Abstract
Although some trials have allowed matched or single human leukocyte antigen (HLA)–mismatched related donors (mmRDs) along with HLA-matched sibling donors (MSDs) for pediatric bone marrow transplantation in early-stage hematologic malignancies, whether mmRD grafts lead to similar outcomes is not known. We compared patients < 18 years old reported to the Center for International Blood and Marrow Transplant Research with acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, and myelodysplastic syndrome undergoing allogeneic T-replete, myeloablative bone marrow transplantation between 1993 and 2006. In total, patients receiving bone marrow from 1208 MSDs, 266 8/8 allelic-matched unrelated donors (URDs), and 151 0-1 HLA-antigen mmRDs were studied. Multivariate analysis showed that recipients of MSD transplants had less transplantation-related mortality, acute graft-versus-host disease (GVHD), and chronic GVHD, along with better disease-free and overall survival than the URD and mmRD groups. No differences were observed in transplant-related mortality, acute and chronic GVHD, relapse, disease-free survival, or overall survival between the mmRD and URD groups. These data show that mmRD and 8/8 URD outcomes are similar, whereas MSD outcomes are superior to the other 2 sources. Whether allele level typing could identify mmRD recipients with better outcomes will not be known unless centers alter practice and type mmRD at the allele level.
Introduction
The benchmark for the best survival of children undergoing myeloablative bone marrow transplantation (BMT) for leukemia has been the results obtained with the use of human leukocyte antigen (HLA)–matched sibling donors (MSDs). This is conventionally referred to as a “6/6” match, that is, a tissue type match at a low level of resolution at the HLA region at HLA-A and -B and at the allelic level at DRB1. In most related cases, this equates to a complete allelic match along both HLA haplotypes, together with the associated genes. Only one-third of patients have an MSD. Alternative donor types are critical for the remaining two-thirds of the population.
In the 1980s and early 1990s, when unrelated transplantation was not an option for most patients, attempts were made to use family donors who were less well matched than an HLA-identical sibling. This resulted in several protocols that offered transplantation with the use of a sibling donor with a single HLA antigen mismatch or a family donor with a zero or one HLA antigen mismatch (mismatched related donors, mmRDs). Some studies suggested that survival rates achieved with the use of these donors were similar to outcomes after MSD transplantation; this was on the basis of a higher risk of graft-versus-host disease (GVHD) being counterbalanced by a reduced risk of relapse, the 2 main adverse outcomes of allogeneic BMT for malignant diseases.1-4 These observations supported the practice of using mmRDs in patients in early-stage disease, similar to MSDs. Over the past 2 decades, however, the indications for transplantation have been refined as results with the use of chemotherapy have improved and risk groups have been defined with more precision. With this in mind, it is important to continuously reevaluate the risk/benefit ratio of subjecting patients to transplantation, including different types of donors, compared with chemotherapy approaches.
Several retrospective pediatric studies have reported series of patients where the outcome is similar for recipients of MSD transplants and unrelated donor (URD) transplants.4-10
Recent publications relating to the use of URD show a clear relationship between the level of HLA-allelic match and outcome.11,12 To clarify data about choice of donors, we organized a study to reevaluate the role of transplantations with the use of mmRD in the current era in a purely pediatric population and to compare outcomes after BMT with the use of an MSD or URD. This study compares the outcomes of BMT with one-antigen mmRDs (5/6 match) or phenotypically matched nonsibling related donors versus MSD versus HLA-A, -B, -C, and DRB1 (8/8) allele-matched URDs in pediatric patients with leukemia and myelodysplasia.
Methods
Data collection
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research affiliation of the International Bone Marrow Transplant Registry, Autologous Blood and Marrow Transplant Registry, and the National Marrow Donor Program (NMDP) established in 2004 that comprises a voluntary working group of > 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic stem cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplantations consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally with yearly follow-up. Computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority, and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the institutional review boards of the NMDP and the Medical College of Wisconsin since 1985. All participants provided informed consent for inclusion of clinical data and biospecimens, in accordance with the Declaration of Helsinki.
Study population
This study was restricted to pediatric patients (< 18 years) with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and myelodysplastic syndrome undergoing a first allogeneic transplantation from 1993 to 2006. In total, transplantations that used 1208 MSDs, 97 one-antigen mmRDs, 54 phenotypically matched nonsibling related donors, and 266 8/8 allele-matched URDs were included in the study. The inclusion and exclusion criteria are shown in detail in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
To reduce heterogeneity in the study population, we limited the study to those patients who received myeloablative conditioning with bone marrow as the only source of cells. Prophylaxis against GVHD was limited to calcineurin inhibitors, with or without methotrexate, and no ex vivo T-cell depletion was permitted.
Data on matched siblings were used only from institutions who also contributed transplantation data for the mismatched related transplant group. Selection of the donor and care of the recipient, including prophylactic therapies, was per an institution's standards. The level of HLA mismatch for one-antigen mismatch and phenotypically matched related group was considered at the 6/6 antigen level at HLA-A, -B, and -DRB1 and was verified by review of HLA reports provided by the reporting center. Allele level typing was rarely obtained by transplantation centers for the mismatch related group; it was reported to CIBMTR in only 2% of cases from 2001 to 2006.
The URD group was restricted to a subset whose typing had been confirmed through the NMDP high-resolution retrospective typing program as being an 8/8 allelic match at HLA-A, -B, -C, and -DRB1 with the patients.13 All surviving unrelated recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the NMDP research program. Approximately 9% of surviving patients would not provide consent for use of the research data. To adjust for the potential bias introduced by exclusion of nonconsenting surviving patients, a corrective action plan modeling process randomly excluded appropriately the same percentage of deceased patients with the use of a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors (CAP modeling).12 Patient-, disease-, and transplantation-related characteristics are listed in Table 1.
Characteristics of pediatric patients with AML, ALL, CML, or MDS; T cell–replete BM grafts and myeloablative conditioning regimen, who underwent MSD transplant vs 1-antigen mmRD transplant vs 8/8 matched URD transplant reported to CIBMTR from 1993 to 2006
| Patient characteristic . | MSD . | mmRD . | URD . | P . | ||
|---|---|---|---|---|---|---|
| MSD vs mmRD . | mmRD vs URD . | MSD vs URD . | ||||
| No. of patients | 1208 | 151 | 266 | |||
| Median age, y, (range) | 9 (< 1-17) | 8 (< 1-17) | 9 (< 1-17) | .220 | .099 | .320 |
| Male sex, n (%) | 729 (60) | 96 (64) | 162 (61) | .444 | .589 | .867 |
| Performance score | .335 | .001 | < .001 | |||
| < 90, n (%) | 154 (13) | 25 (17) | 41 (15) | |||
| ≥ 90, n (%) | 1037 (86) | 125 (83) | 199 (75) | |||
| Unknown, n (%) | 17 (1) | 1 (<1) | 26 (10) | |||
| Disease | .004 | .293 | .001 | |||
| AML, n (%) | 447 (37) | 42 (28) | 67 (25) | |||
| ALL, n (%) | 559 (46) | 67 (44) | 136 (51) | |||
| CML, n (%) | 97 (8) | 23 (15) | 26 (10) | |||
| MDS, n (%) | 105 (9) | 19 (13) | 37 (14) | |||
| Disease status* | .144 | .002 | < .001 | |||
| Early, n (%) | 608 (50) | 69 (46) | 76 (29) | |||
| Intermediate, n (%) | 445 (37) | 54 (36) | 134 (50) | |||
| Advanced, n (%) | 155 (13) | 28 (19) | 56 (21) | |||
| TBI use in conditioning | .387 | < .001 | < .001 | |||
| No, n (%) | 565 (47) | 65 (43) | 53 (20) | |||
| Yes, n (%) | 643 (53) | 86 (57) | 213 (80) | |||
| ATG use in conditioning | < .001 | .949 | < .001 | |||
| No, n (%) | 1191 (99) | 123 (81) | 216 (81) | |||
| Yes, n (%) | 17 (1) | 28 (19) | 50 (19) | |||
| GVHD prophylaxis | .004 | .081 | < .001 | |||
| CSA/FK506 ± others (no MTX), n (%) | 258 (21) | 17 (15) | 17 (6) | |||
| MTX ± CSA/FK506, n (%) | 950 (79) | 134 (85) | 249 (94) | |||
| Median time from diagnosis to transplantation, mo (range) | 7 (< 1 to 153) | 8 (1-94) | 14 (< 1 to 115) | .409 | .002 | < .001 |
| Unknown, n (%) | 3 (< 1) | 0 | 0 | |||
| Donor relationship | ||||||
| Sibling, not identical twin, n (%) | 1208 (100) | 43 (28) | 0 | |||
| Parent of recipient, n (%) | 0 | 88 (58) | 0 | |||
| Child of recipient, n (%) | 0 | 2 (1) | 0 | |||
| Other relative, n (%) | 0 | 18 (12) | 0 | |||
| Unrelated, n (%) | 0 | 0 | 266 (100) | |||
| Donor/recipient sex match | .584 | .001 | < .001 | |||
| Male/male, n (%) | 378 (31) | 47 (31) | 119 (45) | |||
| Male/female, n (%) | 254 (21) | 25 (17) | 58 (22) | |||
| Female/male, n (%) | 351 (29) | 49 (32) | 43 (16) | |||
| Female/female, n (%) | 225 (19) | 30 (20) | 46 (17) | |||
| Donor/recipient CMV match | .001 | < .001 | < .001 | |||
| −/−, n (%) | 382 (32) | 38 (25) | 122 (46) | |||
| −/+, n (%) | 170 (14) | 18 (12) | 52 (20) | |||
| +/−, n (%) | 115 (10) | 30 (20) | 43 (16) | |||
| +/+, n (%) | 500 (41) | 56 (37) | 46 (17) | |||
| Unknown, n (%) | 41 (3) | 9 (6) | 3 (1) | |||
| Median donor age, y, (range) | 10 (< 1 to 45) | 31 (< 1 to 66) | 36 (19-57) | < .001 | < .001 | < .001 |
| Unknown, n (%) | 12 (1) | 4 (3) | 0 | |||
| Year of transplantation | .375 | < .001 | < .001 | |||
| 1993-1999 | 912 (75) | 109 (72) | 119 (45) | |||
| 2000-2006 | 296 (25) | 42 (28) | 147 (55) | |||
| Median follow-up of survivors, mo (range) | 79 (2-171) | 62 (3-177) | 61 (12-168) | |||
| Region of transplant center | ||||||
| United States, n (%) | 316 (26) | 49 (32) | 261 (98) | |||
| Canada, n (%) | 89 (7) | 15 (10) | 0 | |||
| Europe, n (%) | 251 (21) | 27 (18) | 4 (2) | |||
| Asia, n (%) | 53 (4) | 8 (5) | 0 | |||
| Australia/New Zealand, n (%) | 135 (11) | 20 (13) | 0 | |||
| Mideast/Africa, n (%) | 290 (24) | 17 (11) | 0 | |||
| Central/South America, n (%) | 74 (6) | 15 (10) | 1 (< 1) | |||
| Patient characteristic . | MSD . | mmRD . | URD . | P . | ||
|---|---|---|---|---|---|---|
| MSD vs mmRD . | mmRD vs URD . | MSD vs URD . | ||||
| No. of patients | 1208 | 151 | 266 | |||
| Median age, y, (range) | 9 (< 1-17) | 8 (< 1-17) | 9 (< 1-17) | .220 | .099 | .320 |
| Male sex, n (%) | 729 (60) | 96 (64) | 162 (61) | .444 | .589 | .867 |
| Performance score | .335 | .001 | < .001 | |||
| < 90, n (%) | 154 (13) | 25 (17) | 41 (15) | |||
| ≥ 90, n (%) | 1037 (86) | 125 (83) | 199 (75) | |||
| Unknown, n (%) | 17 (1) | 1 (<1) | 26 (10) | |||
| Disease | .004 | .293 | .001 | |||
| AML, n (%) | 447 (37) | 42 (28) | 67 (25) | |||
| ALL, n (%) | 559 (46) | 67 (44) | 136 (51) | |||
| CML, n (%) | 97 (8) | 23 (15) | 26 (10) | |||
| MDS, n (%) | 105 (9) | 19 (13) | 37 (14) | |||
| Disease status* | .144 | .002 | < .001 | |||
| Early, n (%) | 608 (50) | 69 (46) | 76 (29) | |||
| Intermediate, n (%) | 445 (37) | 54 (36) | 134 (50) | |||
| Advanced, n (%) | 155 (13) | 28 (19) | 56 (21) | |||
| TBI use in conditioning | .387 | < .001 | < .001 | |||
| No, n (%) | 565 (47) | 65 (43) | 53 (20) | |||
| Yes, n (%) | 643 (53) | 86 (57) | 213 (80) | |||
| ATG use in conditioning | < .001 | .949 | < .001 | |||
| No, n (%) | 1191 (99) | 123 (81) | 216 (81) | |||
| Yes, n (%) | 17 (1) | 28 (19) | 50 (19) | |||
| GVHD prophylaxis | .004 | .081 | < .001 | |||
| CSA/FK506 ± others (no MTX), n (%) | 258 (21) | 17 (15) | 17 (6) | |||
| MTX ± CSA/FK506, n (%) | 950 (79) | 134 (85) | 249 (94) | |||
| Median time from diagnosis to transplantation, mo (range) | 7 (< 1 to 153) | 8 (1-94) | 14 (< 1 to 115) | .409 | .002 | < .001 |
| Unknown, n (%) | 3 (< 1) | 0 | 0 | |||
| Donor relationship | ||||||
| Sibling, not identical twin, n (%) | 1208 (100) | 43 (28) | 0 | |||
| Parent of recipient, n (%) | 0 | 88 (58) | 0 | |||
| Child of recipient, n (%) | 0 | 2 (1) | 0 | |||
| Other relative, n (%) | 0 | 18 (12) | 0 | |||
| Unrelated, n (%) | 0 | 0 | 266 (100) | |||
| Donor/recipient sex match | .584 | .001 | < .001 | |||
| Male/male, n (%) | 378 (31) | 47 (31) | 119 (45) | |||
| Male/female, n (%) | 254 (21) | 25 (17) | 58 (22) | |||
| Female/male, n (%) | 351 (29) | 49 (32) | 43 (16) | |||
| Female/female, n (%) | 225 (19) | 30 (20) | 46 (17) | |||
| Donor/recipient CMV match | .001 | < .001 | < .001 | |||
| −/−, n (%) | 382 (32) | 38 (25) | 122 (46) | |||
| −/+, n (%) | 170 (14) | 18 (12) | 52 (20) | |||
| +/−, n (%) | 115 (10) | 30 (20) | 43 (16) | |||
| +/+, n (%) | 500 (41) | 56 (37) | 46 (17) | |||
| Unknown, n (%) | 41 (3) | 9 (6) | 3 (1) | |||
| Median donor age, y, (range) | 10 (< 1 to 45) | 31 (< 1 to 66) | 36 (19-57) | < .001 | < .001 | < .001 |
| Unknown, n (%) | 12 (1) | 4 (3) | 0 | |||
| Year of transplantation | .375 | < .001 | < .001 | |||
| 1993-1999 | 912 (75) | 109 (72) | 119 (45) | |||
| 2000-2006 | 296 (25) | 42 (28) | 147 (55) | |||
| Median follow-up of survivors, mo (range) | 79 (2-171) | 62 (3-177) | 61 (12-168) | |||
| Region of transplant center | ||||||
| United States, n (%) | 316 (26) | 49 (32) | 261 (98) | |||
| Canada, n (%) | 89 (7) | 15 (10) | 0 | |||
| Europe, n (%) | 251 (21) | 27 (18) | 4 (2) | |||
| Asia, n (%) | 53 (4) | 8 (5) | 0 | |||
| Australia/New Zealand, n (%) | 135 (11) | 20 (13) | 0 | |||
| Mideast/Africa, n (%) | 290 (24) | 17 (11) | 0 | |||
| Central/South America, n (%) | 74 (6) | 15 (10) | 1 (< 1) | |||
MDS indicates matched sibling donor; mmRD, mismatch related donor/phenotypically matched donor; URD, unrelated donor; CIBMTR, Center for International Blood and Marrow Transplant Research; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; MDS, myelodysplastic syndrome; TBI, total body irradiation; ATG, antithymocyte globulin; GVHD, graft-versus-host disease; CSA, cyclosporine; FK506, tacrolimus; MTX, methotrexate; and CMV, cytomegalovirus.
Early disease is defined as patients with ALL and AML in first complete remission, CML in first chronic phase and myelodysplasia with refractory anemia or acquired idiopathic sideroblastic anemia. Intermediate is defined as ALL and AML in second or greater complete remission, CML in accelerated phase or second or greater chronic phase; and advanced is defined as primary induction failure of ALL and AML, blastic phase of CML, relapse, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, chronic myelomonocytic leukemia and juvenile myelomonocytic leukemia.
Endpoints
The objective of this study was to compare the outcomes of BMT with the use of a one-antigen mmRD or phenotypically matched related donor versus an MSD versus an 8/8 matched URD in pediatric patients with malignant diseases. The main outcomes analyzed were treatment-related mortality (TRM), acute and chronic GVHD, relapse, disease-free survival (DFS), and overall survival (OS). The acute GVHD endpoint referred to the development of grades 2-4 and grades 3-4 according to the Glucksberg criteria.14 Chronic GVHD was diagnosed following the standard definitions.15 Relapse consisted of leukemia relapse or myelodysplastic syndrome recurrence, whereas TRM was death resulting from any cause other than relapse. DFS was defined as survival in complete remission after BMT. For OS, death from any cause was considered an event. All living patients were censored at last follow-up.
Statistical analysis
An analysis was performed to look for differences in outcomes between the phenotypically matched and 1-antigen mmRD groups. Because we were performing a large number of comparisons, P < .01 was considered significant (see Table 2). No statistically significant differences were observed in major outcomes between these 2 donor groups; therefore, they were combined together for the subsequent analysis. For consistency, this group will be referred to as the mmRD group. Frailty models were used to test for center effects, and no statistically significant evidence for center effects was found. Patient-, disease-, and transplant-related variables were compared between any 2 of the 3 groups (MSD, URD, mmRD) with the use of the χ2 statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Occurrence of GVHD, TRM, and disease relapse were calculated with cumulative incidence estimates, taking into account the competing risk.16 Probabilities of DFS and OS were estimated from the time of transplantation with the use of Kaplan-Meier curves.17
Multivariate comparison of phenotypically matched related with 1-antigen mismatched related transplants (RR = 1.00)
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Acute GVHD grade 2-4 | 0.77 (0.52-1.38) | .503 |
| Acute GVHD grade 3-4 | 0.62 (0.32-1.22) | .168 |
| Chronic GVHD | 0.52 (0.28-0.97) | .039 |
| Relapse | 0.56 (0.28-1.13) | .107 |
| TRM | 0.88 (0.46-1.70) | .704 |
| Disease-free survival | 0.72 (0.45-1.17) | .186 |
| Overall survival | 0.85 (0.52-1.38) | .503 |
| Variable . | RR (95% CI) . | P . |
|---|---|---|
| Acute GVHD grade 2-4 | 0.77 (0.52-1.38) | .503 |
| Acute GVHD grade 3-4 | 0.62 (0.32-1.22) | .168 |
| Chronic GVHD | 0.52 (0.28-0.97) | .039 |
| Relapse | 0.56 (0.28-1.13) | .107 |
| TRM | 0.88 (0.46-1.70) | .704 |
| Disease-free survival | 0.72 (0.45-1.17) | .186 |
| Overall survival | 0.85 (0.52-1.38) | .503 |
RR indicates relative risk; CI, confidence interval; GVHD, graft-versus-host disease; and TRM, treatment-related mortality.
In addition to the type of donor (MSD vs mmRD vs URD), which was included in all models, the following variables were analyzed: patient age, sex, performance score, disease, disease stage, use of total body irradiation (TBI) in conditioning, use of antithymocyte globulin in conditioning, GVHD prophylaxis, time between diagnosis and transplantation, donor/recipient sex match, donor/recipient cytomegalovirus status, and year of transplantation (Table 1). Early disease is defined as patients with ALL and AML in first complete remission, CML in first chronic phase, and myelodysplasia with refractory anemia or acquired idiopathic sideroblastic anemia. Intermediate is defined as ALL and AML in second or greater complete remission or as CML in accelerated phase or second or greater chronic phase; and advanced is defined as primary induction failure of ALL and AML, blastic phase of CML, relapse, refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, chronic myelomonocytic leukemia/juvenile myelomonocytic leukemia.
For the multivariate analysis, Cox proportional hazards regression models were applied. The proportional hazards assumption was assessed for each covariate with the use of a time-dependent covariate approach. Covariates that violated the proportional hazard assumption were adjusted by stratification. The interaction between the donor type and the other covariates was then checked. Stepwise forward-backward selection was used to build the models from the prognostic factors under consideration. Because of multiple comparisons included in the statistical analysis, a P < .01 was defined as statistically significant. All P values were 2-sided. All analyses were performed with SAS 9.1 (SAS Institute).
After completion of the main analysis, a subanalysis was performed on that portion of the mmRD in which typing was available at HLA-C antigen. Because these donors could be classified at 4 loci, these 8/8 parents or 7/8 siblings or parents are referred to as the mmRD subgroup. Univariate and multivariate comparisons were performed. Further analysis of patients by allele level typing was not possible, because only a small fraction (∼ 2%) of the sample had been typed at the allele level.
Results
Mismatched related donor group
The group of 151 mmRDs included 43 siblings, 88 parents, 2 children, and 18 other relatives (Table 1). Fifty-four were a 6/6 match, meaning that the reviewed HLA report showed a low-resolution match at A, B, and DRB1. Ninety-seven had a mismatch at one antigen; at the A locus in 48 cases, at B in 19 cases, and at DRB1 in 30 cases. Antigen level HLA-C typing was available in approximately one-half of the cases; being reported in only 46% of donors used between 1993 and 1999 and 61% between 2000 and 2006 (not statistically significant; χ2P = .132). The small sample size precluded the analysis of locus-specific mismatch effects. Because the reported data rarely included allele-level typing, it was not possible to classify these donors by allele-level mismatches. In addition, pedigree data were not available to classify the mismatches as crossover events or as phenotype matches with different haplotypes. A comparison of the phenotypically matched group with the one-antigen mismatched group by multivariate analysis did not show statistically significant differences (Table 2). Note that in general, the relative risks favored the phenotypically matched donors over the one-antigen mismatched donors, but only the P value for chronic GVHD approached but did not exceed our cutoff of .01 for significance. Given the similarity of outcomes of the phenotypically matched and one-antigen mismatched subsets, the groups were pooled as the mmRD group for the main analyses.
Patient, disease, and transplant characteristics
Details of the patient groups are shown in Table 1. Patients receiving an URD transplant had more intermediate/advanced disease (71% vs 50% for MSD vs 55% for mmRD) and had a longer time from diagnosis to transplantation with a median of 14 months compared with 7 months for MSD and 8 months for mmRD. Recipients of URD transplants were more likely to be conditioned with TBI (80%) than the MSD (53%) or mmRD (57%) group. Of note, for the key characteristics mentioned earlier (advanced vs early disease, time to BMT, TBI conditioning), along with other important characteristics (sex mismatches, cytomegalovirus status), the mmRD group most closely resembled the MSD group, suggesting a use pattern of mmRDs similar to MSDs by pediatric centers. The median follow-up for survivors was 79 months for MSDs, 62 months for mmRDs, and 61 months for URDs.
Graft-versus-host disease
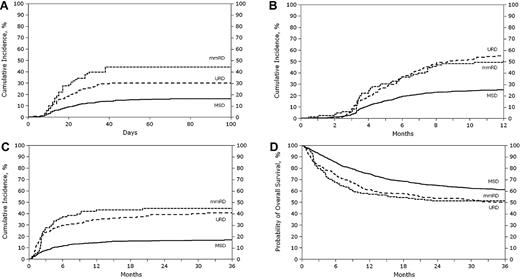
Univariate analysis showed that the probability of grades 2-4 acute GVHD at 100 days in the MSD group was less than that in the mmRD or URD group, with 29% (95% confidence interval [CI], 26%-31%), 56% (95% CI, 48%-64%), and 45%(95% CI, 39%-51%), respectively (Table 3). Similarly, the rate of grades 3-4 acute GVHD at 100 days was less in the MSD group with 10% (95% CI, 8%-11%), compared with 26% (95% CI, 20%-34%) in the mmRD group, and 18% (95% CI, 14%-23%) in the URD group (Figure 1A). Chronic GVHD at 1 year was 15% (95% CI, 13%-17%) for MSD, half that of the 30% (95% CI, 23%-38%) for the mmRD or 33% (95% CI, 27%-39%) for the URD group (Figure 1B). Extensive chronic GVHD at 1 year was 6% (95% CI, 5%-7%) for MSDs, significantly less than the 13% (95% CI, 8%-19%) for mmRDs or 21% (95% CI, 17%-27%) for URDs (Table 3).
Univariate probabilities of outcomes
| Outcome event . | MSD . | mmRD . | URD . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | Probability (95% CI), % . | No. . | Probability (95% CI), % . | No. . | Probability (95% CI), % . | MSD vs mmRD . | mmRD vs URD . | MSD vs URD . | |
| Acute 2-4 GVHD | 1202 | 151 | 265 | ||||||
| At 100 d | 29 (26-31) | 56 (48-64) | 45 (39-51) | < .001 | .031 | < .001 | |||
| Acute 3-4 GVHD | 1206 | 151 | 266 | ||||||
| At 100 d | 10 (8-11) | 26 (20-34) | 18 (14-23) | < .001 | .051 | < .001 | |||
| Chronic GVHD | 1200 | 147 | 263 | ||||||
| At 1 y | 15 (13-17) | 30 (23-38) | 33 (27-39) | < .001 | .573 | < .001 | |||
| Extensive chronic GVHD | 1203 | 149 | 264 | ||||||
| At 1 y | 6 (5-7) | 13 (8-19) | 21 (17-27) | < .001 | .029 | < .001 | |||
| Relapse | 1197 | 148 | 264 | ||||||
| At 1 y | 27 (24-29) | 20 (14-27) | 25 (20-31) | .054 | .189 | .667 | |||
| At 2 y | 33 (30-36) | 25 (18-33) | 30 (25-36) | .046 | .301 | .331 | |||
| At 3 y | 36 (33-39) | 29 (22-37) | 32 (27-38) | .096 | .524 | .229 | |||
| TRM | 1197 | 148 | 264 | ||||||
| At 1 y | 8 (7-10) | 26 (19-33) | 21 (16-26) | < .001 | .253 | < .001 | |||
| At 2 y | 10 (8-11) | 27 (20-34) | 24 (19-29) | < .001 | .485 | < .001 | |||
| At 3 y | 10 (8-12) | 27 (20-34) | 24 (19-30) | < .001 | .615 | < .001 | |||
| Disease-free survival | 1197 | 148 | 264 | ||||||
| At 1 y | 65 (62-67) | 54 (46-62) | 54 (48-60) | .017 | .914 | .001 | |||
| At 2 y | 57 (54-60) | 48 (40-56) | 46 (40-52) | .041 | .754 | .001 | |||
| At 3 y | 54 (51-57) | 44 (36-53) | 43 (37-49) | .035 | .875 | .002 | |||
| Overall survival | 1208 | 151 | 266 | ||||||
| At 1 y | 75 (73-78) | 57 (49-65) | 61 (55-67) | < .001 | .438 | < .001 | |||
| At 2 y | 65 (62-68) | 51 (43-60) | 54 (48-60) | .014 | .669 | < .001 | |||
| At 3 y | 61 (58-64) | 51 (43-60) | 50 (44-56) | .026 | .806 | .001 | |||
| Outcome event . | MSD . | mmRD . | URD . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | Probability (95% CI), % . | No. . | Probability (95% CI), % . | No. . | Probability (95% CI), % . | MSD vs mmRD . | mmRD vs URD . | MSD vs URD . | |
| Acute 2-4 GVHD | 1202 | 151 | 265 | ||||||
| At 100 d | 29 (26-31) | 56 (48-64) | 45 (39-51) | < .001 | .031 | < .001 | |||
| Acute 3-4 GVHD | 1206 | 151 | 266 | ||||||
| At 100 d | 10 (8-11) | 26 (20-34) | 18 (14-23) | < .001 | .051 | < .001 | |||
| Chronic GVHD | 1200 | 147 | 263 | ||||||
| At 1 y | 15 (13-17) | 30 (23-38) | 33 (27-39) | < .001 | .573 | < .001 | |||
| Extensive chronic GVHD | 1203 | 149 | 264 | ||||||
| At 1 y | 6 (5-7) | 13 (8-19) | 21 (17-27) | < .001 | .029 | < .001 | |||
| Relapse | 1197 | 148 | 264 | ||||||
| At 1 y | 27 (24-29) | 20 (14-27) | 25 (20-31) | .054 | .189 | .667 | |||
| At 2 y | 33 (30-36) | 25 (18-33) | 30 (25-36) | .046 | .301 | .331 | |||
| At 3 y | 36 (33-39) | 29 (22-37) | 32 (27-38) | .096 | .524 | .229 | |||
| TRM | 1197 | 148 | 264 | ||||||
| At 1 y | 8 (7-10) | 26 (19-33) | 21 (16-26) | < .001 | .253 | < .001 | |||
| At 2 y | 10 (8-11) | 27 (20-34) | 24 (19-29) | < .001 | .485 | < .001 | |||
| At 3 y | 10 (8-12) | 27 (20-34) | 24 (19-30) | < .001 | .615 | < .001 | |||
| Disease-free survival | 1197 | 148 | 264 | ||||||
| At 1 y | 65 (62-67) | 54 (46-62) | 54 (48-60) | .017 | .914 | .001 | |||
| At 2 y | 57 (54-60) | 48 (40-56) | 46 (40-52) | .041 | .754 | .001 | |||
| At 3 y | 54 (51-57) | 44 (36-53) | 43 (37-49) | .035 | .875 | .002 | |||
| Overall survival | 1208 | 151 | 266 | ||||||
| At 1 y | 75 (73-78) | 57 (49-65) | 61 (55-67) | < .001 | .438 | < .001 | |||
| At 2 y | 65 (62-68) | 51 (43-60) | 54 (48-60) | .014 | .669 | < .001 | |||
| At 3 y | 61 (58-64) | 51 (43-60) | 50 (44-56) | .026 | .806 | .001 | |||
MSD indicates matched sibling donor transplant; mmRD, 1-antigen mismatch related donor/phenotypically matched donor transplant; URD, 8/8 matched unrelated donor transplant; CI, confidence interval; GVHD, graft-versus-host disease; and TRM, treatment-related mortality.
Probabilities of acute, chronic GVHD, TRM, and relapse were calculated with the cumulative incidence estimate. Overall survival and disease-free survival were calculated with the Kaplan-Meier product limit estimate.
Unadjusted curves of acute GVHD grades 3-4, chronic GVHD, TRM, and OS by donor types. (A) Cumulative incidence of acute GVHD grades 3-4. (B) Cumulative incidence of chronic GVHD. (C) Cumulative incidence of TRM. (D) Probability of OS.
Unadjusted curves of acute GVHD grades 3-4, chronic GVHD, TRM, and OS by donor types. (A) Cumulative incidence of acute GVHD grades 3-4. (B) Cumulative incidence of chronic GVHD. (C) Cumulative incidence of TRM. (D) Probability of OS.
Multivariate analysis confirmed lower rates of GVHD in MSD recipients. Patients who received a transplant from an MSD had lower risks of grades 2-4 and 3-4 acute GVHD and chronic GVHD than the mmRD or URD groups (all P < .001; Table 4). URD recipients had a significantly lower risk of acute GVHD grades 2-4 compared with mmRD (relative risk [RR], 0.65; 95% CI, 0.49-0.86; P = .003), but differences in grades 3-4 acute GVHD or chronic GVHD between mmRDs and URDs were not significant.
Multivariate analysis comparing MSD with URD with mmRD
| Outcome . | mmRD vs MSD . | URD vs MSD . | URD vs mmRD . | |||
|---|---|---|---|---|---|---|
| RR (95%CI) . | P . | RR (95%CI) . | P . | RR (95%CI) . | P . | |
| Acute GVHD 2-4* | 2.70 (2.13-3.42) | < .001 | 1.76 (1.42-2.18) | < .001 | 0.65 (0.49-0.86) | .003 |
| Acute GVHD 3-4† | 3.41 (2.38-4.89) | < .001 | 2.54 (1.78-3.64) | < .001 | 0.75 (0.49-1.14) | .177 |
| Chronic GVHD‡ | 3.09 (2.24-4.27) | < .001 | 3.92 (3.00-5.13) | < .001 | 1.27 (0.88-1.82) | .198 |
| TRM§ | 3.01 (2.10-4.33) | < .001 | 3.48 (2.54-4.77) | < .001 | 1.16 (0.77-1.64) | .739 |
| Relapse‖ | 0.90 (0.65-1.24) | .516 | 0.98 (0.76-1.27) | .874 | 1.09 (0.74-1.60) | .661 |
| Disease-free survival¶ | 1.38 (1.09-1.75) | .008 | 1.41 (1.16-1.70) | < .001 | 1.02 (0.77-1.35) | .904 |
| Overall survival# | 1.55 (1.21-1.98) | < .001 | 1.46 (1.18-1.80) | < .001 | 0.94 (0.71-1.26) | .686 |
| Outcome . | mmRD vs MSD . | URD vs MSD . | URD vs mmRD . | |||
|---|---|---|---|---|---|---|
| RR (95%CI) . | P . | RR (95%CI) . | P . | RR (95%CI) . | P . | |
| Acute GVHD 2-4* | 2.70 (2.13-3.42) | < .001 | 1.76 (1.42-2.18) | < .001 | 0.65 (0.49-0.86) | .003 |
| Acute GVHD 3-4† | 3.41 (2.38-4.89) | < .001 | 2.54 (1.78-3.64) | < .001 | 0.75 (0.49-1.14) | .177 |
| Chronic GVHD‡ | 3.09 (2.24-4.27) | < .001 | 3.92 (3.00-5.13) | < .001 | 1.27 (0.88-1.82) | .198 |
| TRM§ | 3.01 (2.10-4.33) | < .001 | 3.48 (2.54-4.77) | < .001 | 1.16 (0.77-1.64) | .739 |
| Relapse‖ | 0.90 (0.65-1.24) | .516 | 0.98 (0.76-1.27) | .874 | 1.09 (0.74-1.60) | .661 |
| Disease-free survival¶ | 1.38 (1.09-1.75) | .008 | 1.41 (1.16-1.70) | < .001 | 1.02 (0.77-1.35) | .904 |
| Overall survival# | 1.55 (1.21-1.98) | < .001 | 1.46 (1.18-1.80) | < .001 | 0.94 (0.71-1.26) | .686 |
MSD indicates matched sibling donor transplant; URD, 8/8 matched unrelated donor transplant; mmRD, 1-antigen mismatch related donor/phenotypically matched donor transplant; RR, relative risk; CI, confidence interval; GVHD, graft-versus-host disease; and TRM, treatment-related mortality.
Adjusted for total body irradiation use and stratified by GVHD prophylaxis.
Adjusted for year of transplantation and stratified by GVHD prophylaxis.
Adjusted for donor-recipient sex match, performance score, recipient age at transplantation, GVHD prophylaxis, and antithymocyte globulin use.
Adjusted for recipient age at transplantation, performance score, disease, donor-recipient sex match, and year of transplantation.
Adjusted for total body irradiation use in conditioning and year of transplantation and stratified by performance score, disease, disease status, and time from diagnosis to transplantation.
Adjusted for donor-recipient cytomegalovirus match and recipient age at transplantation and stratified by disease and disease status.
Adjusted for disease, disease status, recipient age at transplantation, and stratified by performance score, use of antithymocyte globulin in conditioning, and years of transplantation.
Relapse
The cumulative incidence of relapse at 3 years was 36% (95% CI, 33%-39%) after MSD transplantation, compared with 29% (95% CI, 22%-37%) for the mmRD group and 32% (95% CI, 27%-38%) for the URD group (Table 3). The trend toward a lower relapse rate after mmRD transplantation compared with MSD transplantation in univariate analysis (Table 3) was not supported by the multivariate analysis, which adjusted for TBI use and year of transplantation and stratified by performance score, disease, disease status, and time from diagnosis to transplantation (Table 4).
Treatment-related mortality
The cumulative incidence of TRM at 3 years was 10% (95% CI, 8%-12%) for the MSD group, compared with 27% (95% CI, 20%-34%) for the mmRD group and 24% (95% CI, 19%-30%) for the URD group (Table 3; Figure 1C). The results of the multivariate analysis for TRM are shown in Table 4. The rate of TRM for the MSD group was significantly less than the other 2 groups (both P < .001). However, no difference was found between the use of mmRD or URD (RR, 1.16; 95% CI, 0.77-1.64; P = .739). Other factors associated with higher TRM in the multivariate model were older recipient age (11-17 years vs ≤ 10 years; RR, 2.39; 95% CI, 1.83-3.12; P < .001) and lower Lansky/Karnofsky performance score at transplantation (< 90 vs ≥ 90; RR, 1.58; 95% CI, 1.13-2.20; P = .008).
Disease-free survival
Unadjusted DFS rates at 3 years were 54% (95% CI, 51%-57%), 44% (95% CI, 36%-53%), and 43% (95% CI, 37%-49%) for MSD, mmRD, and URD transplantations, respectively (Table 3). Of note, although mmRD recipients had disease risk profiles similar to MSD recipients, mmRD unadjusted DFS tracks more closely with the outcomes of URD recipients, who as a group have higher disease risk. This observation is confirmed with multivariate analysis, which showed that transplantations with MSDs resulted in superior DFS compared with mmRD (RR, 0.72; 95% CI, 0.57-0.92; P = .008) or URD (RR, 0.71; 95% CI, 0.59-0.86; P = .001) transplantation. However, no difference was detected between URD and mmRD (RR, 1.02; 95% CI, 0.77-1.35; P = .904; Table 4).
Overall survival
Unadjusted OS was 61% (95% CI, 58%-64%), 51% (95% CI, 43%-60%), 50% (95% CI, 44%-56%) at 3 years for MSD, mmRD, and URD transplantations, respectively (Table 3). Multivariate analysis shows a lower survival rate with mmRD rather than MSD (RR, 1.55; 95% CI, 1.21-1.98; P < .001; Table 4). No statistical difference was found between URD and mmRD transplantations in OS (RR, 0.94; 95% CI, 0.71-1.26; P = .686).
Subanalysis of mmRD subgroup matched at 7/8 or 8/8 antigens
In the subset of mmRD transplants that had C typing available (mmRD subgroup), there were 29 transplantations involving 7/8 antigen matched siblings or parents and 25 transplantations involving 8/8 matched parental donors. Major outcomes for these 2 groups were similar; therefore, their data were combined for comparison with URD and MSD. In univariate analyses, the probabilities of acute GVHD, chronic GVHD, and TRM in the mmRD subgroup were nearly identical to the respective probabilities for the URD group and worse than those for the MSD group. Survival of the mmRD subgroup appeared closer to the MSD group than the URD group (3-year OS, 61% [95% CI, 48%-74%] vs 61% [95% CI, 58%-64%] MSD vs 50% [95% CI, 44%-56%] URD), but these findings were not significant (MSD vs mmRD subgroup, P = .994; mmRD subgroup vs URD, P = .142). Multivariate analysis that compared the mmRD subgroup (Table 5) confirms that 7/8 and 8/8 mmRDs have rates of acute and chronic GVHD and TRM similar to URDs, but, as in the univariate analysis, their survival rate is not statistically distinguishable, neither from the MSD group nor the URD group.
Multivariate analysis comparing MSD vs URD vs mmRD with known 7/8 or 8/8 matches (mmRD subgroup)
| Outcome . | mmRDsub vs MSD . | URD vs MSD . | URD vs mmRDsub . | |||
|---|---|---|---|---|---|---|
| RR (95%CI) . | P . | RR (95%CI) . | P . | RR (95%CI) . | P . | |
| Acute GVHD 2-4* | 2.09 (1.39-3.14) | < .001 | 1.79 (1.44-2.22) | < .001 | 0.86 (0.55-1.33) | .491 |
| Acute GVHD 3-4† | 2.68 (1.48-4.86) | .001 | 2.64 (1.83-3.78) | < .001 | 0.98 (0.52-1.86) | .960 |
| Chronic GVHD‡ | 3.51 (2.21-5.58) | < .001 | 4.05 (3.12-5.27) | < .001 | 1.15 (0.71-1.88) | .564 |
| TRM§ | 2.16 (1.16-4.00) | .015 | 3.17 (2.33-4.32) | < .001 | 1.47 (0.77-2.79) | .242 |
| Relapse‖ | 0.69 (0.41-1.16) | .162 | 1.05 (0.81-1.35) | .724 | 1.52 (0.86-2.69) | .146 |
| Disease-free survival¶ | 1.05 (0.71-1.57) | .802 | 1.35 (1.12-1.62) | .002 | 1.28 (0.84-1.95) | .256 |
| Overall survival# | 1.17 (0.77-1.77) | .456 | 1.43 (1.15-1.77) | .001 | 1.22 (0.79-1.89) | .368 |
| Outcome . | mmRDsub vs MSD . | URD vs MSD . | URD vs mmRDsub . | |||
|---|---|---|---|---|---|---|
| RR (95%CI) . | P . | RR (95%CI) . | P . | RR (95%CI) . | P . | |
| Acute GVHD 2-4* | 2.09 (1.39-3.14) | < .001 | 1.79 (1.44-2.22) | < .001 | 0.86 (0.55-1.33) | .491 |
| Acute GVHD 3-4† | 2.68 (1.48-4.86) | .001 | 2.64 (1.83-3.78) | < .001 | 0.98 (0.52-1.86) | .960 |
| Chronic GVHD‡ | 3.51 (2.21-5.58) | < .001 | 4.05 (3.12-5.27) | < .001 | 1.15 (0.71-1.88) | .564 |
| TRM§ | 2.16 (1.16-4.00) | .015 | 3.17 (2.33-4.32) | < .001 | 1.47 (0.77-2.79) | .242 |
| Relapse‖ | 0.69 (0.41-1.16) | .162 | 1.05 (0.81-1.35) | .724 | 1.52 (0.86-2.69) | .146 |
| Disease-free survival¶ | 1.05 (0.71-1.57) | .802 | 1.35 (1.12-1.62) | .002 | 1.28 (0.84-1.95) | .256 |
| Overall survival# | 1.17 (0.77-1.77) | .456 | 1.43 (1.15-1.77) | .001 | 1.22 (0.79-1.89) | .368 |
MSD indicates matched sibling donor transplant; URD, 8/8 matched unrelated donor transplant; mmRDsub, 8/8 parents or 7/8 sibs/parents; RR, relative risk; CI, confidence interval; GVHD, graft-versus-host disease; and TRM, treatment-related mortality.
Adjusted for total body irradiation use and stratified by GVHD prophylaxis.
Adjusted for year of transplantation and stratified by GVHD prophylaxis.
Adjusted for donor-recipient sex match, recipient age at transplantation, GVHD prophylaxis, and antithymocyte globulin use.
Adjusted for recipient age at transplantation, performance score, disease, and year of transplantation.
Adjusted for total body irradiation use in conditioning and stratified by performance score, disease, disease status, and interval from diagnosis to transplantation.
Adjusted for recipient age at transplantation and stratified by performance score, disease, and disease stage.
Adjusted for disease, disease status, recipient age at transplantation, and stratified by performance score, use of antithymocyte globulin in conditioning, and years of transplantation.
Discussion
Although transplantation with the use of an MSD provides the best outcome for patients with malignant disease, at most, one-third of patients will have such a donor available and the remaining two-thirds of the population will require an alternative donor type. Within this large registry study of pediatric recipients of related and unrelated BM transplants, we observed that recipients of transplants with MSD grafts have superior outcomes compared with recipients of mmRD or URD grafts. The data also show that recipients receiving 8/8 allelic matched URD transplants produce overall outcomes similar to recipients of mmRD. We limited our analysis to conditions for which pediatric BMT continues to use ablative conditioning regimens without ex vivo T-cell depletion in most cases. Therefore, the groups we analyzed were relatively homogeneous according to indications, preparative regimens, and GVHD prophylaxis and reflect current clinical practice. The data were insufficient to explore differences between types of phenotypic or allelic mismatches within the mmRD group, because only a small number of the mmRDs had allele-level typing available. A subanalysis suggested that the parental donors who were matched at the 8/8 antigens and sibling or parent donors who matched at 7/8 antigens persisted in sharing a clinical course comparable with the 8/8 URD group (similar rates of GVHD and TRM, DFS, and OS), but their DFS and OS were not significantly different from the MSD group. This subanalysis can only hint that 7/8 or 8/8 mmRD may have equivalent survival to an MSD, because there are too few patients in this subgroup to definitively show this improvement in survival. This is shown by the broad CIs and lack of statistical significance in the 3-year OS rates between the mmRD subgroup and both the 8/8 URD and MSD groups. These findings are significant because they call into question the current practice in pediatric transplantation of using an mmRD interchangeably with an MSD.
The study is limited by its retrospective nature and because we do not know the clinical decision making behind any individual BMT. Recipients of 8/8 URD transplants had more intermediate/advanced disease and a longer interval from diagnosis to transplantation than the mmRD and MSD groups (Table 1). This observation suggests that treating clinicians considered an mmRD in the same manner and at the same time as an MSD rather than a URD, particularly because the centers contributing the MSD were restricted to centers that also contributed to the mmRD group. Because the mmRD recipient population more closely resembled the risk profile of the MSD group compared with the higher risk 8/8 URD recipient population, it makes this finding even more striking and adds to the validity of our conclusion. Our analysis suggests that an mmRD should be considered equivalent to an 8/8 matched URD and not to an MSD.
One of the original studies that described the use of mmRD found comparable outcome for MSD and 0-1 locus mismatched donors.1 However, for those patients, receiving transplants between 1975 and 1986 with single-agent methotrexate as GVHD prophylaxis, the outcome for their early-stage disease group was inferior to what we see now, some 20 years later. A previous report in a primarily adult population from the International Bone Marrow Transplant Registry compared the outcome for patients with leukemia who received a transplant with the use of MSDs, mmRDs, or URDs and found superior outcomes for MSDs compared with all alternative donor types.18 Direct comparisons between one-antigen mmRDs and matched URDs found no statistically significant difference in TRM, with outcomes for both inferior to MSD. T-cell depletion was used in 49% of the mmRD transplantations in this series (Szydlo et al18 ), but similar findings were still seen in a large Japanese study of adult patients, all T cell replete.19 Although there are marked differences in the age of the study populations across the 3 studies and the use of T-cell depletion in Szydlo et al,18 the overall effects of donor source on outcome are consistent.
No previous studies have numbers sufficient to address this issue in a pediatric recipient population. In AML, Neudorf et al4 concluded that patients who received grafts from a one-antigen mismatched donor had a DFS that was not statistically different from the DFS for patients who received HLA-identical grafts, but the conclusion was based on only 6 patients. In a high-risk ALL Berlin-Frankfurt-Münster study, transplantation was scheduled to occur if there was an MSD or a “compatible” related donor. The inclusion criteria for such a donor was a 0-1 locus mismatch at 4 loci (antigen at A, B, C; allele at DRB1), that is, minimum of a 7/8 match, rather than potentially a 6/8 match. Of the 77 with a compatible donor, 76 were siblings and only 1 was a compatible related donor.20 Thus, even large, multicenter, prospective studies recruit too few mmRD recipients to allow a precise assessment of outcome with the use of related alternative donors. Although our subanalysis of 54 donors with HLA-C typing available suggests that inclusion of these 0-1 locus of 8 antigen mismatches may produce long-term survival similar to MSD, there are insufficient numbers in this group to show an improvement in survival compared with URD, and other key aspects of their clinical course (GVHD and TRM) are similar to that of a 8/8 URD transplantation, not an MSD transplantation.
One of the traditional factors that drove the use of mismatched donors was the concept that the mismatch generated more graft versus leukemia, which counteracted an increase in TRM due to GVHD. In our study, the relapse rate was not statistically different between the 3 donor groups, even controlling for disease status. In addition, within the comparison of the phenotypically matched related versus the one-antigen mismatch group, there was no significant difference in relapse rates. Previous studies allowed a greater degree of mismatch, and it could be that our better matching means that the difference in relapse rate is less. This is consistent with recent publications; a CIBMTR study published in 2009 did not find an improved graft-versus-leukemia effect with 8/8 allelic matched URD compared with MSD and reported comparable rates of grades 3-4 acute GVHD in the URD and MSD groups.21
The original intent of this study was to look in depth at the mmRD group and to reclassify those who were a 0-1 antigen mismatch at low resolution into 0, 1, 2, 3 of 8 mismatches at the allelic level. However, detailed typing was not available in sufficient numbers of patients to permit the allelic level analysis to proceed (< 2% of donors has allelic typing), showing that most of the pediatric BMT community submitting data to the CIBMTR do not feel allele-level typing is necessary in mmRDs. The relationship between degree of mismatch at the allele level and outcome remains an important one. The small improvement in survival we noted when analyzing the mmRD cohorts with 7/8 and 8/8 antigen level typing suggests that better typing may lead to better outcomes, but larger numbers are needed to prove this assertion. We urge the pediatric BMT community to change their clinical practice to include allele-level HLA typing of all mmRDs and to obtain and report typing at the allele level to registries so that this analysis can be performed.
Our data support the use of an mmRD as an acceptable alternative to an 8/8 allelic matched URD. Depending on local circumstances, it may be faster to identify a family donor, particularly if one can predict that an unrelated search is less likely to produce an 8/8 allelic match. In certain areas of the world, having large extended families and lacking access to URDs may also favor use of an mmRD.22 For pediatric patients with leukemia, if an 8/8 URD is not available, a well-matched cord blood unit with a good cell dose will produce results comparable with an allelic matched URD.23 A cord blood search may also be faster than an extended family search, and cord blood is a cell source that will be considered in future comparative studies.
In summary, use of a 0- or 1-antigen mmRD results in outcomes similar to the use of an allelic 8/8 matched URD. Both have toxicities and complications that are higher than when an MSD is used. If an mmRD is used, the probable clinical course after BMT mirrors that of an unrelated transplantation rather than an MSD transplantation, and this should be the basis of the information and consent process for the family of the patient. Whether more data and further analysis of allele level mismatches will allow us to identify a subgroup of the mmRD cohort that mirrors an MSD transplantation rather than a URD transplantation remains to be seen.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the HSCT Committee of the Children's Oncology Group for generation of the study question and ongoing support of the work through the CIBMTR development and editorial process.
The CIBMTR is supported by the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID; Public Health Service grant/cooperative agreement U24-CA76518); by NHLBI and NCI (grant/cooperative agreement 5U01HL069294); by Health Resources and Services Administration (HRSA/DHHS; contract HHSH234200637015C); by the Office of Naval Research (grants N00014-06-1-0704 and N00014-08-1-0058); and by grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen Inc; anonymous donation to the Medical College of Wisconsin; Astellas Pharma US Inc; Baxter International Inc; Bayer HealthCare Pharmaceuticals; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical Inc; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; CaridianBCT; Celgene Corporation; CellGenix GmbH; Centers for Disease Control and Prevention; Children's Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc; CytoTherm; DOR BioPharma Inc; Dynal Biotech, an Invitrogen Company; Eisai Inc; Enzon Pharmaceuticals Inc; European Group for Blood and Marrow Transplantation; Gamida Cell Ltd; GE Healthcare; Genentech Inc; Genzyme Corporation; Histogenetics Inc; HKS Medical Information Systems; Hospira Inc; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co Ltd; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; MGI Pharma Inc; Michigan Community Blood Centers; Millennium Pharmaceuticals Inc; Miller Pharmacal Group; Milliman USA Inc; Miltenyi Biotec Inc; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics Inc; Otsuka America Pharmaceutical Inc; Pall Life Sciences; Pfizer Inc; Saladax Biomedical Inc; Schering Corporation; Society for Healthcare Epidemiology of America; Soligenix Inc; StemCyte Inc; StemSoft Software Inc; Sysmex America Inc; THERAKOS Inc; Thermogenesis Corporation; Vidacare Corporation; Vion Pharmaceuticals Inc; ViraCor Laboratories; ViroPharma Inc; and Wellpoint Inc.
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
National Institutes of Health
Authorship
Contribution: P.J.S., M.A.P., F.K., and S.R.S. designed the research and drafted the manuscript; K.W.A. and F.K. performed the statistical analysis; and all authors interpreted the data and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Shaw, BMT Services, Children's Hospital at Westmead, Sydney NSW 2145, Australia; e-mail: Peters@chw.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal