permits astrocytoma formation.18,20,23-28 Although NF1 disorders arise from multiple germ layers, regionalized tissue may show increased reliance on NF1 GAP signaling. For example, a recent study has shown increased NF1 protein in astrocytes localized to the optic nerve, brain stem, and cerebellum compared with astrocytes in the neocortex. That study found that NF1-deficient astrocytes in the optic nerve, brain stem, and cerebellum, but not in the neocortex, showed hyperfunctioning phenotypes, perhaps explaining the propensity for localized tumor formation in patients with NF1.29
Recent studies in addition have implicated Nf1 haploinsufficient cell lineages in nonmalignant but common manifestations of NF1, including altered osteoclast-osteoblast interactions in skeletal dysplasia, Nf1+/− endothelial cells in vascular infarcts, and Nf1+/− γ-aminobutyric acid neurons in learning deficiencies.24,30-32 As reviewed in this article, haploinsufficiency of bone marrow–derived cells within the neurofibroma microenvironment critically contributes to tumorigenesis. Broadly, these data emphasize that consideration of molecular mechanisms underlying specific NF1 morbidities requires consideration of intracellular signaling not only in single cell types or in a single germ layer but also within and between multiple cell systems. In the case of the plexiform neurofibroma, it is important to investigate signaling mechanisms within and interactions between the Nf1−/− Schwann cell, the Nf1+/− dermal tissue and vasculature, and the Nf1+/− hematopoietic system.23
Discussion
A question of lineage: mouse modeling unravels a genetic riddle
A series of murine models have indispensably facilitated our understanding of the malignant and nonmalignant manifestations of NF1. Although these models have provided insights into a range of disease phenotypes, we focus our discussion here on the models illuminating the mechanisms of plexiform neurofibroma formation.
The Nf1 heterozygote
The first Nf1 knockout mice, created by Jacks et al33 and Brannan et al,34 carried a targeted disruption of Nf1 exon 31 (Nf1+/n31). This mutation produces protein instability and subsequent degradation. Compared with wild-type (WT) controls, the Nf1+/− animals had a high predisposition to multiple cancers after 1 year of age, experienced a shorter overall lifespan, and occasionally exhibited characteristic NF1 tumors such as pheochromocytomas and myeloid leukemia. The leukemic cells and pheochromocytoma tissue showed LOH in the WT Nf1 allele, consistent with the Nf1 tumor suppressor model. None of these mice, however, developed neurofibromas, Lisch nodules, or café-au-lait macules, hallmark characteristics of human NF1.
This failure to develop key symptoms in the Nf1+/− mouse posed a formidable problem; after all, humans born heterozygous at the NF1 locus ubiquitously have a combination of cutaneous, diffuse, or plexiform neurofibromas. To explain this apparent disconnect, Jacks et al33 hypothesized that the reduced cell number, shorter lifespan, or disparate resistance to Nf1 mutations decreases the probability of a second-hit in the murine system. Problematically, Nf1−/− mice die at embryonic day 13.5 as a result of cardiac developmental defects, complicating the analysis of a germline second-hit. Although the Nf1+/− mouse disappointed as a model for neurofibroma formation, it propelled the stimulus for the development of additional murine models and created a tremendous resource for studying Nf1+/−-dependent physiology in multiple cell lineages, including Schwann cells, fibroblasts, vascular cells, and hematopoietic cells.
The Nf1 chimera
After the observations from the Nf1+/− mouse, Cichowski et al35 devised a murine model to test the hypothesis that Nf1 functions as a tumor suppressor and that LOH precedes tumor formation. They injected Nf1−/− embryonic stem cells into WT blastocysts, creating Nf1−/− chimeric mice. Although high chimerism resulted in early death and low chimerism produced no phenotypic effect, all of the remaining animals in the experimental group (12 of 18) experienced myelodysplasia, neuromotor defects, and the formation of neurofibromas. The neurofibromas arose from multiple nerves (in the tongue, along the dorsal root, and within the limb muscle) and shared gross morphologic, histologic, and electron microscopic features with human neurofibromas. Neurofibroma tissue from chimeric animals carrying an Nf1−/− ES cell–linked β-galactosidase transgene showed near uniform β-gal expression, suggesting tumor dependence on Nf1 nullizygosity. However, this model relied on a large and unpredictable number of Nf1−/− admixed cells throughout the animal and precluded the assessment of admixed Nf1−/− and Nf1+/− cells, a genetic status expected in the human condition. Although the chimera solidified the tumor suppressor effect of Nf1 in vivo and suggested the Schwann cell as the tumor cell of origin, the question of the exact genetic and cell conditions required for neurofibroma formation persisted.
Impact of Nf1 haploinsufficiency in multiple cell lineages
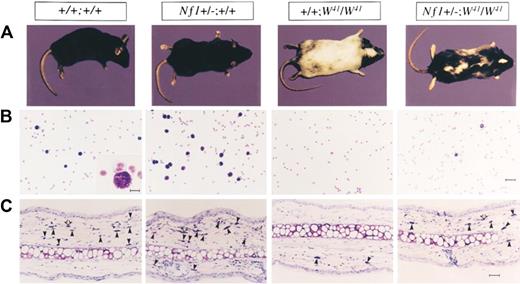
Studies from knockout and chimeric Nf1 mice have shown that LOH in tumorigenic cells concur with NF1 as a tumor suppressor gene. However, the slow growth of neurofibromas and the numerous and pleomorphic nonmalignant manifestations observed in patients with NF1 suggested an NF1 gene dosage effect. Genetic evidence for this dosage effect was first provided by the study of Nf1+/− mast cells and melanocytes, showing that Nf1 heterozygosity, and not solely nullizygosity, produced a stem cell factor (SCF)–dependent hyperactive phenotype (Figure 2).36 In that study, Ingram et al36 intercrossed Nf1+/− mice with c-kit mutant mice (W41/W41), a point mutation that compromises SCF-induced c-kit receptor tyrosine kinase activity by approximately 85%. (The W, or white spotting locus mutation, severely diminishes mast cell differentiation and compromises neural crest–derived melanocyte migration, leading to a white coat color.37-41 ) Dermal tissue and peritoneal fluid from both Nf1+/− and Nf1+/−;W41/W41 mice exhibited increased numbers of mast cells compared with WT and W41/W41 mice, respectively. Likewise, bone marrow from Nf1+/− and Nf1+/−;W41/W41 mice formed increased numbers of mast cell colonies in semisolid media compared with WT and W41/W41 mice, respectively. SCF induced Nf1+/− and Nf1+/−;W41/W41 mast cell proliferation at nearly twice the rate as WT and W41/W41 cells, respectively, and both Nf1+/− and Nf1+/−;W41/W41 mast cells showed increased SCF-dependent survival. Most strikingly, the Nf1+/−;W41/W41 animals showed a black-white mottling of the W41/W41 mouse's normally albino coat, showing that Nf1 haploinsufficiency modulated the fates of both mast cells and the neural crest–derived melanocytes, a lineage known to express high levels of neurofibromin. Thus, Nf1 haploinsufficiency partially or fully restored multiple loss-of-function phenotypes associated with the W mutation in vitro and in vivo.
Effect of haploinsufficiency of Nf1 on coat color and total numbers of cutaneous and peritoneal mast cells. (A) Coat color pattern of a representative mouse from each of the following genotypes: +/+;+/+, Nf1+/−;+/+, +/+;W41/W41, and Nf1+/−;W41/W41. Haploinsufficiency at Nf1 partially corrects the coat color deficiency in mice homozygous for the W41 allele in a C57BL/6 genetic background. (B) Representative cytospins from peritoneal lavages stained for mast cells from individual mice of the 4 Nf1 and W genotypes. Peritoneal cells were stained with toluidine blue to quantify the total number of mast cells per peritoneal lavage. A WT mouse (original magnification ×200). Bar (inset), 10 μm. Bar (far right), 30 μm. (C) Representative ear biopsies stained for cutaneous mast cells from individual mice of the 4 Nf1 and W genotypes. Specimens were stained with hematoxylin-eosin to assess routine histology and with Giemsa to identify mast cells. Ear biopsies were stained with Fontana-Masson to differentiate melanin-containing cells from mast cells. Cutaneous mast cells (Giemsa-positive, Fontana-Masson–negative) were quantitated in a blinded fashion by counting the distal 5 mm of ears. Black arrows indicate Giemsa-positive mast cells, and open arrows indicate Fontana-Masson melanin–containing cells. Bar, 35 μm. Originally published in Ingram et al.36
Effect of haploinsufficiency of Nf1 on coat color and total numbers of cutaneous and peritoneal mast cells. (A) Coat color pattern of a representative mouse from each of the following genotypes: +/+;+/+, Nf1+/−;+/+, +/+;W41/W41, and Nf1+/−;W41/W41. Haploinsufficiency at Nf1 partially corrects the coat color deficiency in mice homozygous for the W41 allele in a C57BL/6 genetic background. (B) Representative cytospins from peritoneal lavages stained for mast cells from individual mice of the 4 Nf1 and W genotypes. Peritoneal cells were stained with toluidine blue to quantify the total number of mast cells per peritoneal lavage. A WT mouse (original magnification ×200). Bar (inset), 10 μm. Bar (far right), 30 μm. (C) Representative ear biopsies stained for cutaneous mast cells from individual mice of the 4 Nf1 and W genotypes. Specimens were stained with hematoxylin-eosin to assess routine histology and with Giemsa to identify mast cells. Ear biopsies were stained with Fontana-Masson to differentiate melanin-containing cells from mast cells. Cutaneous mast cells (Giemsa-positive, Fontana-Masson–negative) were quantitated in a blinded fashion by counting the distal 5 mm of ears. Black arrows indicate Giemsa-positive mast cells, and open arrows indicate Fontana-Masson melanin–containing cells. Bar, 35 μm. Originally published in Ingram et al.36
Concomitant investigations showed Nf1 haploinsufficient phenotypes in other cell lineages. Atit et al42 demonstrated that Nf1+/− C57BL/6 mice, a strain typically resistant to chemical-induced carcinogenesis, uniformly developed papillomas subsequent to treatment with dimethylbenzanthracene and 12-O-tetradecanoyl-13-acetylphorbol. This papilloma formation showed Nf1 haploinsufficient keratinocytes aberrantly proliferating in response to chemical injury. Likewise, Bajenaru et al43,44 found hyperactivity in Nf1 haploinsufficient astrocytes, a pertinent discovery considering the predisposition of patients with NF1 to low-grade astrocytomas. These studies have shown that Nf1+/− astrocytes proliferated more quickly than WT cells, grew autonomously in vitro, and exhibited increased activation of Ras pathways.
Taken together, these data from in vitro and in vivo studies of Nf1+/− melanocytes, mast cells, keratinocytes, and astrocytes, all of which are lineages relevant to manifestations of NF1, argued that genetic haploinsufficiency of a tumor suppressor could modulate cellular phenotypes.
Generation of a conditional Nf1 knockout
The early embryonic deaths of Nf1−/− mice generated with the use of traditional homologous recombination prompted Zhu et al21,45 to create a conditional knockout murine model of NF1. In this model, loxP sites flank Nf1 exon 31 and 32 (Nf1flox/flox), allowing for Cre-recombinase–mediated gene deletion. To test the hypothesis that Nf1 LOH in the Schwann cell results in neurofibroma formation, the investigators crossed the Nf1flox/flox mouse with a mouse expressing Cre protein under control of the Krox20 promoter (Krox20cre). Because Krox20 specifically promotes transcription in approximately 5% to 10% of Schwann cells, the Nf1flox/flox;Krox20cre mouse provides limited Schwann cell Nf1 nullizygosity while circumventing the problem of embryonic lethality in the Nf1−/− animal.
The Nf1flox/flox;Krox20cre mouse, however, failed to develop the hallmark neurofibroma. The investigators hypothesized that neurofibroma formation requires not only Schwann cell LOH but also haploinsufficiency in the microenvironment. To test this hypothesis, the investigators intercrossed their Nf1flox/flox;Krox20cre mouse with an Nf1+/− mouse, creating the Nf1flox/−;Krox20cre animal. In this model the Schwann cell loses both alleles of Nf1. Concurrently, all other cells remain functionally Nf1+/− (ie, Nf1flox/−). The Nf1flox/−;Krox20cre mouse develops normally until 10 to 12 months of age, at which time it experiences an enlargement of peripheral nerves along the spinal dorsal root ganglia which are phenotypically similar to human plexiform neurofibroma tissue. Histology showed disassociated Schwann cells, pervasive collagen bundles, fibroblastic proliferation, and an abundant infiltration of mast cells. Of critical importance, these pathologic features remain consistently absent in mice with Nf1−/− Schwann cells and a WT background (Nf1flox/flox;Krox20cre).
In this mouse model, Zhu et al21,45 demonstrated that neurofibroma formation requires not only LOH in the Schwann cells but also Nf1 haploinsufficiency in the supporting tissue. Importantly, the tumors in Nf1flox/−;Krox20cre mice are infiltrated with an abundance of mast cells and are histologically reminiscent of human neurofibroma tissue. Thereby, the Nf1flox/−;Krox20cre mouse recapitulates the human condition with 100% penetrance, granting scientists studying NF1 indispensable insights into neurofibroma pathogenesis, elegantly showing microenvironment requirements for neurofibroma development, and providing foundational evidence implicating the Nf1+/− mast cell (reviewed in Le and Parada20 ).
Interactions in the Nf1 haploinsufficient microenvironment
After the creation of the Nf1flox/−Krox20cre in vivo plexiform neurofibroma model, subsequent discoveries further elucidated intercellular and intracellular mechanisms of tumor formation. It is well established that mast cells differentiate, proliferate, and secrete cytokines in response to SCF, a secreted ligand to the c-kit receptor tyrosine kinase.46 Importantly, SCF mRNA can be detected in neurofibroma tissue, and both normal and dysplastic Schwann cells can secrete SCF.19,39,47 Moreover, evidence in other experimental models has implicated inflammatory cells such as mast cells in the growth, vascularization, and spread of neoplastic conditions,48-55 and Ingram et al36 had shown that Nf1+/− mast cells are hypersensitive to SCF.
Given these data, investigators studying NF1 have increasingly sought to elucidate the interactions through which Schwann cells, mast cells, fibroblasts, and endothelial cells interact within the neurofibroma and to identify critical molecular and cellular events in neurofibroma pathogenesis.
Schwann cells
In 2001, Mashour et al56 showed that Nf1−/− Schwann cells directly promoted the proliferation of endothelial cells and fibroblastoid cells in vitro. That study implicated the abnormal secretion of fibroblast growth factor 2 (FGF-2), platelet-derived growth factor (PDGF), and midkine from nullizygous Schwann cells as effectors of in vitro cellular mitogenesis. These initial data suggested the potential for Schwann cell–driven cellular growth. Subsequently, Yang et al57 provided mechanistic insight into the aberrant Schwann cell activity driving neurofibroma inflammation. This study found that Nf1−/− Schwann cells secrete pathologic concentrations of SCF and, in turn, the secreted SCF potently recruits Nf1+/− mast cells. Schwann cells derived from Nf1−/− mouse embryos proliferated more quickly than WT cells, displayed an irregular structure, and secreted approximately 6 times as much soluble SCF as did Nf1+/− and WT Schwann cells. Conditioned media from Nf1−/− Schwann cells promoted chemotaxis of Nf1+/− mast cells at twice the rate as WT mast cells. Recombinant SCF reproduced these results in vitro, and the genetic disruption of c-kit or the addition of c-kit receptor blocking antibodies prevented mast cell chemotaxis in response to Schwann cell–conditioned media. Taken in the context of Nf1flox/−Krox20cre tumor model, these data suggested a potentially important interaction between the Nf1−/− Schwann cell and the Nf1+/− mast cell.
SCF- and Nf1-dependent mast cell biochemistry
SCF aberrantly secreted from Nf1−/− Schwann cells promotes a hyperactive SCF-responsive phenotype in Nf1+/− mast cells, and this Nf1-dependent mast cell pathophysiology stems from deregulated Ras signaling. In response to multiple distinct growth factors and cytokines, Ras activates to its GTP-bound state and initiates a series of signal transduction cascades.58-61 Neurofibromin, a highly conserved GAP encoded by 350 kilobases of genomic DNA located on human chromosome 17q11.2 (chromosome 11 in mice) and closely related to the yeast gene products IRA1 and IRA2, converts active Ras from its GTP-bound state to its inactive guanine diphosphate–bound state.10,13,15,20,62,63 Loss of GAPs can induce neoplasia through increased cellular growth, proliferation, and migration.54 Loss of the NF1 GAP in hematopoietic cells, including but not limited to mast cells, increases the latency and potency of GTP-bound Ras and phosphorylated downstream effectors within the Raf–mitogen-activated protein (MAP)/extracellular signal-related kinase (ERK) kinase (MEK)–ERK and phosphoinositide-3-kinase (PI3K)–Rac–p21-activated kinase (Pak)–P38 pathways.36,57,59,64-70
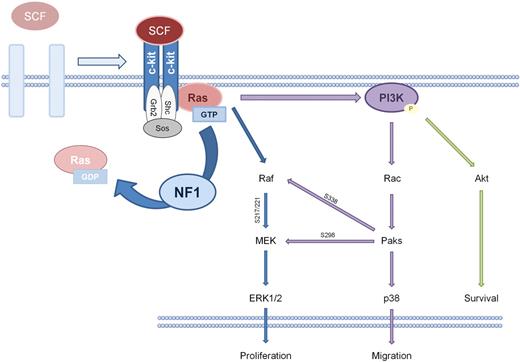
SCF-dependent c-kit signaling in the mast cell hinges specifically on K-ras pathways.67 Immunoprecipitation experiments indicate that SCF:c-kit–activated Ras induces a cascade ultimately phosphorylating and activating p44/p42 (ERK1/2), p38, and AKT.36,68,71,72 In vitro studies of mast cells generated from the marrow of various knockout mice and cells treated with chemical inhibitors of MEK, PI3K, and p38 MAP kinase (MAPK; PD98059, LY294002, and SB203580, respectively) indicate that the Raf-MEK-ERK pathway primarily modulates proliferation and cytokine synthesis, whereas the PI3K-Rac2-Pak-p38 pathway additionally modulates F-actin rearrangement and cellular motility.67,68,70-72 Biochemical studies show that the classical Raf-MEK-ERK cascade and the PI3K-dependent cascade crosstalk through the activity of the Paks. Pak phosphorylates Raf1 at serine 338, which primes Raf1 for increased phosphorylation of MEK at serine 217/222.68 Pak1 can also directly phosphorylate MEK at serine 298. Both of these events potentiate the phosphorylation of ERK1 and ERK2. ERK1 and ERK2 translocate to the nucleus where they phosphorylate progrowth transcription factors such as Elk1 and potentially regulate the G1-to-S phase transition.68,72-74 Additional data indicate that SCF-dependent activation of Rac2, the highly expressed hematopoietic Rho GTPase isoform, phosphorylates Akt, which modulates the Bcl-2 family of proteins to prevent apoptosis, increasing mast cell survival.71 Because neurofibromin negatively regulates Ras-GTP and its multiple downstream targets, these data mechanistically explain aberrant function in the Nf1+/− mast cell, providing potential therapeutic targets along the Raf-MEK-ERK and PI3K-Rac-Pak-p38 pathways (Figure 3).
Schematic of SCF:c-kit signaling in the mast cell. On SCF binding at the c-kit receptor tyrosine kinase (RTK), c-kit dimerizes and autophosphorylates. This phosphorylation promotes the conversion of Ras–guanine diphosphate (GDP) to Ras-GTP, which activates PI3K, MAPK, and Rho GTPase signaling pathways. NF1 potentiates the hydrolysis of Ras-GTP to Ras-GDP, the inactive form of Ras.
Schematic of SCF:c-kit signaling in the mast cell. On SCF binding at the c-kit receptor tyrosine kinase (RTK), c-kit dimerizes and autophosphorylates. This phosphorylation promotes the conversion of Ras–guanine diphosphate (GDP) to Ras-GTP, which activates PI3K, MAPK, and Rho GTPase signaling pathways. NF1 potentiates the hydrolysis of Ras-GTP to Ras-GDP, the inactive form of Ras.
Fibroblasts
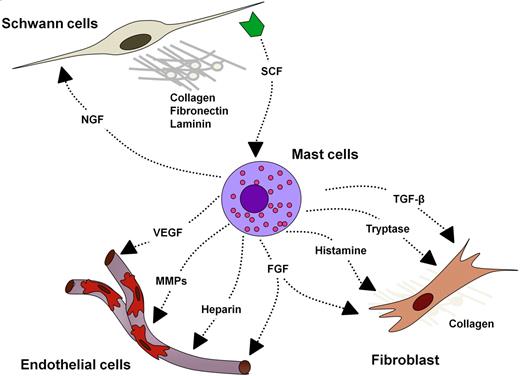
In 2006, Yang et al75 demonstrated another critical neurofibroma microenvironment interaction: SCF-stimulated Nf1+/− mast cells potentiate Nf1+/− fibroblast functions. Fibroblasts comprise a major cellular portion of the neurofibroma, and their secreted collagen accounts for nearly one-half of the dry tumor weight.76 Fibroblasts migrate, proliferate, and synthesize collagen in response to transforming growth factor β (TGF-β). Yang et al75 demonstrated that Nf1+/− mast cells secreted 2.5-fold higher TGF-β than WT mast cells in vitro. Likewise, Nf1+/− fibroblasts cocultured with Nf1+/− mast cells showed the greatest ability to contract collagen in an in vitro lattice system, representing positive cooperation in the remodeling of the extracellular matrix. Nf1+/− mast cell–conditioned media compared with WT-conditioned media induced higher fibroblast bioactivity as measured by proliferation, migration, and collagen production. The study confirmed this heightened fibroblast bioactivity to be TGF-β dependent by neutralizing Nf1+/− mast cell–conditioned media with TGF-β–blocking antibody. These data supported the growing idea that the Nf1+/− mast cell is the critical effector in the paracrine induction of neurofibroma pathogenesis. Interestingly, TGF-β–dependent Nf1+/− fibroblast hyperactivity appears to result from increased kinase activity of c-abl secondary to increased Ras-GTP. Accordingly, imatinib mesylate (Gleevec) inhibits both in vitro collagen production and in vivo fibroblast migration in the dermal tissue of mice given subcutaneous TGF-β or Nf1+/− mast cell–conditioned media (Figure 4).
Potential cellular interactions in the plexiform neurofibroma microenvironment. NGF indicates nerve growth factor; and MMP, matrix metalloproteinase.
Potential cellular interactions in the plexiform neurofibroma microenvironment. NGF indicates nerve growth factor; and MMP, matrix metalloproteinase.
An SCF/c-Kit signaling axis in the Nf1+/− bone marrow cells is required for plexiform neurofibroma formation in a murine model
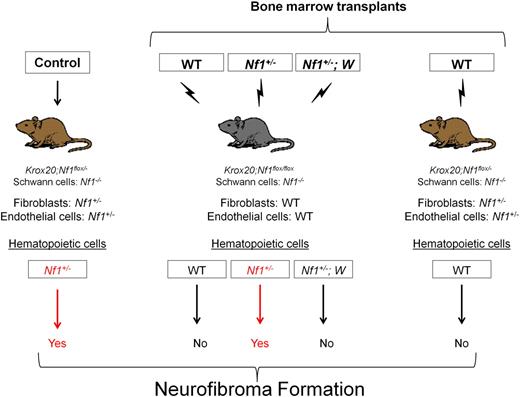
In light of the conditional plexiform neurofibroma model (Nf1flox/−;Krox20cre) and the data implicating interactions between Schwann cells, mast cells, and fibroblasts, Yang et al23 tested the hypothesis that Nf1+/− mast cells are the critical effectors of neurofibroma pathogenesis. The investigators transplanted bone marrow from Nf1+/− mice into lethally irradiated Nf1flox/flox;Krox20cre mice.23 An enhanced green fluorescent protein reporter gene carried by the donors ensured that engrafting hematopoietic cells, and not isolated host progenitor cells, had successfully reconstituted the recipient's bone marrow. These transplants created mice with approximately 10% of Schwann cells Nf1 nullizygous, bone marrow cells Nf1 haploinsufficient, and all other cells functionally wild type. Within 6 months, the Nf1flox/flox;Krox20cre mice reconstituted with Nf1+/− marrow developed neuromotor defects, weight loss, dorsal root ganglia thickening, and an increased mortality rate comparable to the tumorigenic Nf1flox/−;Krox20cre mice. By comparison, Nf1flox/flox;Krox20cre mice reconstituted with WT marrow did not exhibit any of these defects. In a critical converse experiment, transplantation of WT bone marrow into the Nf1flox/−;Krox20cre mouse prevented the normally reliable tumorigenesis of the conditional knockout model. These data validated the hypothesis that Nf1 haploinsufficiency in the marrow microenvironment is both required and sufficient for neurofibroma formation (Figure 5).
To test the hypothesis that SCF-recruited and -stimulated mast cells are the principal hematopoietic effectors of the neurofibroma microenvironment, Yang et al next examined the effect of c-kit inhibition on tumor formation.23 They transplanted bone marrow harboring both the Nf1+/− mutation and 2 different c-kit gene mutations (W41/W41 or Wv/Wv) compromising kinase activity 85% and 92% to 95%, respectively. Nf1flox/flox;Krox20cre mice reconstituted with Nf1+/−;W marrow failed to exhibit the neuromotor defects, dorsal root ganglia enlargement, and mast cell infiltration found in the Nf1flox/−Krox20cre neurofibroma model and the Nf1flox/floxKrox20cre mouse reconstituted with Nf1+/− marrow. Importantly, Southern blot analysis of genomic DNA isolated from the bone marrow and individual myeloid colonies (granulocyte-macrophage colony-forming unit) isolated from the bone marrow of recipients confirmed greater than 95% Nf1+/−;W marrow (donor) engraftment efficiency. These data have shown that neurofibroma formation in a murine model depends on Nf1 LOH in the Schwann cell and c-kit–dependent Nf1 haploinsufficiency in the bone marrow.
Correspondingly, models using other Schwann cell–limited Cre promoters (periostin-Cre, P0-Cre, and tamoxifen-inducible PLPCre) require Nf1+/− and c-kit–dependent hematopoietic contributions.23 However, it should be noted that widespread Nf1−/− deletion in glial cells at the appropriate murine developmental timing (embryonic day 12.5), such as that driven by Dhh-Cre, permits plexiform and dermal neurofibroma formation despite a WT cellular background.77 This model may provide an important explanation for sporadic plexiform neurofibromas occurring in persons without genetic neurofibromatosis type 1.
Pharmacologic studies support SCF/c-kit axis as critical to the neurofibroma microenvironment
Given the evidence that the hematopoietic system and the c-kit pathway contribute to the Krox20cre murine plexiform neurofibroma formation, Yang et al proceeded to treat a cohort of adult Nf1flox/−;Krox20cre mice with the tyrosine kinase inhibitor imatinib mesylate (Gleevec).23 Imatinib mesylate potently inhibits the c-kit, PDGF-β, and bcr/abl receptor tyrosine kinases and currently carries approval from the Food and Drug Administration for the treatment of chronic myelogenous leukemia, other hematologic malignancies, and some solid tumors.78 Mice were followed to an age at which they would be expected to have multiple plexiform neurofibromas, as evidenced by fluorodeoxyglucose (FDG) uptake in positron emission tomography (PET). Further volumetric analysis performed with FDG-PET showed that imatinib mesylate reduced tumor volume and metabolic activity in the Nf1flox/−;Krox20cre mice approximately 50%. By contrast, the placebo-treated controls showed a small increase in FDG uptake in PET. Histologic samples from nerve roots of the treatment group showed regularly patterned Schwann cells and tissue free of mast cell infiltrate, whereas tissue from the placebo group showed characteristic stigmata of neurofibromas.23
Hypothetically, imatinib mesylate prevents mast cell proliferation, infiltration, and exacerbation of the nascent tumor by inhibiting the c-kit receptor tyrosine kinase and, subsequently, inflammation driven by Nf1 haploinsufficient mast cells. Further, imatinib mesylate may reinforce its effect through inhibition of the PDGF receptor and c-abl tyrosine kinases, signaling molecules potentially important to vascular and fibroblastic aberrancies in Nf1+/− tissue.24,75
Imatinib mesylate reduces a highly morbid neurofibroma in a pediatric patient
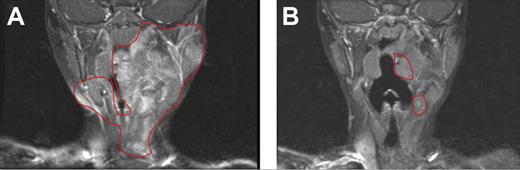
On the basis of these murine experiments implicating c-kit–dependent Nf1+/− marrow cells and the efficacy of imatinib mesylate, clinicians treated a 3-year-old girl with a highly vascularized, nonresectable, and progressively growing neurofibroma. The girl presented as an infant with several hallmark signs of NF1 and a histologically confirmed neurofibroma first appearing at 6 months of age. The tumor had progressively enlarged to encompass the left floor of her mouth, tongue, and mastoid bone and was encasing her carotid artery and jugular vein. At the time of treatment, the tumor had severely compressed her airway, leading to drooling, sleeplessness, and anorexia. After discussing the risks and potential benefits of experimental medical therapy with her physician and her parents, the patient received 350 mg/m2/dose imatinib mesylate. After 3 months of treatment, magnetic resonance imaging showed a remarkable 70% reduction in tumor volume, and the complications associated with the airway compression had resolved. After the tumor ceased its regression and appeared stable, treatment was terminated.23 The patient remains stable and in relatively good health (Figure 6). Encouraged by the mouse model and the successful medical treatment of this index patient, a phase 2 clinical trial involving patients with NF1 with highly morbid neurofibromas has been initiated.
Evaluation of imatinib mesylate efficacy in an index patient with a plexiform neurofibroma. Coronal magnetic resonance imaging scans (T1-weighted images with gadolinium contrast and fat saturation) of the head and oropharynx of a patient with NF1 patient with a plexiform neurofibroma before (A) and 3 months after (B) treatment with imatinib mesylate. The region of the tumor in the respective images is indicated. Reprinted from Yang et al23 with permission from Elsevier.
Evaluation of imatinib mesylate efficacy in an index patient with a plexiform neurofibroma. Coronal magnetic resonance imaging scans (T1-weighted images with gadolinium contrast and fat saturation) of the head and oropharynx of a patient with NF1 patient with a plexiform neurofibroma before (A) and 3 months after (B) treatment with imatinib mesylate. The region of the tumor in the respective images is indicated. Reprinted from Yang et al23 with permission from Elsevier.
Other cell lineages and future directions
Although we have principally discussed interactions among Schwann cells and the hematopoietic microenvironment in the context of neurofibroma formation, other lineages in the microenvironment are clearly involved in tumor progression and are potential targets for drug treatment. Neoangiogenesis is required for tumor expansion and metastasis in multiple human cancers,54,79 and previous data indicated that Nf1−/− Schwann cells can initiate neoangiogenesis by secreting vascular endothelial growth factor (VEGF).80 Likewise, Munchhof et al25 demonstrated that Nf1−/−-cultured Schwann cell media placed intradermally into Nf1+/− mice produced hyperactive angiogenic responses similar to the intradermal placement of VEGF and basic FGF (bFGF). In vitro, VEGF and bFGF treatment of murine and human NF1-cultured endothelial cells increased cell proliferation and migration and showed hyperphosphorylation of ERK1 and ERK2. The addition of a MEK inhibitor (PD98059) diminished these effects, including the vascularization of Matrigel plugs treated with VEGF, bFGF, or Schwann cell–conditioned media and placed within the skin of experimental mice. Similarly, Nf1+/− vascular smooth muscle cells showed increased migration and proliferation, providing an additional Ras-dependent mechanism enabling neoangiogenesis.24 However, many of the NF1-dependent biochemical mechanisms within nonneoplastic tissue such as the vasculature need further exploration.
In addition, although the experimental data to date show a clear role for c-kit/SCF signaling in the initiation of tumorigenesis, the exact processes within mast cells and potentially other c-kit responsive hematopoietic cells that promote this pathologic progression still need to be defined. Nf1+/− mast cells secrete a number of matrix metalloproteinases, cytokines (eg, interleukin-6, tumor necrosis factor-α, CCL2, CCL3, CCL4, and C5a), and growth factors (eg, nerve growth factor, VEGF, PDGF, bFGF, and TGF-β). These secreted factors, along with factors secreted by the Schwann cell, stimulate fibroblasts, endothelial cells, and smooth muscle cells, promoting extracellular matrix remodeling, collagen deposition, chemotaxis, neoangiogenesis, and generalized inflammation.19,23,36,46,57,67,68,72,75 Whether a subset of these secreted molecules has a particularly seminal role in the initiation of tumorigenesis is an area of experimentation that may provide more specific therapies and potentially inform about other neoplastic conditions potentiated by inflammatory microenvironments.52,53,55 Importantly, a deeper understanding of the cellular and biochemical mechanisms inducing tumor regression during imatinib mesylate treatment would directly inform, and perhaps improve, the medical management of human patients with established plexiform neurofibromas. Finally, although mast cell inflammation underpins the murine plexiform neurofibroma microenvironment and undoubtedly contributes to the human condition, we have not explored cellular events required for the transition to the malignant peripheral nerve sheath tumor. The continuing refinement of murine models, the use of mouse genetics, and advances in vivo experimental imaging are important tools that may help provide additional insights into these processes.
Acknowledgments
This work was supported, in part, by National Institutes of Health–National Cancer Institute (RO1 CA074177-11A1/D and P50 NS052606-04; F.-C.Y. and D.W.C.). K.S. was additionally supported by the Howard Hughes Medical Institute and a predoctoral fellowship from the National Institutes of Health (grant T32 CA111198).
National Institutes of Health
Authorship
Contribution: K.S. conducted literature review and analysis, prepared the figures, and composed the paper; F.-C.Y. offered critical insight and analysis; and D.W.C. offered critical insight and analysis and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: D. Wade Clapp, Cancer Research Institute, 1044 W Walnut St, Bldg R4, Rm 402B, Indianapolis, IN, 46202; e-mail: dclapp@iupu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal