Abstract
Double-unit cord blood transplantation (DCBT) appears to enhance engraftment despite sustained hematopoiesis usually being derived from a single unit. To investigate DCBT biology, in vitro and murine models were established using cells from 39 patient grafts. Mononuclear cells (MNCs) and CD34+ cells from each unit alone and in DCB combination were assessed for colony-forming cell and cobblestone area-forming cell potential, and multilineage engraftment in NOD/SCID/IL2R-γnull mice. In DCB assays, the contribution of each unit was measured by quantitative short tandem repeat region analysis. There was no correlation between colony-forming cell (n = 10) or cobblestone area-forming cell (n = 9) numbers and clinical engraftment, and both units contributed to DCB cocultures. In MNC transplantations in NOD/SCID/IL2R-γnull mice, each unit engrafted alone, but MNC DCBT demonstrated single-unit dominance that correlated with clinical engraftment in 18 of 21 cases (86%, P < .001). In contrast, unit dominance and clinical correlation were lost with CD34+ DCBT (n = 11). However, add-back of CD34− to CD34+ cells (n = 20) restored single-unit dominance with the dominant unit correlating not with clinical engraftment but also with the origin of the CD34− cells in all experiments. Thus, unit dominance is an in vivo phenomenon probably associated with a graft-versus-graft immune interaction mediated by CD34− cells.
Introduction
Cord blood (CB) is an alternative source of allogeneic hematopoietic stem cells for the transplantation of patients lacking suitable human leukocyte antigen (HLA)-matched related or unrelated volunteer donors.1-5 Although CB transplantation has the advantage of a reduced stringency of required HLA match, it is limited by the low total nucleated cell (TNC) and CD34+ cell dose, resulting in an increased risk of delayed or failed engraftment1-5 and restricting the use of CB transplantation in larger children and adults. A strategy to augment engraftment, and the use of CB transplantation in adults, is to combine 2 units from 2 different donors in a double-unit graft.6-9 Although data from controlled trials are not yet available to prove that double-unit CB transplantation (DCBT) is more efficacious than a single unit in adults and larger children, engraftment and survival with this approach are encouraging despite sustained engraftment being accounted for by only one unit in almost all patients.6,9 However, the mechanism responsible for unit dominance is unknown. The biology of double-unit transplantations is of even greater interest given the recent reports suggesting that DCBT is associated with a reduced risk of relapse.10,11
We therefore investigated the mechanisms responsible for single-unit dominance using aliquots of cells from each unit of 39 DCB grafts. Units were evaluated alone and in DCB combination using in vitro colony-forming cell (CFC) progenitor and week 5 cobblestone area-forming cell (CAFC) stem cell assays, and by 4- to 8-week engraftment in NOD/SCID/IL2R-γnull (NSG) mice.12 We examined 2 hypotheses: (1) single-unit dominance after DCBT is determined by the stem cell and hematopoietic progenitor cell content of each unit; and (2) single-unit dominance after DCBT is the result of a graft-versus-graft interaction mediated by CD34− cells. Our study is the first to use samples from a large series of clinical DCBTs and correlate the laboratory findings with patient engraftment.
Methods
Patients
Thirty-nine patients (median age, 42 years; range, 1-66 years; median weight, 72 kg; range, 8-105 kg) with high-risk hematologic malignancies (7 acute lymphoblastic leukemia, 8 acute myeloid leukemia, 3 other acute leukemias or advanced myelodysplasia, 14 non-Hodgkin lymphoma/chronic lymphocytic leukemia, and 7 Hodgkin lymphoma) were transplanted with DCB grafts using myeloablative (n = 26) or nonmyeloablative (n = 13) conditioning according to age, diagnosis, extent of prior therapy, and comorbidities.9 The 78 transplanted units were 6 of 6 (n = 4), 5 of 6 (n = 44), and 4 of 6 (n = 30) HLA-A, -B antigen, and -DRB1 allele matched to the recipients, respectively. The median infused TNC dose of the larger unit was 2.48 × 107/kg (range, 1.42-11.33 × 107/kg), and the smaller unit was 1.93 × 107/kg (range, 1.27-7.09 × 107/kg). All patients provided informed consent before transplantation in accordance with the Declaration of Helsinki. Patients also signed Institutional Review Board-approved consent for the study of ≤ 5% of each CB unit in the laboratory and the analysis of transplantation outcome for research purposes.
Cell preparations
Cells from each unit were processed for laboratory studies on the same day as clinical transplantation. Mononuclear cells (MNCs) were isolated from each CB unit by density gradient separation with Ficoll-Hypaque. CD34+ cells were positively selected using MACS immunomagnetic MicroBeads and passage through MACS separation columns (Miltenyi Biotec). Except when the cell number was limited (see experiments 1 and 4 in Table 5) to increase CD34+ purity, 2 consecutive passages were done for 85% to 90% enrichment. The CD34− fractions were collected for use in the murine studies.
CFC assays
For assessment of the progenitor cell potential of each CB unit alone and in DCB coculture, CFC assays were established using MNCs or enriched CD34+ cells cultured in triplicate in 35-mm culture dishes (Figure 1) with 1 mL of semisolid medium containing 1.2% methylcellulose, 30% fetal bovine serum, 57.2mM 2-mercaptoethanol, 2mM l-glutamine, and 0.5mM hemin, and cytokines. Cytokines included 20 ng/mL granulocyte colony-stimulating factor, 20 ng/mL c-kit ligand, 20 ng/mL interleukin-3, and 6 U/mL erythropoietin. Each unit alone and DCB cocultures were plated at 2 different cell concentrations (10 000 and 100 000 MNCs, and 500 and 1000 CD34+ cells per dish). With DCB cocultures, the cells were plated in the same ratio as transplanted into the patient according to the infused TNCs per kilogram. Each unit alone and DCB combinations were also incubated for 16 to 20 hours to allow cell-cell contact in Quality Biological serum-free 60 medium supplemented with 90 ng/mL Flt3 ligand, c-kit ligand, and thrombopoietin to preserve stem and progenitor cell viability. Cells were then plated in methylcellulose. Dishes were incubated at 37°C in a 5% CO2 humidified incubator. Total CFC consisted of burst-forming units-erythroid, granulocyte-macrophage progenitors, and colony-forming units (CFUs) granulocyte-erythrocyte-macrophage-megakaryocyte progenitors, and were scored 14 days after plating.
Methodology of in vitro assays. MNCs or CD34+ cells from each unit alone and in DCB combination were plated in methylcellulose and on MS5 stroma for CFC and CAFC assays, respectively. CFC assay was also performed after overnight incubation with thrombopoietin (TPO), kit ligand (KL), and Fms-like tyrosine kinase 3 ligand (Flt3L). CFC and CAFC were harvested from DCB cocultures for DNA extraction and quantitative PCR of short tandem repeats to establish donor origin. (Bottom left photograph) Composite of 4 images taken from the CFC experiments. Photomicrograph taken with Nikon Eclipse Ti-S microscope; objectives: 4× for CFC scoring, 10× and 20× for CAFC scoring. A Spot camera (Diagnostic Instruments) with Spot Version 4.0.2 software was used.
Methodology of in vitro assays. MNCs or CD34+ cells from each unit alone and in DCB combination were plated in methylcellulose and on MS5 stroma for CFC and CAFC assays, respectively. CFC assay was also performed after overnight incubation with thrombopoietin (TPO), kit ligand (KL), and Fms-like tyrosine kinase 3 ligand (Flt3L). CFC and CAFC were harvested from DCB cocultures for DNA extraction and quantitative PCR of short tandem repeats to establish donor origin. (Bottom left photograph) Composite of 4 images taken from the CFC experiments. Photomicrograph taken with Nikon Eclipse Ti-S microscope; objectives: 4× for CFC scoring, 10× and 20× for CAFC scoring. A Spot camera (Diagnostic Instruments) with Spot Version 4.0.2 software was used.
CAFC assays
The stroma-based (CAFC) assay at week 5 was used as the surrogate assay for hematopoietic stem cells in each CB unit alone and in DCB combination.13,14 MS5, the murine bone marrow (BM)–derived fibroblastic stromal cell line, was cultured and propagated to 70% to 80% confluence in T12.5-cm2 tissue-culture flasks in α-Eagle minimum essential medium with 10% fetal bovine serum, 1% penicillin and streptomycin supplemented with 57.2mM 2-mercaptoethanol. MNCs or CD34+ cells in 2 different concentrations (10 000 and 100 000 MNCs, or 1000 and 2500 CD34+ cells per flask) from each CB unit and in DCB combination (in the same TNC per kilogram ratio as transplanted into the patient) were cocultured in duplicate with MS5 in long-term culture Gartner medium (α-minimum essential medium containing 12.5% fetal bovine serum, 12.5% horse serum, 1% penicillin and streptomycin, 200mM l-glutamine, 57.2μM 2-mercaptoethanol, and 1μM hydrocortisone). The culture flasks were incubated at 37°C in a 5% CO2 humidified incubator with weekly half-medium changes. CAFCs (defined as a clone of at least 8 tightly packed cells beneath the MS5 stroma monolayer) were scored at week 5 using inverted phase-contrast microscopy (Figure 1).
Xenotransplantation
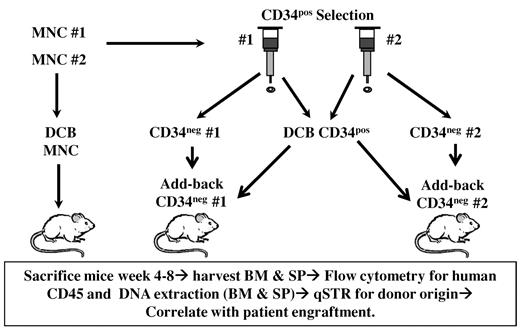
NSG mice12 at 8 to 12 weeks of age were maintained at the Rockefeller Research Laboratory animal facility under pathogen-free conditions and provided with water and irradiated sterile diet supplemented with amoxicillin during the first week after irradiation. Mice were transplanted via tail vein injection within 5 to 6 hours of thaw of the CB units and within 24 hours of sublethal irradiation (2.8-3 Gy). A total of 1 × 106 MNCs per mouse was injected when transplanting each CB unit alone with MNCs. In DCB combination, a total of 1 × 106 MNCs was injected per mouse in the same TNC per kilogram ratio as transplanted into the patient. Murine DCB CD34+ experiments used a cell dose that varied according to the cell number obtained with immunoselection and was a median total of 8.4 × 104 CD34+ cells per mouse (range, 5.5-24.2 × 104 CD34+ cells per mouse). To further investigate the possibility of graft-versus-graft immune interactions, we performed CD34+ DCBTs with add-back of a fixed dose of 1 × 106 CD34− cells from one unit (ie, DCB CD34+ + CD34− unit 1, and DCB CD34+ + CD34− unit 2; Figure 2). The cell yield from the research aliquot of each unit determined the number of experimental conditions tested with each murine experiment.
Methodology of murine experiments. NSG mice were sublethally irradiated and transplanted by tail vein injection.
Methodology of murine experiments. NSG mice were sublethally irradiated and transplanted by tail vein injection.
Mice were killed at a median of 6 weeks (range, 4-8 weeks) according to animal health, and human cell engraftment was analyzed via flow cytometry on BD FACSCalibur using CellQuest (BD Biosciences) after staining with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC)–conjugated antihuman antibodies for human CD45 (PE) in BM, spleen, and thymus, as well as human CD34, CD56, CD20 (APC), CD19 (PE), and CD33 (FITC) in the BM and spleen, plus CD3 (FITC), CD4 (APC), CD7 (PE), and CD8 (FITC) in the thymus. Human FcR blocking reagent was used to block nonspecific binding of antibodies to human Fc receptor–expressing cells. The flow cytometry data were analyzed with FlowJo software Version 7.2.2.
Assessment of donor origin in patients and in the correlative laboratory studies
In the patients, clinical engraftment of each unit was evaluated by testing DNA from peripheral blood or BM on days 21, 28, 60, and 100 and later time points after transplantation with quantitative polymerase chain reaction (PCR) for informative short tandem repeat regions that distinguished each donor and the recipient.6 Complete donor chimerism was defined as 100% total donor engraftment, whereas partial donor chimerism was less than 100% total donor engraftment. This total donor engraftment was composed of either one unit or both units combined. Single-unit dominance was engraftment of one unit alone without any contribution of the other unit.
In both in vitro and in vivo DCB laboratory assays, DNA was extracted from CFC, CAFC, and mouse BM and spleen (5-10 × 106 cells). The contribution of each unit to coculture and murine engraftment was measured using identical quantitative PCR methodology as was used in the patients.6 In mice, the percentage of each unit that contributed to the total human engraftment was analyzed. Therefore, the percentage murine engraftment of each unit always added up to 100%.
Statistical analyses
Results are shown as mean values plus or minus SEM from independent experiments or mean values with a range. A paired t test was used to determine group differences. The binomial distribution was used to test whether the agreement between human and murine engraftment occurred by chance (P = .5).
Results
DCBT is associated with a high incidence of sustained donor engraftment with the engrafting unit having a higher infused CD3+ dose per kilogram
Analysis of the day 21 BM revealed that donor hematopoiesis was present in all patients. It was accounted for by a single unit in 30 of 39 (77%) patients, whereas both units were present in 9 of 39 (23%) patients, although one unit was dominant. Donor engraftment, as evidenced by sustained donor-mediated neutrophil recovery, was seen in 37 of 39 (95%) of patients. The median day to a sustained absolute neutrophil count of ≥ 0.5 × 109/L was 23 days (range, 12-37 days) in recipients of myeloablative, and 15 days (range, 7-43 days) in recipients of nonmyeloablative conditioning. Of the 2 patients with clinical graft failure, the first was 100% donor in the BM on day 21 with a single unit but had early posttransplantation sepsis with multiorgan failure and did not achieve an absolute neutrophil count of ≥ 0.5 × 109/L before death. The second patient had early secondary graft failure in the setting of human herpes virus 6 viremia.
In the 24 recipients of myeloablative conditioning with sustained donor engraftment, the median donor chimerism of the engrafting unit in the day 21 posttransplantation BM was 100% (range, 63%–100%) and remained 100% at all subsequent evaluations. In the 13 recipients of nonmyeloablative conditioning, the median donor chimerism of the engrafting unit was 80% (range, 22%–100%). However, the engrafting unit achieved a median of 88% engraftment in the peripheral blood by day 28, and 100% from day 60 and thereafter. Overall, sustained hematopoiesis was derived from a single unit in all but one case. This patient has had complete donor chimerism (100% total donor), but this has been composed of both units and sustained for 3 years after transplantation, to date.
Excluding the single patient with sustained coengraftment of both units, the engrafting and nonengrafting units had similar mean infused TNC doses of 2.60 × 107/kg (range, 1.40-11.30) versus 2.45 × 107/kg (range, 1.30-7.30 × 107/kg, P = .440), and similar mean infused CD34+ cell doses of 1.12 × 105/kg CD34+ cells (range, 0.20-4.00 × 105/kg CD34+ cells) versus 1.08 × 105/kg cells (range, 0.15-6.40 × 105/kg CD34+ cells, P = .848). However, the mean infused CD3+ dose of the engrafting unit of 4.43 × 106/kg CD3+ cells (range, 1.70-10.60 × 105/kg cells) was higher than the mean of 3.47 × 106/kg (range, 0.11-12.00 × 106/kg) of the nonengrafting unit (P = .020).
The in vitro hematopoietic potential of each unit as measured by CFC formation does not explain clinical unit dominance
Ten CFC experiments were performed: 5 using MNCs and 5 with CD34+ cells. CFC formation was present in all 3 conditions: clinically engrafting unit alone, clinically nonengrafting unit alone, and DCB combination. There was no correlation between which unit engrafted in the patients and the total CFC content of either unit using MNCs or CD34+ cells, nor was there any augmentation of CFC formation with DCB cocultures (Table 1). The representation of each unit in CFC DCB cocultures was concordant with the clonogenic efficiency of each unit alone. Hence, the unit with the higher number of total CFCs dominated in CFC DCB coculture. However, these results did not correlate with the engrafting unit in the patient. The mean percentage of total CFCs derived from the clinically engrafting unit in DCB cocultures was 53% plus or minus 12% and did not differ from that of the nonengrafting unit. In addition, the calculated infused CFC per kilogram was not predictive of which unit would engraft (data not shown). Preincubation with cytokines before CFC assay did not significantly alter CFC numbers or the result of DCB assays with the mean percentage of the clinically engrafting unit in DCB cocultures being 54% ± 10% (Table 1).
In vitro CFC and CAFC formation with each unit alone and in DCB combination: in vitro hematopoietic potential of CB units does not explain clinical unit dominance
| Assay . | Plated cells . | N . | CFC or CAFC/1000 cells (mean ± SEM) . | Both units present in DCB cocultures (yes or no) . | |
|---|---|---|---|---|---|
| Clinically engrafting unit versus clinically nonengrafting unit . | DCB (P for comparisons with each unit cultured alone) . | ||||
| CFC (GM, BFUe, GEMM) | |||||
| Direct plating | MNCs | 5 | 6 ± 2 vs 4 ± 1 (P = .206) | 5 ± 1 (P = .479 and P = .061) | Yes (mean percentage of clinically engrafting unit only 53% ± 12%) |
| CD34+ | 5 | 144 ± 28 vs 138 ± 29 (P = .885) | 165 ± 27 (P = .504 and P = .165) | ||
| Overnight incubation | MNCs | 5 | 6 ± 2 vs 3 ± 1 (P = .196) | 5 ± 1 (P = .476 and P = .236) | Yes (mean percentage of clinically engrafting unit only 54% ± 10%) |
| CD34+ | 5 | 135 ± 30 vs 127 ± 30 (P = .838) | 151 ± 28 (P = .377 and P = .297) | ||
| CAFC | MNCs | 4 | 0.7 ± 0.5 vs 0.4 ± 0.05 (P = .479) | 0.6 ± 0.3 (P = .464 and P = .500) | See Table 2 |
| CD34+ | 5 | 33 ± 11 vs 24 ± 9 (P = .266) | 23 ± 5 (P = .294 and P = .711) | ||
| Assay . | Plated cells . | N . | CFC or CAFC/1000 cells (mean ± SEM) . | Both units present in DCB cocultures (yes or no) . | |
|---|---|---|---|---|---|
| Clinically engrafting unit versus clinically nonengrafting unit . | DCB (P for comparisons with each unit cultured alone) . | ||||
| CFC (GM, BFUe, GEMM) | |||||
| Direct plating | MNCs | 5 | 6 ± 2 vs 4 ± 1 (P = .206) | 5 ± 1 (P = .479 and P = .061) | Yes (mean percentage of clinically engrafting unit only 53% ± 12%) |
| CD34+ | 5 | 144 ± 28 vs 138 ± 29 (P = .885) | 165 ± 27 (P = .504 and P = .165) | ||
| Overnight incubation | MNCs | 5 | 6 ± 2 vs 3 ± 1 (P = .196) | 5 ± 1 (P = .476 and P = .236) | Yes (mean percentage of clinically engrafting unit only 54% ± 10%) |
| CD34+ | 5 | 135 ± 30 vs 127 ± 30 (P = .838) | 151 ± 28 (P = .377 and P = .297) | ||
| CAFC | MNCs | 4 | 0.7 ± 0.5 vs 0.4 ± 0.05 (P = .479) | 0.6 ± 0.3 (P = .464 and P = .500) | See Table 2 |
| CD34+ | 5 | 33 ± 11 vs 24 ± 9 (P = .266) | 23 ± 5 (P = .294 and P = .711) | ||
Data are mean ± SEM of the total number of colonies (CFCs or CAFCs) per 1000 MNCs or CD34+ cells from independent experiments.
GEMM indicates granulocyte-erythrocyte-macrophage-megakaryocyte.
The in vitro hematopoietic potential of each unit as measured by CAFC formation does not explain clinical unit dominance
Nine CAFC experiments were performed (4 using MNCs and 5 using CD34+ cells). CAFC formation was seen when plating the clinically engrafting unit alone, the clinically nonengrafting unit alone, and in DCB coculture (Table 1). There was no correlation between clinical engraftment and the CAFC content of either unit using MNCs or CD34+ cells, and there was no augmentation of CAFC number with DCB cocultures. Single-unit dominance (Table 2) was present in 3 of 9 DCB cocultures (2 with MNCs and 1 with CD34+), whereas both units were present in 6 of 9, with the mean percentage of the clinically engrafting unit in these 6 cocultures being 53% ± 11%. Furthermore, the contribution of each unit was similar when measured in the adherent population and the supernatant of DCB CAFC cocultures (n = 4). The calculated infused CAFC per kilogram in each unit was also not predictive of clinical engraftment (data not shown).
Results of CAFC DCB assays: no correlation with clinical engraftment (n = 9)
| Condition . | n (%) . | Comment . |
|---|---|---|
| Single-unit dominance | 3/9 (33%) (2 MNCs, 1 CD34+) | Correlated with clinically engrafting unit in only 1 of 3 experiments. |
| Both units present | 6/9 (67%) | No unit dominance: mean percentage of clinically engrafting unit was only 53% ± 11%. |
| Condition . | n (%) . | Comment . |
|---|---|---|
| Single-unit dominance | 3/9 (33%) (2 MNCs, 1 CD34+) | Correlated with clinically engrafting unit in only 1 of 3 experiments. |
| Both units present | 6/9 (67%) | No unit dominance: mean percentage of clinically engrafting unit was only 53% ± 11%. |
Data are number of positive/total number of experiments.
The clinically engrafting unit had similar engraftment in NSG mice as the nonclinically engrafting unit when each was transplanted alone
Transplantation studies were undertaken in NSG mice with cells from each unit of 31 DCBT recipients. We observed high levels of human engraftment as shown with the 12 MNC experiments in Table 3. Figure 3 shows representative flow cytometric analysis of multilineage human engraftment in NSG mice transplanted with a double-unit graft. First, MNCs were transplanted from each unit alone from 20 double-unit grafts. The mean human engraftment was 19% ± 5% in the BM and 35% ± 6% in the spleen, when transplanting the clinically engrafting unit alone. This was similar to the human engraftment seen in murine transplantations of the clinically nonengrafting unit alone of 18% ± 5% in the BM (P = .563), and 31% ± 6% in the spleen (P = .489).
Results of NSG murine transplantations using MNCs (n = 12): high levels of human engraftment were observed with the clinically engrafting unit transplanted alone, the clinically nonengrafting unit transplanted alone, and with DCB combination
| Experiment no. . | Mean human CD45+ percentage engraftment . | |||||
|---|---|---|---|---|---|---|
| Clinically engrafting unit alone . | Clinically nonengrafting unit alone . | DCB transplantation . | ||||
| BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | |
| 1 | 31 | 34 | 27 | 33 | 24 | 30 |
| 2 | 12 | 12 | 10 | 2 | 2 | 16 |
| 3 | 5 | 6 | 1 | 12 | 15 | 2 |
| 4 | 21 | 29 | 17 | 24 | 20 | 26 |
| 5 | 33 | 52 | 20 | 32 | 31 | 45 |
| 6 | 7 | 11 | 7 | 14 | 15 | 25 |
| 7 | 25 | 44 | 46 | 57 | 45 | 72 |
| 8 | 22 | 53 | 48 | 78 | 27 | 22 |
| 9 | 88 | 74 | 68 | 70 | 38 | 82 |
| 10 | 44 | 87 | 53 | 52 | 8 | 25 |
| 11 | Pre | Pre | 3 | 13 | 4 | 15 |
| 12 | 15 | 14 | 3 | 5 | 7 | 6 |
| Experiment no. . | Mean human CD45+ percentage engraftment . | |||||
|---|---|---|---|---|---|---|
| Clinically engrafting unit alone . | Clinically nonengrafting unit alone . | DCB transplantation . | ||||
| BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | |
| 1 | 31 | 34 | 27 | 33 | 24 | 30 |
| 2 | 12 | 12 | 10 | 2 | 2 | 16 |
| 3 | 5 | 6 | 1 | 12 | 15 | 2 |
| 4 | 21 | 29 | 17 | 24 | 20 | 26 |
| 5 | 33 | 52 | 20 | 32 | 31 | 45 |
| 6 | 7 | 11 | 7 | 14 | 15 | 25 |
| 7 | 25 | 44 | 46 | 57 | 45 | 72 |
| 8 | 22 | 53 | 48 | 78 | 27 | 22 |
| 9 | 88 | 74 | 68 | 70 | 38 | 82 |
| 10 | 44 | 87 | 53 | 52 | 8 | 25 |
| 11 | Pre | Pre | 3 | 13 | 4 | 15 |
| 12 | 15 | 14 | 3 | 5 | 7 | 6 |
Mice were killed at a median of 6 weeks after transplantation.
Pre indicates premature mice mortality.
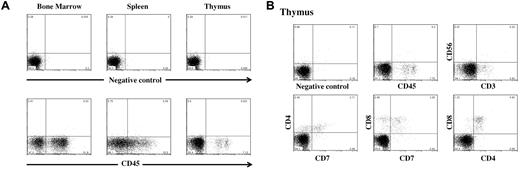
Representative flow cytometric analysis of human cell engraftment of an NSG mouse transplanted with human double-unit CB and gated on live cells. (A) Fluorescence-activated cell sorter analysis of the murine BM, spleen, and thymus after staining with PE-conjugated anti–human CD45 antibodies. (Bottom panel) Percentage of human CD45+ cells (51.8% in BM, 32.5% in spleen, and 7.1% in thymus). Analysis of murine BM revealed that the majority (71%) of human CD45+ cells were CD19+ (B lymphocytes), 6% CD33+ (myeloid cells), 3% CD34+, and less than 1% CD56+ (NK cells; data not shown). Staining for monocytes, erythroid cells, and megakaryocytes was not performed. (B) Human T-cell engraftment in the murine thymus. Of the human CD45+ cells, the majority were CD4+/CD8+.
Representative flow cytometric analysis of human cell engraftment of an NSG mouse transplanted with human double-unit CB and gated on live cells. (A) Fluorescence-activated cell sorter analysis of the murine BM, spleen, and thymus after staining with PE-conjugated anti–human CD45 antibodies. (Bottom panel) Percentage of human CD45+ cells (51.8% in BM, 32.5% in spleen, and 7.1% in thymus). Analysis of murine BM revealed that the majority (71%) of human CD45+ cells were CD19+ (B lymphocytes), 6% CD33+ (myeloid cells), 3% CD34+, and less than 1% CD56+ (NK cells; data not shown). Staining for monocytes, erythroid cells, and megakaryocytes was not performed. (B) Human T-cell engraftment in the murine thymus. Of the human CD45+ cells, the majority were CD4+/CD8+.
MNC DCBT in NSG mice was associated with single-unit dominance and correlated with clinical engraftment
The percentage of the total human engraftment accounted for by each unit after murine DCBTs using MNCs from 21 DCB grafts are shown in Tables 4 and 5 (12 MNC experiments in Table 4 and 9 MNC experiments in Table 5). Engraftment of a single unit was observed in 18 of 21 (86%) murine MNC transplantations, whereas both units engrafted in 3 experiments (experiment 8 in Table 4 and experiments 7 and 11 in Table 5). Notably, 16 patients had single-unit engraftment with the same unit also engrafting in the mice, and an additional 2 patients had predominance of one unit with the same unit predominating in the mouse (experiment 8 in Table 4 and experiment 7 in Table 5). Thus, murine engraftment using MNCs showed unit predominance in 20 of 21 (95%), and this was with a single unit in 18 of 21 (86%). Further, murine MNC engraftment correlated with clinical engraftment in 18 of 21 (86%) patients (P < .001). Three murine experiments did not correlate: in one, the mice engrafted with the clinically nonengrafting unit (experiment 12 in Table 4); in another, one mouse engrafted with the clinically engrafting unit and the other engrafted with the nonengrafting unit (experiment 5 in Table 5); and in a third, the mice engrafted with both units (experiment 11 in Table 5).
Percentage engraftment of clinically engrafting unit after murine MNC DCB transplantation (n = 12) and correlation with patient engraftment: MNC DCB transplantation shows unit dominance and clinical correlation
| Experiment no. . | Clinically engrafting unit in murine BM, % . | Clinically engrafting unit in murine spleen, % . | Clinically engrafting unit in patient, % . |
|---|---|---|---|
| 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 |
| 3 | 100 | 100 | 100 |
| 4 | 100 | 100 | 100 |
| 5 | 100 | 100 | 100 |
| 6 | 100 | 100 | 100 |
| 7 | 100 | 100 | 100 |
| 8 | 83 unit 1* (17 unit 2) | 91 unit 1* (9 unit 2) | 61 unit 1, 39 unit 2* (3 y: 69 unit 1, 31 unit 2) |
| 9 | 100 | 100 | 100 |
| 10 | 100 | 100 | 100 |
| 11 | 100 | Inadequate | 100 |
| 12 | 0 (100)† | Inadequate | 100 |
| Experiment no. . | Clinically engrafting unit in murine BM, % . | Clinically engrafting unit in murine spleen, % . | Clinically engrafting unit in patient, % . |
|---|---|---|---|
| 1 | 100 | 100 | 100 |
| 2 | 100 | 100 | 100 |
| 3 | 100 | 100 | 100 |
| 4 | 100 | 100 | 100 |
| 5 | 100 | 100 | 100 |
| 6 | 100 | 100 | 100 |
| 7 | 100 | 100 | 100 |
| 8 | 83 unit 1* (17 unit 2) | 91 unit 1* (9 unit 2) | 61 unit 1, 39 unit 2* (3 y: 69 unit 1, 31 unit 2) |
| 9 | 100 | 100 | 100 |
| 10 | 100 | 100 | 100 |
| 11 | 100 | Inadequate | 100 |
| 12 | 0 (100)† | Inadequate | 100 |
Inadequate indicates that the DNA sample was inadequate to quantify engraftment of each unit.
The mice had coengraftment of both units, and the patient had sustained coengraftment of both units for more than 3 years to date. Unit 1 was the predominating unit, but there was also sustained coengraftment of unit 2.
As indicated in parentheses, mice engrafted with the clinically nonengrafting unit.
Percentage donor chimerism of clinically engrafting unit after DCB transplantation of MNCs, CD34+ cells, and CD34+ cells + add-back of CD34− cells from the clinically engrafting or the clinically nonengrafting unit in NSG mice (n = 11)*: DCB transplantation with MNCs, but not CD34+ cells, is associated with unit dominance and correlates with patient engraftment, whereas unit dominance is restored with add-back of CD34− to CD34+ DCB from either unit, but engraftment in this setting is dictated by the origin of the CD34− cells
| Experiment no. . | Mouse ID . | Clinically engrafting unit in mice after MNC DCB, % . | Clinically engrafting unit in mice after CD34+ DCB, % . | Clinically engrafting unit in mice after CD34+ DCB + add-back CD34− from engrafting unit, % . | Clinically engrafting unit in mice after CD34+ DCB + add-back CD34− from nonengrafting unit,* % . | Clinically engrafting unit in patient on day 21 after DCB transplantation, % . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | |||
| 1 | M1 | Not done | Not done | 33 (67) | 36 (64) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 29 (71) | 36 (64) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||||
| M3 | 29 (71) | 53 (47) | 100 (0) | 100 (0) | Pre | Pre | ||||
| 2 | M1 | Not done | Not done | 23 (77) | 36 (64) | Pre | Pre | 0 (100) | 0 (100) | 100 |
| M2 | 41 (59) | 50 (50) | Pre | Pre | Pre | Pre | ||||
| M3 | Pre | Pre | Pre | Pre | Pre | Pre | ||||
| 3 | M1 | 100 (0) | 100 (0) | 43 (57) | 36 (64) | 100 (0) | 100 (0) | 28 (72) | Inadequate | 100 |
| M2 | 100 (0) | 100 (0) | 46 (54) | 48 (52) | 100 (0) | 100 (0) | 23 (77) | 0 (100) | ||
| M3 | Pre | Pre | Pre | Pre | 100 (0) | 100 (0) | Pre | Pre | ||
| 4 | M1 | 100 (0) | 100 (0) | 86 (14) | 81 (19) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 62 (38) | 75 (25) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | Pre | Pre | Pre | Pre | 0 (100) | 0 (100) | ||
| 5 | M1 | 0 (100) | 0 (100) | 100 (0) | 100 (0) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 100 (0) | 100 (0) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 64 (36) | 100 (0) | Pre | Pre | Pre | Pre | ||
| 6 | M1 | 100 (0) | 100 (0) | 86 (14) | 83 (17) | 100 (0) | 100 (0) | 13 (87) | 0 (100) | 100 |
| M2 | Pre | Pre | 93 (7) | 80 (20) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 79 (21) | 93 (7) | Pre | Pre | Pre | Pre | ||
| 7 | M1 | 92 (8) | Inadequate | 44 (56) | 39 (61) | Pre | Pre | 33 (67) | 8 (92) | 37 (14) (50% host); blood 100% engrafting unit by day 96 |
| M2 | Pre | Pre | 51 (49) | 43 (57) | Pre | Pre | 50 (50) | 18 (82) | ||
| M3 | Pre | Pre | 42 (58) | 46 (54) | Pre | Pre | 6 (94) | Inadequate | ||
| 8 | M1 | 100 (0) | 100 (0) | 5 (95) | 16 (84) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 14 (86) | 7 (93) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 7 (93) | 10 (90) | Pre | Pre | 0 (100) | 0 (100) | ||
| 9 | M1 | 100 (0) | 100 (0) | 100 (0) | 67 (33) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 100 (0) | 71 (29) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | 67 (33) | 73 (27) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| 10 | M1 | 100 (0) | 100 (0) | 25 (75) | 19 (81) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 63 (26) (11% host); blood 100% engrafting unit by day 102 |
| M2 | 100 (0) | 100 (0) | 72 (28) | 48 (52) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | 0 (100) | 33 (67) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| 11 | M1 | 15 (85) | 29 (71) | 88 (12) | 92 (8) | 83 (17) | 100 (0) | 81 (19) | 17 (83) | 100 |
| M2 | 72 (28) | 71 (29) | Pre | Pre | Pre | Pre | 12 (88) | 0 (100) | ||
| M3 | 90 (10) | 59 (41) | Pre | Pre | Pre | Pre | Pre | Pre | ||
| Experiment no. . | Mouse ID . | Clinically engrafting unit in mice after MNC DCB, % . | Clinically engrafting unit in mice after CD34+ DCB, % . | Clinically engrafting unit in mice after CD34+ DCB + add-back CD34− from engrafting unit, % . | Clinically engrafting unit in mice after CD34+ DCB + add-back CD34− from nonengrafting unit,* % . | Clinically engrafting unit in patient on day 21 after DCB transplantation, % . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | BM . | Spleen . | |||
| 1 | M1 | Not done | Not done | 33 (67) | 36 (64) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 29 (71) | 36 (64) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||||
| M3 | 29 (71) | 53 (47) | 100 (0) | 100 (0) | Pre | Pre | ||||
| 2 | M1 | Not done | Not done | 23 (77) | 36 (64) | Pre | Pre | 0 (100) | 0 (100) | 100 |
| M2 | 41 (59) | 50 (50) | Pre | Pre | Pre | Pre | ||||
| M3 | Pre | Pre | Pre | Pre | Pre | Pre | ||||
| 3 | M1 | 100 (0) | 100 (0) | 43 (57) | 36 (64) | 100 (0) | 100 (0) | 28 (72) | Inadequate | 100 |
| M2 | 100 (0) | 100 (0) | 46 (54) | 48 (52) | 100 (0) | 100 (0) | 23 (77) | 0 (100) | ||
| M3 | Pre | Pre | Pre | Pre | 100 (0) | 100 (0) | Pre | Pre | ||
| 4 | M1 | 100 (0) | 100 (0) | 86 (14) | 81 (19) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 62 (38) | 75 (25) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | Pre | Pre | Pre | Pre | 0 (100) | 0 (100) | ||
| 5 | M1 | 0 (100) | 0 (100) | 100 (0) | 100 (0) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 100 (0) | 100 (0) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 64 (36) | 100 (0) | Pre | Pre | Pre | Pre | ||
| 6 | M1 | 100 (0) | 100 (0) | 86 (14) | 83 (17) | 100 (0) | 100 (0) | 13 (87) | 0 (100) | 100 |
| M2 | Pre | Pre | 93 (7) | 80 (20) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 79 (21) | 93 (7) | Pre | Pre | Pre | Pre | ||
| 7 | M1 | 92 (8) | Inadequate | 44 (56) | 39 (61) | Pre | Pre | 33 (67) | 8 (92) | 37 (14) (50% host); blood 100% engrafting unit by day 96 |
| M2 | Pre | Pre | 51 (49) | 43 (57) | Pre | Pre | 50 (50) | 18 (82) | ||
| M3 | Pre | Pre | 42 (58) | 46 (54) | Pre | Pre | 6 (94) | Inadequate | ||
| 8 | M1 | 100 (0) | 100 (0) | 5 (95) | 16 (84) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 14 (86) | 7 (93) | Pre | Pre | 0 (100) | 0 (100) | ||
| M3 | Pre | Pre | 7 (93) | 10 (90) | Pre | Pre | 0 (100) | 0 (100) | ||
| 9 | M1 | 100 (0) | 100 (0) | 100 (0) | 67 (33) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 100 |
| M2 | 100 (0) | 100 (0) | 100 (0) | 71 (29) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | 67 (33) | 73 (27) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| 10 | M1 | 100 (0) | 100 (0) | 25 (75) | 19 (81) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | 63 (26) (11% host); blood 100% engrafting unit by day 102 |
| M2 | 100 (0) | 100 (0) | 72 (28) | 48 (52) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| M3 | 100 (0) | 100 (0) | 0 (100) | 33 (67) | 100 (0) | 100 (0) | 0 (100) | 0 (100) | ||
| 11 | M1 | 15 (85) | 29 (71) | 88 (12) | 92 (8) | 83 (17) | 100 (0) | 81 (19) | 17 (83) | 100 |
| M2 | 72 (28) | 71 (29) | Pre | Pre | Pre | Pre | 12 (88) | 0 (100) | ||
| M3 | 90 (10) | 59 (41) | Pre | Pre | Pre | Pre | Pre | Pre | ||
Pre indicates premature mice mortality; and Inadequate, inadequate murine DNA sample.
The murine percentage donor chimerism of the clinically nonengrafting unit is shown in parentheses.
Similar engraftment after MNC DCBT with half as many cells as when that unit was transplanted alone
In 10 evaluable MNC experiments (experiments 1-10, Table 3), the clinically engrafting unit efficiently engrafted mice when transplanted alone and was the sole engrafting unit in MNC DCBTs as shown in Table 4. In these 10 experiments, the mean human engraftment of the clinically engrafting unit when transplanted alone was 29% ± 8% in the BM and 40% ± 9% in the spleen. When this unit was infused as part of a DCBT, the percentage murine engraftment of this unit was not statistically different from when it was transplanted alone at 22% ± 4% in the BM (P = .337) and 34% ± 8% in the spleen (P = .479). However, this engraftment was achieved with approximately half as many cells of that unit as the mean proportion of that unit infused in the DCBTs was 0.52.
CD34+ selection abrogates unit dominance and clinical correlation
The results of 11 DCBTs using CD34+ cells are shown in Table 5 (n = 11). In contrast to the single-unit engraftment in 86% of mice receiving MNC DCBTs, both units engrafted in 10 of 11 (91%) after DCBT of isolated CD34+ cells, with a loss of clinical correlation given the mice engrafted with both units. Indeed, chimerism in the mice correlated with clinical engraftment in only 2 of 3 mice of one experiment (experiment 5, column 2, Table 5; P = not significant). Overall, the mean percentage of the clinically engrafting unit after CD34+ DCBT was 56% ± 9% in the BM and 58% ± 9% in the spleen.
Donor engraftment after CD34+ DCBT with CD34− add-back of one unit is dictated by the origin of the CD34− cells
CD34+ DCBTs with add-back of CD34− cells were done using cells from the DCB grafts of 11 patients with 20 interpretable experiments (Table 5). In 9 of 9 (100%) experiments, single-unit dominance from the clinically engrafting unit was restored with add-back of CD34− cells from the clinically engrafting unit (P = .004). Conversely, however, in 11 of 11 (100%) experiments, single-unit dominance by the clinically nonengrafting unit was induced completely (n = 9) or predominantly (n = 2) by add-back of CD34− cells from the clinically nonengrafting unit (P = .001). The single exception was in the BM of the first mouse in experiment 11 (Table 5). Thus, in these experiments, murine engraftment was dictated by the origin of the CD34− cells regardless of which unit engrafted in the patients. A summary of murine experiment results is shown in Table 6.
Summary of murine DCB transplantation results and correlation with clinical engraftment
| Infused cell type . | N . | Single-unit dominance . | Correlation of murine engraftment with clinically engrafting unit . |
|---|---|---|---|
| DCB MNCs | 21 | 18 of 21 (86%) | 18 of 21 (86%) |
| DCB CD34+ | 11 | 1 of 11 (9%) in 2 of 3 mice | 1 of 11 (9%) in 2 of 3 mice |
| DCB CD34+ + add-back CD34− from clinically | 9 | 9 of 9 (100%) in BM or spleen or both | 9 of 9 (100%) clinically engrafting unit (CD34− origin) |
| engrafting unit | |||
| DCB CD34+ + add-back CD34− from clinically nonengrafting unit | 11 | 9 of 11 (82%) in BM or SP or both, with remaining 2 predominantly nonengrafting unit | 0 of 11 (0%) clinically engrafting unit; 11 of 11 (100%) engrafted predominantly or exclusively with clinically nonengrafting unit (CD34− origin) |
| Infused cell type . | N . | Single-unit dominance . | Correlation of murine engraftment with clinically engrafting unit . |
|---|---|---|---|
| DCB MNCs | 21 | 18 of 21 (86%) | 18 of 21 (86%) |
| DCB CD34+ | 11 | 1 of 11 (9%) in 2 of 3 mice | 1 of 11 (9%) in 2 of 3 mice |
| DCB CD34+ + add-back CD34− from clinically | 9 | 9 of 9 (100%) in BM or spleen or both | 9 of 9 (100%) clinically engrafting unit (CD34− origin) |
| engrafting unit | |||
| DCB CD34+ + add-back CD34− from clinically nonengrafting unit | 11 | 9 of 11 (82%) in BM or SP or both, with remaining 2 predominantly nonengrafting unit | 0 of 11 (0%) clinically engrafting unit; 11 of 11 (100%) engrafted predominantly or exclusively with clinically nonengrafting unit (CD34− origin) |
Discussion
Transplantation of double-unit CB grafts is being investigated as a strategy to overcome the low TNC dose of single CB units in adults and larger children,6-9 but the mechanism of single-unit dominance is poorly understood. Yoo et al have reported a higher number of postthaw CFU-granulocyte-macrophage in the engrafting units.15 Scaradavou et al have reported that units with a low percentage of viable CD34+ cells had poor engraftment potential, and this correlated with a lower CFU content.16 Barker et al reported that neither the infused TNC or CD34+ cell doses nor the donor-recipient disparity of HLA-A and -B antigens, and -DRB1 alleles predicted which of the 2 CB units would engraft. The only factor correlating with unit engraftment in this study was a higher CD3+ cell dose.6 Interestingly, in the current series, a higher infused CD3+ dose was again shown to correlate with unit dominance supporting a role for T cells in unit dominance.
Our study further investigates double-unit biology in the laboratory using cells from clinical CB grafts and correlates the findings with the engraftment of those units in the patients. We demonstrated that the success of clinical engraftment is not determined by the stem/progenitor cell content as quantified by the CFC or CAFC content of either unit. Further, we could not demonstrate single-unit dominance using in vitro assays, as both units contributed approximately equally in cocultures. In the CFC assays, this could have been accounted for by the culture conditions. Semisolid media and low cell densities could preclude the cell-cell interactions necessary for T lymphocyte and/or NK-cell cytotoxicity, and in the CAFC assays the use of hydrocortisone and horse serum could profoundly suppress T lymphocyte and NK viability and function. We conclude from the in vitro studies that the overall hematopoietic potential of each unit does not explain clinical unit dominance. This does not exclude the possibility that in human transplantation there could be an engraftment advantage to a high potency unit when coupled with a unit with poor viability as recently reported by Scaradavou et al.16 However, it does suggest that, if hematopoietic potency of each unit is adequate, then other factors determine unit dominance.
For in vivo studies, we used the NSG model to facilitate multilineage engraftment of human hematopoietic cells12 and found high levels of human engraftment. In these murine experiments, we found that, although each unit engrafted alone, DCBT with MNCs demonstrated both single-unit dominance and a close correlation between mice and patients. That this was mediated by CD34− cells was demonstrated when unit dominance was lost with CD34+ selection and then restored with add-back of CD34− cells to CD34+ DCBTs. However, unlike murine DCBT with MNCs, engraftment after murine CD34+ DCBTs with CD34− add-backs of one unit correlated not with clinical engraftment but with the origin of the CD34− cells. Thus, absolute unit dominance is an in vivo phenomenon and represents a graft-versus-graft immune interaction mediated by CD34− cells. No definitive statement can be made as to whether DCBT enhances engraftment of the engrafting unit. It is notable that a comparable level of engraftment of the clinically engrafting unit was seen when half the number of these cells were infused in the context of a double-unit graft compared with when that unit was transplanted alone. We have previously observed a linear relationship in the human hematopoietic engraftment obtained in the bone marrow and spleen of irradiated NSG mice using similar numbers of CB cells (both MNCs and CD34+) as transplanted in this DCB study (M.A.M., unpublished observations, 2010) with maximum engraftment levels of 80% to 90%. This supports the interpretation that the comparable level of engraftment obtained with approximately half as many cells of the engrafting unit in the DCB setting may be the result of graft-versus-graft interactions that promote enhanced HSC engraftment. However, further studies will be needed to confirm this preliminary observation, and the only way to definitively answer this question would be to perform transplantation at limiting dilutions.
Hexner et al have reported that donor T cells exert effects independent of host resistance via their direct or indirect interactions with donor progenitor cells and their microenvironment.17 These investigators transplanted immunodeficient mice in limiting dilutions and showed enhanced engraftment with the addition of T cells compared with T-depleted MNCs, and specifically found that CD3/CD28 costimulated CB T cells possess graft-facilitating properties in a model where there is minimal host resistance to engraftment. They postulated that an alloresponse between the 2 units of a double-unit graft could therefore accelerate the engraftment of the engrafting unit. In the DCB experiments of Kim et al,18 single-unit dominance in NOD/SCID mice was observed after cotransplantation of MNCs. In contrast, single-unit dominance was lost and mixed chimerism achieved with either lineage depletion of each unit or cotransplantation of mesenchymal stromal cells expanded from third-party BM.18 Similarly, Yahata et al found unit dominance was mediated by CD34− cells, and specifically by a combination of CD4+ and CD8+ cells.19 That unit dominance is an immunologic phenomenon is further supported by Delaney et al who found that sustained engraftment was mediated by a T replete–unmanipulated CB unit rather than by the expanded but T cell–depleted unit in their report of 9 patients enrolled in a phase 1 study of a double-unit CB transplantation with ex vivo expansion.20 This group has also recently reported that single-unit dominance in human DCBT is associated with the presence of a specific CD8+ T lymphocyte response from the engrafting unit directed against the nonengrafting unit.21
Our results are consistent with those previous reports but provide, for the first time, a murine DCBT model using MNCs from clinical specimens that correlates with patient engraftment. Although interactions between CD34+ cells did not induce unit dominance, cells in the CD34− fraction of either unit can induce selective engraftment of that unit. The close correlation between the engrafting unit in the NSG mice, which do not have T and NK cells to resist engraftment, and that detected in the transplanted patients suggests that unit dominance reflects interactions between the units rather than an alloreaction against one unit mediated by residual host T or NK cells. Although it has been well documented that NSG mice support T-cell development,22,23 unit dominance in our model is observed before the time required for the emergence of postthymic effector T cells. The NSG model also supports alloreactive T-cell function from adult peripheral blood as reflected by mortality from graft-versus-host disease with as few as 2.5 × 106 MNCs.24,25 Hence, we hypothesize that T cells present in the CB at infusion both account for human unit dominance and are supported by the NSG mouse. Our model will be used to determine the specific cell populations within the CD34− fraction that mediate unit dominance and investigate the genetically determined allo-disparities on the nonengrafting unit that are targeted by the engrafting unit. Although results of these studies will not probably enable the prediction of which unit will be dominant before transplantation, they should further enhance the understanding of the biology of these transplantations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marcel van den Brink, James Young, Andromachi Scaradavou, Sharon Avery, Kang Zhang, Maureen Sullivan, Lingbo Shen, Paulo Salazar, Shieh Jae-Hung, Sheik Baksh, Jennifer Wilshire, Jan Hendrix, staff of the MSKCC Cytotherapy Laboratory, and Katharine H. deBeer for their assistance with this work.
This work was supported in part by the Gabrielle's Angel Foundation for Cancer Research (J.N.B.), the Memorial Sloan-Kettering Cancer Center Society (J.N.B.), the Translational and Integrative Medicine Research Grant (J.N.B.), the National Cancer Institute, National Institutes of Health (P01 CA23766; J.N.B., R.J.O.), and the Gar Reichman Fund of the Cancer Research Institute (M.A.M.).
National Institutes of Health
Authorship
Contribution: L.K.E. and S.C. performed the research, interpreted the data, and wrote the manuscript; A.B.-d.L., M.H., and M.E.A. performed the research; G.H. assisted in data analysis; R.J.O. interpreted the data and contributed to the manuscript; J.N.B. directed the clinical transplantation; and J.N.B. and M.A.M. designed the study, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juliet N. Barker, Department of Medicine, Allogeneic Bone Marrow Transplantation Service, Memorial Sloan-Kettering Cancer Center, Box 259, 1275 York Ave, New York, NY 10065; e-mail: barkerj@mskcc.org.
References
Author notes
L.K.E. and S.C. contributed equally to this study and are first authors.
J.N.B. and M.A.M. contributed equally to this study and are senior authors.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal