Abstract
Cellular senescence is a mechanism to inhibit the growth of mammalian cells after oncogenic activation, or in response to damage or stress. We describe here the identification of a novel gene, SENEX, that regulates stress induced premature senescence pathways in endothelial cells (ECs) involving p16INK4a and retinoblastoma protein activation. Endogenous levels of SENEX remain unchanged during replicative senescence but are regulated by H2O2-mediated stress. In contrast to that previously described for senescence in other cell types, the SENEX induced senescent ECs are profoundly anti-inflammatory. The cells are resistant to tumor necrosis factor (TNF)α–induced apoptosis, adhesion of neutrophils and mononuclear cells, and the surface (but not cytoplasmic) expression of endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. Furthermore they are resistant to thrombin induced vascular leak. Senescent ECs such as those lining atherosclerotic lesions may therefore function to limit the inflammatory response. SENEX is also essential for EC survival since depletion either ectopically by siRNA or by high- dose H2O2 treatment causes apoptosis. Together, these findings expand our understanding of the role of senescence in the vasculature and identify SENEX as a fulcrum for driving the resultant phenotype of the endothelium after activation.
Introduction
Cellular senescence together with apoptosis is viewed as a major pathway to control cell proliferation and suppress tumorigenesis.1,2 Recent evidence suggests that the senescence program may have a broader role, as an active mechanism to limit disease progression.3 The recognition and impact of senescence on the vascular system is only just emerging. Increased numbers of senescent endothelial cells (ECs) are found in mature atherosclerotic plaques, in vessels from diabetic patients, in postangioplastic restenotic vessels, in coronary vessels of patients with ischemic heart disease, and in hypertensive patients (reviewed in Voghel et al4 ). Senescent ECs have also been identified in the tumor vasculature in glioma.5 However, the causes and consequences of these senescent ECs in the different pathologies have not been clearly defined.
The recognition of senescent cells relies on several specific criteria. The cells exit the cell cycle but remain viable, they exhibit a large flattened morphology,6 and show accumulation of senescence-associated β-galactosidase (SA-β-gal) activity.7 In addition, they show altered genetic profiles which are likely to be cell type specific.8 There are 2 broad forms of senescence, replicative and stress induced. Replicative senescence (RS) is mediated through the shortening of telomeres that occurs during each cell division. This shortening eventually registers as DNA damage and triggers ataxia telangiectasia mutated kinase (ATM) activation and initiates a program of cell cycle arrest.9 Stress induced premature senescence (SIPS) is induced by oncogene activity,10 oxidative stress,11 or suboptimal culture conditions,12 and occurs independent of a change in telomere length.13 Senescence is mediated through the p53 pathway which transactivates the cyclin-dependent kinase inhibitor p2114,15 or through the p16 INK4a(p16) pathway to inhibit the cyclin-dependent kinases 2 and 4, preventing phosphorylation of the retinoblastoma protein (Rb)16,17 and thus silencing genes involved in proliferation. Although it was originally thought that the 2 signaling pathways delineate the 2 types of senescence, this is no longer the case, and the p53 and Rb pathways can show molecular cross talk or their contribution to senescent development can be cell type and signal dependent.18 Furthermore, some stimulants in some cells activate both pathways.19
Oxidative stress with the generation of reactive oxygen species (ROS) occurs in many senescence-associated vascular diseases.20 ROS, accumulated with age, are involved in age associated degenerative diseases21 and tumor development.22 Furthermore, ROS induce cell senescence and apoptosis. Indeed, there is a common theme in terms of signaling pathways and phenotypic changes seen during aging, senescence and cancer development. Of the functional ROS, H2O2 is perhaps the most important since it is also a known signaling molecule involved in the proliferative response to growth factors such as platelet-derived growth factor and epidermal growth factor, but can also induce apoptosis and senescence.
Given the impact of senescence on the biologic function and the potential for its manipulation in damaged or aged vasculature, we sought to discover genes regulating this process that could provide novel diagnostic and therapeutic tools. We describe one such gene, named SENEX for senescence gene (based on the Latin SENEX for “old man”), which provides a unique gatekeeper function in the SIPS and apoptosis pathways in ECs. To date, this is one of the few genes described which has the dual capacity to regulate this arm of cellular responses and suggests that modulation of the levels of SENEX are crucial in determining vascular function.
Methods
Replicative senescence
ECs were maintained under subconfluent conditions at all times and passaged every 3-4 days. Cells were lifted using 0.5% (wt/vol) trypsin and between 0.6 × 106 and 1 × 106 cells were replated onto fresh 75-cm2 flasks.
Adenovirus production and generation of human umbilical vein endothelial cells overexpressing SENEX
The AdEasy system (Qbiogene) was used to produce recombinant adenovirus carrying human SENEX or empty vector (EV) according to the Qbiogene Version 1.4 AdEasy Vector system manual. Equivalent plaque forming units (pfu) per cell were adjusted to yield a similar level of green fluorescent protein (GFP) expression as determined by flow cytometry.
Proliferation assay
Human umbilical vein endothelial cells (HUVECs) were plated at 3 × 103 cells per well in 96-well plates and cell numbers assessed with the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (Promega) on day 0 and day 3.
Plasmid transfection
pcDNA3-SENEX constructs were transfected into HUVECs using the Amaxa nucleofector kit according to the manufacturer's protocol (Amaxa Biosystems).
β-galactosidase activity
Acidic β-galactosidase activity was detected using the manufacturer's method (Cell Signaling Technology).
Relative quantitative reverse transcription polymerase chain reaction
Total RNA was isolated using the RNeasy mini prep kit (QIAGEN). Details of the reaction are given in supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Immunoprecipitation and immunoblotting
Immunoprecipitations and immunoblotting were performed as given in supplemental data.
Immunostaining
HUVECs were plated on fibronectin-coated labtek slides (In Vitro Technologies) at 6 × 104 for 24 hours. Monolayers cultured on the slides were preincubated with tumor necrosis factor-α (TNF-α; 5 ng/mL) for 5 hours and then washed. The cells were fixed in 4% paraformaldehyde/phosphate-buffered saline for 10 minutes, and permeabilized by treatment with 0.1% Triton X-100. Primary mouse monoclonal antibodies targeting endothelial leukocyte adhesion molecule 1 (E-selectin) and vascular cell adhesion molecule 1 (VCAM1) were used at 50 μg/mL and binding detected by incubation with Alexa 594 fluorophore-coupled secondary antibody (Invitrogen).
Flow cytometric analysis
Cells were stained for E-selectin and VCAM1 expression using specific antibodies and APC Goat anti-mouse Ig (BD Biosciences) secondary antibody and analyzed using a FACS Canto and FACS Diva for data acquisition (BD Biosciences) and FlowJo Version 8.8.6 software (TreeStar) for data analysis.
Caspase 3 activity assay
Cell lysates were prepared and the caspase 3 assay was performed essentially as given in the manufacturer's protocol (Calbiochem-Novabiochem). Fluorescence was measured at excitation and emission wavelengths of 385 nm and 460 nm, and normalized for the protein concentration.
Telomere length analysis
Genomic DNA was extracted from cells using a DNA extraction kit (QIAGEN). Twenty micrograms of DNA was digested with Hinfl and RsaI (Boehringer Mannheim). The digested DNA was quantified by Nanodrop (Thermo Scientific) and 1.0 μg was electrophoresed through a 0.8% agarose gel in 1× Tris-acetate-ethylenediaminetetraacetic-acid (TAE) buffer at 2 V/cm for 17 hours. The gel was dried at 60°C for 2 hours, denatured for 30 to 60 minutes in 0.5M NaOH and 1.5M NaCl, and neutralized for 30 to 60 minutes in 1M Tris-HCl, pH 8.0, and 1.5M NaCl. The gel was then hybridized to a [γ-32P]dATP 5′ end-labeled telomeric oligonucleotide probe [γ-32P-(TTAGGG)3]. Hybridization and washing were carried out as described.23 The gel was autoradiographed on Kodak XAR-5 X-ray film for 12-24 hours at room temperature.
siRNA transfection
HUVECs were transfected with validated Stealth siRNAs (50nM; Invitrogen) in parallel with corresponding nonspecific Stealth siRNA negative control (Invitrogen). The cells were transfected using HiPerFect transfection reagent according to the manufacturer's protocol (QIAGEN).
Transwell permeability assay
Polycarbonate membrane (3 μm) transwells (Corning Incorporated) were coated with 50 μg/mL fibronectin. HUVECs (3 × 105, passage 2) were added to each well and incubated for 24 hours. Another 1 × 105 cells were added to each well to produce a confluent monolayer. Details of the permeability assay are given in supplemental information.
Neutrophil and mononuclear cell isolation
Neutrophils and mononuclear cells were prepared from fresh blood from healthy volunteers. Blood was dextran sedimented, and cells were separated by Histopaque (Sigma-Aldrich) gradient centrifugation. The buffy coat was collected to obtain mononuclear cells and the neutrophils were purified from the cell pellet with hypotonic lysis of the remaining red cells.
Aortic sections from mice
Male apoE−/− gene knockout mice (8-9 weeks of age) were fed a Western diet24 for 1 (n = 20) or 5 months (n = 10). Details of analysis are in supplemental data. All mice studies were approved by the University of Sydney Animal Ethics Committee.
Statistical analysis
Statistical analyses using a 2-tailed Student t test and 2-way analysis of variance with Bonferroni posttest were performed using Prism software (Version 4; GraphPad Software). Data that satisfy confidence levels of P < .05, .01, or .001 are noted. Data are presented as means ± SEM.
Results
Isolation of SENEX
A PCR-based suppression subtractive hybridization approach was used to isolate genes involved in the process of capillary tube formation.25 One clone had an interesting and unique expression pattern in that it was down regulated during the stage of lumen formation but up-regulated thereafter. The cDNA was isolated and cloned from ECs and a search of the National Center for Biotechnology Information (NCBI) in February 2004 identified a 3503-bp cDNA (RefSeq NM_33515, that encoded a hypothetical protein of 663 aa (75 kD) RefSeq NP_277050. Western blot analysis shows a molecular weight of approximately 80 kDa, suggesting it may be altered by posttranslational modifications. This protein contains a putative RhoGAP domain spanning amino acids 340-520 but does not contain any other known protein domains. The gene has been classified in RefSeq as ARHGAP18.
SENEX in angiogenesis
To determine whether this gene (SENEX) is important in EC function, HUVECs were infected with adenovirus containing constructs of SENEX in the antisense orientation or EV as control, and analyzed for effects on capillary tube formation on Matrigel. The EV control cells formed tubes normally (Figure 1Ai). In contrast, cells infected with antisense containing adenovirus failed to form capillary tubes (Figure 1Aii) with changes evident as early as 45 minutes after plating. Similar morphologic changes were seen when siRNA transfected cells were tested in the Matrigel assay (supplemental Figure 1A). Although they initially aligned, the antisense or siRNA transfected cells failed to join and were unable to form tubes and the cells appeared to undergo apoptosis. This apoptosis was confirmed with the use of siRNA where there was a depletion of SENEX mRNA expression by 70% (supplemental Figure 1B) and at the protein level by 75% (supplemental Figure 1C). Such depletion caused a > 2-fold increase in apoptosis as measured by caspase 3 activity (supplemental Figure 1D) and confirmed with DAPI (4′,6-diamidino-2-phenylindole) stain (supplemental Figure 1E). Similar changes on caspase and DAPI staining were seen with knockdown of SENEX using the anti-sense adenovirus system (data not shown). Thus SENEX expression is essential for EC survival. Interestingly, the mRNA expression profile as determined by Virtual Northern blots for the expression of this gene during tube formation showed that it was down- regulated at the 3- to 6-hour time points (supplemental Figure 2A-C) where apoptosis is known to occur and contribute to lumen formation.26
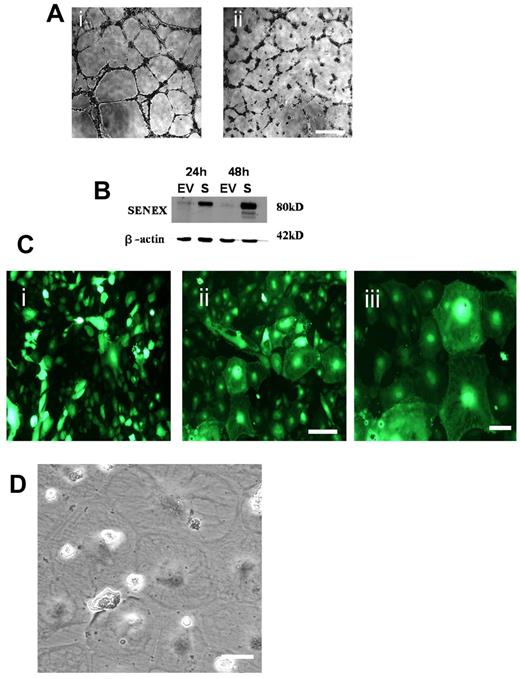
SENEX regulates angiogenesis and is required for EC survival. (A) HUVECs were infected with EV (i) or SENEX anti sense (ii) containing adenovirus. After 24 hours cells were plated onto Matrigel, and capillary tube formation was observed over a 24-hour time course. Photographs taken 12 hours after plating are shown. This is a representative of 3 similar experiments performed. Bars = 500 μm. (B) Expression levels of SENEX protein in HUVECs at 24 and 48 hours after infection with EV (EV) or SENEX (S) in the sense orientation in adenovirus and detected by Western blot analysis. Protein molecular masses (in kDa) appear on the right. This is a representatative of 5 experiments. (C) HUVECs were infected with EV (i) or SENEX (ii) containing adenovirus. Infected cells are visualized with green fluorescent protein (GFP). Photographs were taken after 48 hours. Bar = 220 μm. (iii) Enlarged area of cells from (B). Bar = 80 μm. (D) Phase contrast photograph of several enlarged cells. Bar = 80 μm.
SENEX regulates angiogenesis and is required for EC survival. (A) HUVECs were infected with EV (i) or SENEX anti sense (ii) containing adenovirus. After 24 hours cells were plated onto Matrigel, and capillary tube formation was observed over a 24-hour time course. Photographs taken 12 hours after plating are shown. This is a representative of 3 similar experiments performed. Bars = 500 μm. (B) Expression levels of SENEX protein in HUVECs at 24 and 48 hours after infection with EV (EV) or SENEX (S) in the sense orientation in adenovirus and detected by Western blot analysis. Protein molecular masses (in kDa) appear on the right. This is a representatative of 5 experiments. (C) HUVECs were infected with EV (i) or SENEX (ii) containing adenovirus. Infected cells are visualized with green fluorescent protein (GFP). Photographs were taken after 48 hours. Bar = 220 μm. (iii) Enlarged area of cells from (B). Bar = 80 μm. (D) Phase contrast photograph of several enlarged cells. Bar = 80 μm.
SENEX causes senescence
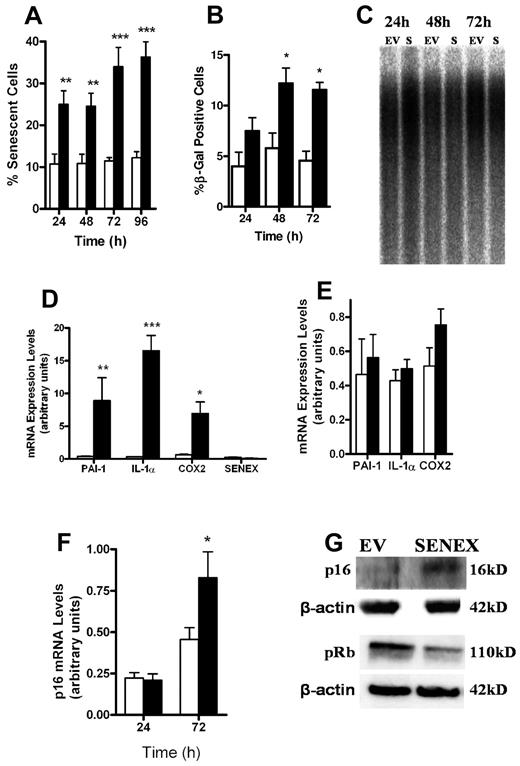
Using the adenovirus system for gene delivery of the sense construct we routinely achieved overexpression of 5- to 10-fold after 24 hours and 15- to 25-fold after 48 hours compared with basal levels of SENEX when the dose of virus was adjusted to achieve an infectivity of 18 pfu per cell (Figure 1B). This level of overexpression did not affect capillary tube formation within 48 hours of infection (supplemental Figure 3A). However, under normal culture conditions large flattened cells which often contained large vacuoles and exhibited polyploidy became apparent, reminiscent of senescent cells (Figure 1C-D). To confirm the possibility of senescence, the cells were stained for the classic marker of senescence, senescence-associated β-galactosidase (SA-β-gal). Infection with SENEX adenovirus significantly increased the number of HUVECs with SA-β-gal activity compared with the EV (Figure 2A). Furthermore the number of cells which exhibited polyploidy was enhanced in the SENEX overexpressing cells and increased with time (Figure 2B-C). Overexpression of SENEX inhibited the proliferation of ECs (Figure 2D) consistent with senescent cells that enter a state of irreversible growth arrest. There was a 61% ± 4% reduction in cell numbers (n = 4) with a 5.8% ± 2% increase in cells in the G1 phase and a 3% ± 1% decrease in the S and G2 phases as assessed by flow cytometry at 24 hours after transfection, confirming the arrest is at the G1 phase of the cell cycle. SENEX induced senescent cells also showed the characteristic decrease in endothelial nitric oxide synthase (eNOS) expression (Figure 2E) as has been reported previously for senescent EC.27 Finally, the change in morphology to the large flattened cell shape was associated with an increase in SENEX expression since cells that were infected (as detected by GFP expression) but were morphologically normal did not express enhanced levels of SENEX (supplemental Figure 3B). The induction of senescence was independent of the use of adenovirus since a similar morphology in the cells was obtained when overexpression was achieved through transfection of plasmid (supplemental Figure 3C). Thus, on the basis of morphology, expression of β-gal, cell-cycle arrest and eNOS expression overexpression of SENEX induces senescence in ECs.
SENEX overexpression induces senescence characteristics. (A) HUVECs were infected with EV (i) or SENEX (ii) containing adenovirus. After 72 hours cells were stained for acidic β-galactosidase. Bars = 220 μm. (B) HUVECs were infected with SENEX containing adenovirus. The cells were stained with DAPI every 24 hours for the next 3 days. Top panel shows the cells at 24 hours with the GFP field with cells exhibiting polyploidy marked with an arrow. DAPI stain is shown in the bottom panel. Bar = 100 μm. (C) SENEX overexpression induces polyploidy. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for polyploidy. At least 1000 cells were counted each day in each group for each of 3 individual cell lines and are presented as a percentage of the senescent cells. The mean ± SEM is shown. **P < .01 and ***P < .001 compared with EV. (D) SENEX overexpression inhibits EC proliferation. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for cell proliferation after 4 days using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. OD490nm for cells at day 0 and day 4 are given. Results are the mean ± SEM of a pool of 3 experiments where each experiment used a different HUVEC line, and each group in each experiment was done in 4 replicates. *P < .05 compared with EV on day 4. (E) SENEX overexpressing cell lysate (from adenovirus infected cells) was used for Western blotting with eNOS antibodies. β-actin was used as a loading control. This is a representative of 3 experiments.
SENEX overexpression induces senescence characteristics. (A) HUVECs were infected with EV (i) or SENEX (ii) containing adenovirus. After 72 hours cells were stained for acidic β-galactosidase. Bars = 220 μm. (B) HUVECs were infected with SENEX containing adenovirus. The cells were stained with DAPI every 24 hours for the next 3 days. Top panel shows the cells at 24 hours with the GFP field with cells exhibiting polyploidy marked with an arrow. DAPI stain is shown in the bottom panel. Bar = 100 μm. (C) SENEX overexpression induces polyploidy. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for polyploidy. At least 1000 cells were counted each day in each group for each of 3 individual cell lines and are presented as a percentage of the senescent cells. The mean ± SEM is shown. **P < .01 and ***P < .001 compared with EV. (D) SENEX overexpression inhibits EC proliferation. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for cell proliferation after 4 days using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. OD490nm for cells at day 0 and day 4 are given. Results are the mean ± SEM of a pool of 3 experiments where each experiment used a different HUVEC line, and each group in each experiment was done in 4 replicates. *P < .05 compared with EV on day 4. (E) SENEX overexpressing cell lysate (from adenovirus infected cells) was used for Western blotting with eNOS antibodies. β-actin was used as a loading control. This is a representative of 3 experiments.
SENEX is a member of the RhoGAP family of proteins. To determine whether the GTPase-activating protein domain is essential for SENEX induced senescence, an R365A mutant was generated. This mutation eliminated the Rho activity (data not shown). However, overexpression of this mutant protein was still able to confer the senescence phenotype on ECs (supplemental Figure 3D) suggesting that the GTPase-activating protein domain is not essential for this aspect of its function.
SENEX induces stress induced premature senescence
SENEX induced senescence within 24 hours using gene delivery through adenovirus, as judged by either the increase in cell size (Figure 3A) or positive staining for SA-β-gal (Figure 3B). The difference in degree of senescence obtained by these 2 methods may reflect the observation that the extremely large, highly flattened cells have a very low to undetectable level of staining for SA-β-gal whereas intermediate size cells are highly positive for the stain. At present we do not know the reason for this discrepancy. It may be a loss of the lysosomal compartment with increase in size or may simply be a function of the “flatness” of the cell. Given the rapidity of this induction, it is unlikely that it is a replicative form of senescence and this was confirmed using 2 criteria. Firstly, we investigated telomere length by Southern blot analysis in SENEX induced senescent ECs. Cells were infected with adenovirus containing either EV or SENEX. Seventy-two hours later, senescent cells were enriched by light trypsinization and then analyzed for telomere length. We found that there was no change in telomere length of HUVECs (Figure 3C) overexpressing SENEX compared with control EV. Secondly, we induced replicative senescent in the HUVECs by repeatedly passaging the cells. Senescence was evident after 15-20 passages. These replicative cells displayed increased expression of 3 genes considered markers of replicative senescent ECs, plasminogen activator inhibitor-1 (PAI-1)28 (although changes in PAI have been reported in SIPS induced through high glucose29 and with hydrogen peroxide treatment30 ), interleukin-1α (IL1-α)31 and cycloxygenase 2 (COX2).32 However, there was no concurrent increase in SENEX expression (Figure 3D). Furthermore, when senescence was induced by SENEX, there was no significant change in the levels of PAI-1, COX2, or IL-1α (Figure 3E). Together, these results suggest that SENEX is not involved in the RS pathway.
SENEX activates the p16 senescence pathway. (A) HUVECs were infected with EV (□) or SENEX (■) containing adenovirus. Photographs were taken at 24-hour time intervals for 4 days. The number of senescent cells, based on an enlarged morphology, were counted and presented as a percentage of total cells counted. At least 1000 cells were counted for each of the 3 individual HUVEC lines. The mean ± SEM is shown. **P < .01 and ***P < .001 compared with EV. (B) EV (□) and SENEX (■) overexpressing cells were harvested after 24, 48, and 72 hours of culture and stained for SA-β-gal expression. At least 1000 cells on each day were counted. The percentage of cells positive ± SEM is given for 3 different HUVEC lines analyzed. *P < .05 compared with EV. (C) EV and SENEX (S) overexpressing cells were harvested after 24, 48, and 72 hours of culture, and telomere length was measured by Southern blot analysis. (D) Replicative senescence in HUVECs was induced through constant passaging and the cells were harvested when the senescent morphology was evident in the majority of cells. mRNA expression was measured by Q-RT-PCR of COX2, IL-1α, PAI-1, and SENEX and standardized to cyclophilin A. The mean ± SEM of 6 replicates from 2 lines of HUVECs is shown. Early-passage cells (□) and late-passage senescent cells (■). *P < .05, **P < .01, and ***P < .001 compared with early passage. (E) SENEX (■) and EV (□) infected cells were harvested when at least 50% senescence was seen and mRNA levels determined for PAI-1, COX-2, and IL-1α by Q-RT-PCR. There were no significant differences seen between the EV and SENEX groups. This is a representative experiment of 2. (F) HUVECs were infected with SENEX or EV adenovirus for 24 and 72 hours. Total RNA was extracted and Q-RT-PCR used to determine levels of p16 standardized to Cyclophilin A. This is a representative of 4 experiments. Results are the mean ± SEM of 3 replicates of each group. *P < .05 compared with EV. (G) Total protein from 24-hour overexpressing SENEX or EV control cells was used for Western blotting using p16 or phosphorylated Rb specific antibodies. β-actin was used as a loading control. This is a representative of 3-4 experiments performed using different EC isolates.
SENEX activates the p16 senescence pathway. (A) HUVECs were infected with EV (□) or SENEX (■) containing adenovirus. Photographs were taken at 24-hour time intervals for 4 days. The number of senescent cells, based on an enlarged morphology, were counted and presented as a percentage of total cells counted. At least 1000 cells were counted for each of the 3 individual HUVEC lines. The mean ± SEM is shown. **P < .01 and ***P < .001 compared with EV. (B) EV (□) and SENEX (■) overexpressing cells were harvested after 24, 48, and 72 hours of culture and stained for SA-β-gal expression. At least 1000 cells on each day were counted. The percentage of cells positive ± SEM is given for 3 different HUVEC lines analyzed. *P < .05 compared with EV. (C) EV and SENEX (S) overexpressing cells were harvested after 24, 48, and 72 hours of culture, and telomere length was measured by Southern blot analysis. (D) Replicative senescence in HUVECs was induced through constant passaging and the cells were harvested when the senescent morphology was evident in the majority of cells. mRNA expression was measured by Q-RT-PCR of COX2, IL-1α, PAI-1, and SENEX and standardized to cyclophilin A. The mean ± SEM of 6 replicates from 2 lines of HUVECs is shown. Early-passage cells (□) and late-passage senescent cells (■). *P < .05, **P < .01, and ***P < .001 compared with early passage. (E) SENEX (■) and EV (□) infected cells were harvested when at least 50% senescence was seen and mRNA levels determined for PAI-1, COX-2, and IL-1α by Q-RT-PCR. There were no significant differences seen between the EV and SENEX groups. This is a representative experiment of 2. (F) HUVECs were infected with SENEX or EV adenovirus for 24 and 72 hours. Total RNA was extracted and Q-RT-PCR used to determine levels of p16 standardized to Cyclophilin A. This is a representative of 4 experiments. Results are the mean ± SEM of 3 replicates of each group. *P < .05 compared with EV. (G) Total protein from 24-hour overexpressing SENEX or EV control cells was used for Western blotting using p16 or phosphorylated Rb specific antibodies. β-actin was used as a loading control. This is a representative of 3-4 experiments performed using different EC isolates.
SENEX activates the p16 pathway
The p53/p21 pathway and the p16/Rb pathway have been implicated in senescence induction. ECs were harvested 48 hours after infection with the SENEX adenovirus at a time when 40%-50% of the cells displayed a senescent morphology. Immunoblotting showed that there was no change in the protein expression of p53 or p21 (supplemental Figure 4). SENEX overexpression, however, did induce an increase in both the mRNA (Figure 3E) and protein levels for p16 (Figure 3F) and there was a decrease in the protein expression of the hyperphosphorylated Rb (Figure 3G). These results indicate that SENEX activates the p16/pRb pathway.
H2O2 induces SENEX expression and senescence
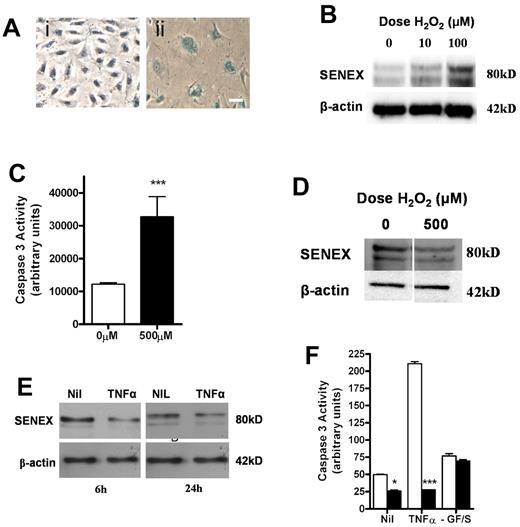
H2O2, a ROS implicated in cardiovascular disease and cancer, is a known inducer of senescence when delivered in a subcytotoxic dose.33 The senescence that results from H2O2 occurs through the oxidative stress pathway, without a shortening in telomere length,11 similar to that seen for SENEX. For this reason, we investigated whether H2O2 regulates SENEX expression. ECs were exposed to subcytotoxic concentrations of H2O2 (10 and 100μM) for 2 hours and the cells cultured in fresh normal medium for a further 24 to 48 hours. H2O2 induced senescence as judged by β-gal staining (Figure 4A). Furthermore, these cells showed an increase in SENEX protein (Figure 4B).
SENEX expression is regulated by H2O2 and TNFα. (A) HUVECs were stimulated with 100μM H2O2 for 2 hours and then placed in normal HUVEC medium for 24 hours (ii) or kept in normal HUVEC medium for 24 hours (i). Cells were then stained for β galactosidase activity. Bar = 100 μm. (B) HUVECs were stimulated as in panel A and then placed in normal HUVEC medium for 24 hours. SENEX expression was measured by Western blot with β-actin used as a loading control. This is a representative of 3 experiments. (C) HUVECs were treated with 500μM H2O2 for 6 hours and then were assessed for apoptosis using the caspase 3 activity assay. The mean ± SEM of 6 replicates from 3 lines of HUVECs is shown. ***P < .001 compared with no H2O2 treatment. (D) HUVECs were treated as in panel C. SENEX expression was measured by Western blot with β-actin as a loading control. This is a representative of 3 experiments. (E) Cells were treated with 10 ng/mL of TNFα in normal HUVEC medium for 24 hours. Protein lysates were harvested at 6 and 24 hours and the SENEX protein levels were measured using Western blotting. β-actin was used as a loading control. This is a representative of 3 experiments. (F) HUVECs were infected with SENEX (■) and EV (□) adenovirus for 24 hours. They were treated with 10 ng/mL TNFα in normal HUVEC medium for 24 hours or cultured in serum-free HUVEC medium for 24 hours or left untreated. Protein lysates were harvested and apoptosis measured using a caspase 3 activity assay. The mean ± SEM of 6 replicates from 3 lines of HUVECs is shown. *P < .05 and ***P < .001 compared with EV.
SENEX expression is regulated by H2O2 and TNFα. (A) HUVECs were stimulated with 100μM H2O2 for 2 hours and then placed in normal HUVEC medium for 24 hours (ii) or kept in normal HUVEC medium for 24 hours (i). Cells were then stained for β galactosidase activity. Bar = 100 μm. (B) HUVECs were stimulated as in panel A and then placed in normal HUVEC medium for 24 hours. SENEX expression was measured by Western blot with β-actin used as a loading control. This is a representative of 3 experiments. (C) HUVECs were treated with 500μM H2O2 for 6 hours and then were assessed for apoptosis using the caspase 3 activity assay. The mean ± SEM of 6 replicates from 3 lines of HUVECs is shown. ***P < .001 compared with no H2O2 treatment. (D) HUVECs were treated as in panel C. SENEX expression was measured by Western blot with β-actin as a loading control. This is a representative of 3 experiments. (E) Cells were treated with 10 ng/mL of TNFα in normal HUVEC medium for 24 hours. Protein lysates were harvested at 6 and 24 hours and the SENEX protein levels were measured using Western blotting. β-actin was used as a loading control. This is a representative of 3 experiments. (F) HUVECs were infected with SENEX (■) and EV (□) adenovirus for 24 hours. They were treated with 10 ng/mL TNFα in normal HUVEC medium for 24 hours or cultured in serum-free HUVEC medium for 24 hours or left untreated. Protein lysates were harvested and apoptosis measured using a caspase 3 activity assay. The mean ± SEM of 6 replicates from 3 lines of HUVECs is shown. *P < .05 and ***P < .001 compared with EV.
Conversely, high-dose H2O2 is known to induce EC apoptosis,34 which we confirm (Figure 4C), and this is associated with an inhibition in the expression of SENEX (Figure 4D). Since reduction in SENEX levels per se induced apoptosis, this duality places an experimental challenge in demonstrating the necessity of SENEX for H2O2 induced senescence and awaits the development, in ECs, of an inducible system that gives a time-dependent control of its expression.
SENEX is also a TNFα responsive gene. TNFα treatment caused a down-regulation of SENEX expression (Figure 4E and supplemental Figure 5A) and induced EC apoptosis as measured by caspase 3 levels (Figure 4F). However, overexpression of SENEX protected against this TNFα-induced apoptosis (Figure 4F). Interestingly, SENEX overexpression did not protect against serum deprivation (Figure 4F) nor did the level of SENEX change with serum stimulation (supplemental Figure 5B).
SENEX induced senescent ECs show anti-inflammatory properties
To determine the inflammatory phenotype of the SENEX-induced ECs, we tested their capacity to support neutrophil adhesion. There was little or no neutrophil or mononuclear cell adhesion to unstimulated EV or SENEX-infected cells (data not shown). As shown in Figure 5A and supplemental Figure 6A, there was a striking lack of neutrophil adhesion to TNFα-stimulated morphologically enlarged SENEX-infected senescent ECs. Cells transfected with EV adenovirus and then stimulated with TNFα supported neutrophil and mononuclear cell adhesion (supplemental Figure 6B). Cells that had been transfected with SENEX, but did not show the change in cell size in general, displayed levels of neutrophil attachment similar to that seen with uninfected cells (supplemental Figure 6A arrows). Quantification based on the number of neutrophils attached per large senescent cell versus nonenlarged ECs occupying the same area showed a 75% inhibition in the capacity of neutrophils to adhere to senescent ECs (Figure 5A,C). There was no preferential binding of the neutrophils to the junctions of the senescent cells and we saw little or no neutrophil transmigration across the senescent ECs (data not shown). Consistent with this observation there was a significant decrease in the amount of IL-8 mRNA levels (supplemental Figure 6C). A similar lack of mononuclear cell adhesion (Figure 5B,D) and transmigration (not shown) was observed.
SENEX regulates adhesion. (A) SENEX- and EV-infected cells were stimulated with TNFα (5 ng/mL) for 5 hours. Adhesion of neutrophils was then assessed (ii). This is a representative of 3 experiments. Arrows show senescent cells from the corresponding fluorescence view in subpanel i. Bar = 220 μm. (B) HUVECs were treated as in panel A. Adhesion of mononuclear cells was then assessed (ii). This is a representative of 3 experiments. Arrows show senescent cells from the corresponding fluorescence view (i). Bar = 220 μm. (C) From the photographs taken in panel A, counts were made of the number of adherent neutrophils on a senescent cell (■).The number of adherent neutrophils were then counted in the same surface area on neighbouring nonsenescent cells (□). This is a representative of 102 senescent cells and the corresponding area of nonsenescent cells from 3 HUVEC lines. ***P < .001 compared with EV. (D) From the photographs in panel B, counts were taken of the number of adherent mononuclear cells on a senescent cell (■). The number of adherent mononuclear cells was then counted in the same surface area on neighbouring nonsenescent cells (□). This is a representative of the mean ± SEM of 42 senescent cells from 3 HUVEC lines. ***P < .001 compared with EV.
SENEX regulates adhesion. (A) SENEX- and EV-infected cells were stimulated with TNFα (5 ng/mL) for 5 hours. Adhesion of neutrophils was then assessed (ii). This is a representative of 3 experiments. Arrows show senescent cells from the corresponding fluorescence view in subpanel i. Bar = 220 μm. (B) HUVECs were treated as in panel A. Adhesion of mononuclear cells was then assessed (ii). This is a representative of 3 experiments. Arrows show senescent cells from the corresponding fluorescence view (i). Bar = 220 μm. (C) From the photographs taken in panel A, counts were made of the number of adherent neutrophils on a senescent cell (■).The number of adherent neutrophils were then counted in the same surface area on neighbouring nonsenescent cells (□). This is a representative of 102 senescent cells and the corresponding area of nonsenescent cells from 3 HUVEC lines. ***P < .001 compared with EV. (D) From the photographs in panel B, counts were taken of the number of adherent mononuclear cells on a senescent cell (■). The number of adherent mononuclear cells was then counted in the same surface area on neighbouring nonsenescent cells (□). This is a representative of the mean ± SEM of 42 senescent cells from 3 HUVEC lines. ***P < .001 compared with EV.
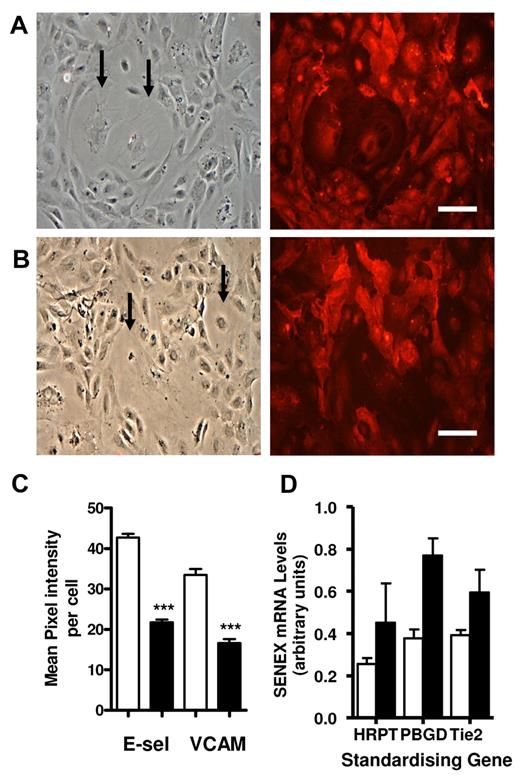
Neutrophil attachment is mediated predominantly through the adhesion molecule E-selectin.35,36 The SENEX-induced senescent cells displayed little or no cell-surface expression of E-selectin (Figure 6A) or VCAM1 (Figure 6C) in response to TNFα stimulation. The results are quantified in Figure 6E. Furthermore, flow cytometric analysis of E-selectin surface expression also demonstrated a significant reduction in expression levels of VCAM1 (Figure 6F) and E-selectin expression (Figure 6G). The small increase in basal E-selectin on the SENEX-overexpressing cells was not reproducible. However, many of the senescent cells contained intracellular levels of E-selectin and VCAM1 (Figure 6B and D, respectively) although there were also cells that failed to express any intracellular levels of E-selectin or VCAM1. Thus, these results would suggest that the TNFα signaling pathway to induce adhesion molecules is functional but that there is an inhibition of translocation of these 2 adhesion molecules to the cell surface. Furthermore, the lack of cell-surface expression of the adhesion molecules was associated with the large flattened cell morphology. Cells transfected with SENEX but that exhibited normal cell morphology displayed surface expression of E-selectin and VCAM1.
SENEX inhibits adhesion molecule expression and permeability. HUVECs were infected with SENEX and EV adenovirus for 24 hours. They were treated with 5 ng/mL TNFα in normal HUVEC medium for 5 hours and then stained for surface expression of E-selectin (A) or VCAM1 (C) (right panels); bar = 220 μm. GFP photos of the area photographed for adhesion molecule expression is given in the left-hand panels. The cells were also permeabilized and stained for intracellular E-selectin (B) and VCAM1 (D); bar = 220 μm. (E) Senescent and nonsenescent cells were analyzed for cell-surface expression of E-selectin and VCAM1. The mean pixel intensity per cell was measured using ImageJ for senescent cells (■) and for nonsenescent cells (□). The E-selectin data are a representative of the mean ± SEM of 25 senescent cells and nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. The VCAM1 data are a representative of the mean ± SEM of 14 senescent cells and the corresponding area of nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. (F) EV- and SENEX-infected cells were left for 5 days and then were either stimulated or unstimulated with 5 ng/mL TNFα for 4 hours, then stained for VCAM1 expression. Cells were analyzed for VCAM1 expression. No TNFα: purple line = EV, blue line = SENEX; TNFα stimulated: green line = EV, red line = SENEX cells. This is a representative of 3 experiments. (G) E-selectin expression performed as for panel F. (H) HUVEC monolayers seeded on a transwell membrane were infected with EV- (□) or SENEX (■)–expressing adenovirus. Passage of FITC-dextran through the membrane in response to thrombin was tested 48 hours later. This is a representative experiment of 3 showing the average of 4 transwells per condition ± SEM. *P < .05 compared with EV group with thrombin.
SENEX inhibits adhesion molecule expression and permeability. HUVECs were infected with SENEX and EV adenovirus for 24 hours. They were treated with 5 ng/mL TNFα in normal HUVEC medium for 5 hours and then stained for surface expression of E-selectin (A) or VCAM1 (C) (right panels); bar = 220 μm. GFP photos of the area photographed for adhesion molecule expression is given in the left-hand panels. The cells were also permeabilized and stained for intracellular E-selectin (B) and VCAM1 (D); bar = 220 μm. (E) Senescent and nonsenescent cells were analyzed for cell-surface expression of E-selectin and VCAM1. The mean pixel intensity per cell was measured using ImageJ for senescent cells (■) and for nonsenescent cells (□). The E-selectin data are a representative of the mean ± SEM of 25 senescent cells and nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. The VCAM1 data are a representative of the mean ± SEM of 14 senescent cells and the corresponding area of nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. (F) EV- and SENEX-infected cells were left for 5 days and then were either stimulated or unstimulated with 5 ng/mL TNFα for 4 hours, then stained for VCAM1 expression. Cells were analyzed for VCAM1 expression. No TNFα: purple line = EV, blue line = SENEX; TNFα stimulated: green line = EV, red line = SENEX cells. This is a representative of 3 experiments. (G) E-selectin expression performed as for panel F. (H) HUVEC monolayers seeded on a transwell membrane were infected with EV- (□) or SENEX (■)–expressing adenovirus. Passage of FITC-dextran through the membrane in response to thrombin was tested 48 hours later. This is a representative experiment of 3 showing the average of 4 transwells per condition ± SEM. *P < .05 compared with EV group with thrombin.
Inflammation is also associated with an increase in the permeability of the endothelium. As measured by the passage of FITC-dextran across the monolayer, the SENEX-induced senescent ECs had a lower response to the permeability inducing agent thrombin than did the control EV-infected cells (Figure 6H). There was no alteration in the levels of PAR-1, the protease-activated receptor for thrombin with SENEX overexpression (data not shown).
Similar to SENEX-induced senescent cells, H2O2-induced senescent cells also displayed this anti-inflammatory phenotype as there was no cell-surface expression of E-selectin or VCAM1 after TNFα stimulation (Figure 7A-C) confirming the biologic importance of the program induced by SENEX. Together, these results demonstrate that SENEX-induced senescent ECs have a profound inhibition of their inflammatory state.
H2O2 induced senescent ECs are non inflammatory. HUVECs were stimulated with 100μM H2O2 for 2 hours and then placed in normal HUVEC medium for 24 hours. They were then replated into Labtek slides and 24 hours later stimulated with 5 ng/mL TNFα for 5 hours then stained for E-selectin (A) or VCAM1 (B) (right panels); Bar = 220 μm. Phase contrast photographs of the area for adhesion molecule expression are given in the left panels. (C) Senescent and nonsenescent cells were analyzed for cell surface expression of E-selectin and VCAM1. The mean pixel intensity per cell was measured using ImageJ for senescent cells (■) and for neighboring nonsenescent cells (□). The E-selectin data are a representative of the mean ± SEM of 33 senescent cells and nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. The VCAM1 data are a representative of the mean ± SEM of 17 senescent cells and the corresponding area of nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. (D) Total RNA was extracted from the aortas of male ApoE−/− mice that were on a Western diet for 1 (□) and 5 months (■). The RNA was subjected to reverse transcriptase followed by Q-RT-PCR, as described in Methods. mRNA expression of Senex was assessed and standardized to Pbgd, Hrpt, and Tie2. Quantification of Senex levels was standardized to the 3 housekeeping genes.
H2O2 induced senescent ECs are non inflammatory. HUVECs were stimulated with 100μM H2O2 for 2 hours and then placed in normal HUVEC medium for 24 hours. They were then replated into Labtek slides and 24 hours later stimulated with 5 ng/mL TNFα for 5 hours then stained for E-selectin (A) or VCAM1 (B) (right panels); Bar = 220 μm. Phase contrast photographs of the area for adhesion molecule expression are given in the left panels. (C) Senescent and nonsenescent cells were analyzed for cell surface expression of E-selectin and VCAM1. The mean pixel intensity per cell was measured using ImageJ for senescent cells (■) and for neighboring nonsenescent cells (□). The E-selectin data are a representative of the mean ± SEM of 33 senescent cells and nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. The VCAM1 data are a representative of the mean ± SEM of 17 senescent cells and the corresponding area of nonsenescent cells from 2 HUVEC lines. ***P < .001 compared with nonsenescent cells. (D) Total RNA was extracted from the aortas of male ApoE−/− mice that were on a Western diet for 1 (□) and 5 months (■). The RNA was subjected to reverse transcriptase followed by Q-RT-PCR, as described in Methods. mRNA expression of Senex was assessed and standardized to Pbgd, Hrpt, and Tie2. Quantification of Senex levels was standardized to the 3 housekeeping genes.
Increases in oxidative stress are associated with atherosclerosis and are postulated to lead to EC damage and aging. Furthermore, senescent ECs, as judged by β-galactosidase positivity, have been detected in human atherosclerotic plaques27 and in vascular cells in injured rabbit carotid arteries.37 To determine whether SENEX is regulated during atherosclerosis, we investigated the mRNA levels in the aortic region of apoE gene knockout mice fed a Western diet. Sections were taken from the aorta. No change is seen in SENEX levels after 1 month of Western diet. However, after 5 months on the diet, there is an increase in the levels of Senex in relation to Pbgd, Hrpt or Tie2 (Figure 7D). Tie2 levels themselves did not significantly alter between 1 and 5 months of the diet, (supplemental Figure 7) indicating that there was very little change in the number of ECs covering the lesion.
Discussion
The induction of senescence by SENEX in ECs is a striking and robust observation, with overexpression resulting in 35% senescence in 3 days and this proportion increases over several weeks. Furthermore, SENEX-induced senescent cells exhibit the established senescence criteria of inhibited proliferation, a flattened, large vacuolated cellular morphology, polyploidy, positive staining with the senescence marker, β-galactosidase, and a reduction in the endothelial specific nitric oxide synthase, eNOS.
The p53/p21/Rb and p16/Rb axes are both important signaling pathways involved in the induction of senescence.38 SENEX activates the p16/Rb pathway by increasing both p16 mRNA and protein levels together with the p16 downstream mediator of cell- cycle arrest Rb. In contrast, SENEX overexpression did not alter the expression of either p53 or p21. Finally, we show that SENEX induces SIPS since it fails to affect telomere length, induces senescence within a few days and does not induce the usual RS gene profile in ECs. Consistent with this, SENEX is not induced upon RS formation in our ECs.
The most notable feature of SENEX-induced senescent cells and contrary to SIPS induced in other cell types,3 is their anti-inflammatory nature. SENEX induced senescent ECs are not activated by TNFα to support neutrophil or mononuclear cell adhesion and have a reduced synthesis of IL-8. Furthermore, the cells have an enhanced barrier function and protect against TNFα- induced apoptosis. Thus, the phenotype of SENEX-induced senescence appears beneficial and protective. The lack of cell-surface expression of TNFα induced E-selectin and VCAM1, although there is some induction of these proteins would suggest that the signaling pathway is partially intact but there is a block in the translocation of these proteins to the cell surface. Since H2O2- induced senescent ECs display a similar phenotype the results suggests that SIPS in ECs displays an anti-inflammatory nature. Thus, our findings of SENEX mRNA induction at sites of atherosclerosis suggest a relevant physiologic role perhaps to limit or attenuate the chronic inflammatory response that is characteristic of such atherosclerotic regions. The decrease in eNOS observed in our SENEX-induced senescent cells would appear contradictory. However, since it is the “available” NO derived from eNOS rather than eNOS expression per se which is critical, further work is required to delineate the role of NO in our observed phenotype. This anti-inflammatory phenotype of SIPS induced ECs is in contrast to SIPS induced in other cell types where a strongly proinflammatory phenotype has been reported. Indeed senescence is associated with the secretion of multiple factors (hence the name, senescence-associated secretory phenotype)39 which are strongly proinflammatory. These include increased levels of IL-8, IL-6, monocyte chemotactic protein 1, and shed proteins such as uPAR, VCAM1-1, and ICAM-1.40,41 The resultant proinflammatory phenotype may have specific disease related consequences. For example, senescence in tumors may not only inhibit tumor growth but may also recruit in immune cells. The stimulation of immune cells, particularly the innate immune system contributes to tumor clearance. Induction of senescence in hepatic stellate cells prevents the progression of liver fibrosis by inhibiting the proliferation of these activated cells. The senescent stellate cells up-regulate a pattern of gene expression associated with enhanced immune surveillance and indeed these senescent cells are sensitive to natural killer–mediated killing as a mechanism for their removal from the fibrotic lesion.3 Thus although senescence was originally considered to be a mechanism, together with apoptosis, for controlling cell proliferation and malignant transformation, the data would suggest that senescence plays a broader role in disease progression or resolution. Our results would also suggest that SIPS-induced ECs are unique in the phenotype of senescence which they display.
This anti-inflammatory phenotype of our SIPS ECs is in contrast to that reported for RS in ECs. EC senescence is induced by a small number of gene products including AKT,42 Rac1,43 Duffy Antigen/Receptor for Chemokines (DARC)43 and the metabolite homocysteine.44 These agents selectively activate RS, mediated through the p53/p21 pathway. Their consequence on the EC phenotype would indicate that the endothelium is rendered inflammatory as judged by increased monocyte adhesion with up-regulation of adhesion molecule expression43,44 and up-regulation of IL-8.45 Interestingly, IL-8 is not only a cytokine responsible for neutrophil transendothelial cell migration46 but is also a downstream effector of C-reactive protein, an activator of ECs and involved in promoting the inflammatory response during atherogenesis.47 Thus, our work reported here and these previous publications would suggest that the mechanism and signaling pathway (RS versus SIPS) activated to achieve senescence in ECs clearly impacts on the consequence to cellular function (inflammatory versus anti-inflammatory, respectively).
Another notable feature of SENEX in ECs is its regulation. Firstly, it is highly regulated during angiogenesis, being down-regulated at a time of lumen formation where apoptosis is involved and up-regulated when vessels are being stabilized. Secondly, SENEX is up-regulated in the process of H2O2-mediated senescence and down-regulated at higher doses when H2O2 causes apoptosis. Finally, SENEX is TNFα responsive, there being a potent down-regulation within 6 hours of TNFα. Thus SENEX appears at the cross roads of inflammation, angiogenesis and stress-induced pathways.
SENEX is of further interest since its expression is essential for EC survival. In addition, SENEX overexpression inhibits TNFα- induced EC apoptosis. These studies were performed at times where there is a significant level of senescent cells and could be merely explained by the previously documented, antiapoptotic phenotype of senescent cells.48 However, the failure of SENEX to protect against growth factor and serum deprivation suggests that the protection seen in TNFα-treated cells is through SENEX effects on specific signaling pathways, not a general effect of the senescence. These signaling pathways are currently being investigated. The fact that overexpression of SENEX induces senescence whereas depletion induces apoptosis highlights the fact that the SENEX dosage (or a more complicated change in its subcellular distribution) governs a balance between these 2 vital cellular mechanisms. The changes induced by oxidative stress in the form of H2O2 support this notion, since low doses of H2O2 induce SENEX and senescence, whereas higher doses inhibit SENEX expression and induce apoptosis. H2O2 has been implicated in senescence induction in other cell types. Although in primary fibroblasts H2O2 targets TGFβ and caveolin-1 to induce the senescence phenotype,49 these downstream targets are not involved in apoptosis signaling. Thus, the characterization of SENEX shows novel features of being highly expressed in EC but that further changes regulate a senescence/apoptosis arm. To our knowledge this is the first gene shown to regulate this key functional axis and implies a major role in homeostasis and disease development.
The structure function analysis of SENEX is still to be elucidated. SENEX is a member of the RhoGAP family of proteins. However, mutation of one of the essential amino acids in the RhoGAP domain that eliminates the Rho activity, does not affect the senescence-inducing capacity of the protein. Thus, it is likely that SENEX can exert multiple functions. In this regard, it is interesting to note that SENEX is predicted to interact with the RNA binding protein MPP6 (www.thebiogrid.org/SearchResults/summary/119675) and MPP6 interacts with the RNA polymerase II–binding protein, Che-1. Che-1 is a protein involved in the control of cell proliferation through interactions with Rb and regulation of the transcription of E2F target genes. More recently Che-1 has been shown to regulate DNA damage and cell-cycle checkpoint control.50 Further investigations are under way to determine whether such potential interactions are involved in the senescence-inducing ability of SENEX.
In summary, we have described the gene SENEX as a major fulcrum for the fine tuning of function in ECs, regulating senescence and apoptosis. The identification of SENEX as a senescence-inducing gene, specifically through the SIPS pathway, allows us to probe the consequences of this on cellular function and its role in disease. Our results indicate that SIPS not only limits excessive proliferation but also activates an anti-inflammatory profile in ECs. Thus while SENEX-mediated senescence is likely to be beneficial in limiting vascular disease, we would predict that excessive and prolonged accumulation of these cells in the vasculature may ultimately result in chronic vascular dysfunction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Professor John Harlan for initial discussions, Li Wang, Jenny Drew, and Anna Sapa for preparation of the ECs, Ben Wu for help with the resection of mouse aortas, and the staff at Royal Prince Alfred Hospital, Sydney and Women's and Children's Hospital, Adelaide for collection of the umbilical cords. We gratefully acknowledge Milena Stankovic, Elizabeth MacMillan, and Eliana Della Flora in the initial work in this area.
This work was supported by grants from the National Health and Medical Research Council of Australia Program Grant No. 349332 and Project Grant No. 570764 and the National Heart Foundation of Australia, Project Grant No. GO8 S3753. J.R.G. and R.S. are Medical Foundation Fellows, Sydney Medical School, University of Sydney. R.S. is also a National Health and Medical Research Senior Principal Research Fellow and Professorial Research Fellow, University of Sydney.
Authorship
Contribution: P.C. designed and performed experiments and contributed to writing the manuscript; C.H. performed experiments and contributed to writing of the manuscript; M.G., Y.L., K.B., and R.S. performed experiments or analyzed data; X.L. and P.B. contributed valuable reagents; and M.A.V. and J.R.G. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jennifer R Gamble, Centenary Institute, Locked Bag No. 6, Newtown, 2042, New South Wales, Australia; e-mail: j.gamble@centenary.org.au; or Mathew Vadas, Centenary Institute, Locked Bag No. 6, Newtown, 2042, New South Wales, Australia; e-mail: m.vadas@centenary.org.au.
References
Author notes
C.N.H. and M.G. contributed equally to this work.

![Figure 2. SENEX overexpression induces senescence characteristics. (A) HUVECs were infected with EV (i) or SENEX (ii) containing adenovirus. After 72 hours cells were stained for acidic β-galactosidase. Bars = 220 μm. (B) HUVECs were infected with SENEX containing adenovirus. The cells were stained with DAPI every 24 hours for the next 3 days. Top panel shows the cells at 24 hours with the GFP field with cells exhibiting polyploidy marked with an arrow. DAPI stain is shown in the bottom panel. Bar = 100 μm. (C) SENEX overexpression induces polyploidy. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for polyploidy. At least 1000 cells were counted each day in each group for each of 3 individual cell lines and are presented as a percentage of the senescent cells. The mean ± SEM is shown. **P < .01 and ***P < .001 compared with EV. (D) SENEX overexpression inhibits EC proliferation. HUVECs, infected with EV (□) or SENEX (■) containing adenovirus, were assessed for cell proliferation after 4 days using the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. OD490nm for cells at day 0 and day 4 are given. Results are the mean ± SEM of a pool of 3 experiments where each experiment used a different HUVEC line, and each group in each experiment was done in 4 replicates. *P < .05 compared with EV on day 4. (E) SENEX overexpressing cell lysate (from adenovirus infected cells) was used for Western blotting with eNOS antibodies. β-actin was used as a loading control. This is a representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/19/10.1182_blood-2009-11-252700/5/m_zh89991059740002.jpeg?Expires=1769168957&Signature=Kt8U81ElWdpBDuZc3Nd6A7nVMO6UHNxak2knCnvS5IH1cV7F1nHq1TCqoR1eR3Q4offRGx1VV9ja17GY7tsBPcEgR9qNqT0JnVQalAVl1KMXmp2E2spgg00xw~MJ6eOKdsKtMloqyC8j28Oa7QpnCvO7tb872X16O2DWixFrcr~HtqoqFCq4O0wXCSrIzeBa5PrAU1C4jHYpvGEkUCuvs10PSnrXyABvq0ItHEFD-8cXgJDCTclbK51S9zPTr2nJu48GuZIrFUSsmKvt86xQI7YSM4fTc~CXPM9XlCW6Za6HcC1jqmGES52tBxLxpQjgU-E7zHGr9ZX-Lj75uxDcGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal