Abstract
Human multipotent mesenchymal stromal cells (MSCs) suppress proliferation and alloreactivity of T cells. Several signaling molecules and enzymes contribute to this effect. We focused on carbohydrate-protein interactions and investigated whether lectins are involved in immune modulation by MSC. Gene expression profiling of MSCs revealed that one of the most important lectins in this setting, galectin-1, was highly expressed. Galectin-1 protein was detected intracellularly and on the cell surface of MSCs. In addition, galectin-1 was released into the cell culture supernatant by MSCs. To analyze the functional role of galectin-1, a stable knockdown of galectin-1 in MSCs with use of a retroviral transfection system was established. Galectin-1 knockdown in MSCs resulted in a significant loss of their immunomodulatory properties, compared with MSCs infected with nontargeting control sequences. The galectin-1 knockdown partially restored the proliferation of CD4+ and CD8+ T cells. By contrast, the effect of MSCs on nonalloreactive natural killer (NK) cells was unaffected by down-regulation of galectin-1 expression. Furthermore, MSC-derived galectin-1 significantly modulated the release of cytokines involved in graft-versus-host disease (GVHD) and autoimmunity (eg, tumor necrosis factor-α [TNFα], IFNγ, interleukin-2 [IL-2], and IL-10. These results identify galectin-1 as the first lectin mediating the immunomodulatory effect of MSCs on allogeneic T cells.
Introduction
Multipotent human mesenchymal stromal cells (MSCs) are plastic-adherent cells with triangular and fibroblastoid morphologic features, which have the capacity to differentiate into osteo-blasts, adipocytes, and chondrocytes. Furthermore, they express the cell-surface markers CD73, CD90, and CD105, while being negative for CD34, CD45, and human leukocyte antigen (HLA-DR).1 Various tissues including bone marrow, fat, umbilical cord blood, or fetal tissues have been used as sources for MSCs.2 Not only are MSCs immunoprivileged because they are poorly lysed by T cells, but they also have strong immunosuppressive effects.3-6 Therefore, MSCs are of clinical interest for both regenerative medicine and immune suppression. MSCs have been used successfully for treatment of graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation.7 In addition, data from mouse models for rheumatoid arthritis or multiple sclerosis suggest that MSCs may be effective in the treatment of autoimmune diseases.8 In vitro studies have analyzed the immunomodulatory effects of MSCs in detail and showed that MSCs inhibit the proliferation of T cells independent of the T-cell stimulus (ie, the suppression was observed when T cells were activated by alloantigens, mitogens, or through T-cell receptor engagement by anti-CD3 in combination with anti-CD28 antibodies).4-6 Moreover, the inhibition is MHC independent.9,10 MSCs do not only inhibit T-cell proliferation directly, but also impair dendritic cell function (ie, MSCs interfere with dentritic cell [DC] differentiation, maturation, and activation), which is shown by compromised antigen presentation and altered cytokine release.11-14 MSCs also suppress the proliferation of NK cells and modulate the functions of B cells.2 Most studies agree in that no cell-cell contact is required for the MSC-mediated immunosuppressive effects.15 Instead, soluble molecules are thought to mediate this inhibition. Possible candidate molecules include transforming growth factor-β (TGFβ), hepatocyte growth factor,5 indoleamine-2,3-dioxygenase (IDO),16 prostaglandin E2 (PGE2),11,17,18 insulin-like growth factor–binding proteins,19 heme oxygenase-1 (HO),20 and HLA-G5.21 Moreover, the mechanism of the suppressive effects may be species dependent, as some molecules were described to be involved in suppressive effects of mouse MSCs such as nitric oxide (NO).22 Another novel candidate molecule recently described to be involved in suppressive effects of mouse MSCs is the MSC-derived CC chemokine ligand CCL2; the proteolytically processed form of CCL2 modulates immunoglobulin production by plasma cells and is involved in the beneficial effects of MSCs in experimental autoimmune encephalomyelitis.23,24 However, blocking any single one of these molecules did not completely abrogate the immunosuppressive functions of MSCs, indicating that several signaling pathways are involved and some important mediators may have not been identified yet. Several aspects, first of all, the induction of maternal tolerance toward the fetus during pregnancy, focused our attention on the evolutionarily conserved family of β-galactoside-binding proteins, the galectins.
The family of human galectins consists of 15 members, of which 11 were found to be expressed in various human tissues.25 They are widely distributed in lymphoid and nonlymphoid tissues and are key players in the regulation of cellular homeostasis involved in the innate and adaptive immune response.26 Galectins are up-regulated for example in activated T and B cells, regulatory T cells, or inflammatory macrophages.27 They are synthesized at free ribosomes in the cytoplasm but can also be released by cells in a nonclassic way bypassing the endoplasmatic reticulum and the golgi apparatus.26 Although some family members are immunostimulatory, galectin-1, the 14.5-kDa prototype member of the galectin family, has been described as a negative regulator of immune responses.28 Galectin-1 was found to exist as a monomer or as a noncovalently associated homodimeric form.29 It has antiproliferative effects on activated T cells30,31 and even causes them to undergo apoptosis in many settings.32 Furthe-rmore, galectin-1 supports the survival of naive T cells without promoting cell proliferation.33 Th2 cells are protected from galectin-1–mediated cell death, whereas stimulated Th1 cells are eliminated.34 Along this line, galectin-1 inhibits the secretion of cytokines typical of Th1 and Th17 cells while promoting Th2-type cytokine secretion.33,35 Moreover, it was shown that galectin-1 has regulatory functions during fetomaternal tolerance because it prevents fetal loss in stress-challenged pregnancies by modulating the Th1/Th2 cytokine balance and by inducing tolerogenic cells.36 Decidual NK cells may also use galectin-1 to negatively regulate decidual T cells.37 Accordingly, it has been demonstrated recently that galectin-1 has an essential function in the generation of tolerogenic DC.38
Here, we report that galectin-1 is highly expressed in MSCs; it is located intracellularly but is also released by MSCs in high amounts. Specific knockdown experiments in human MSCs identified galectin-1 as a key mediator of MSC-induced immune modulation with differential effects on lymphocyte subpopulations and their cytokine profile.
Methods
MSC isolation and cell culture
MSCs were derived from excessive material of standard bone marrow biopsies (Institutional Review Board [IRB] approval 241/2005V). Excess material was used after informed consent in accordance with the Declaration of Helsinki and approval by the University Children's Hospital Tübingen's IRB. Red blood cells were removed by ammonium chloride lysis for 10 minutes at room temperature. After washing with HANKS (Biochrom), cells were resuspended in FFPP medium and plated in culture flasks. FFPP medium consisted of low-glucose Dulbecco modified Eagle medium (DMEM; LG-DMEM; Lonza) supplemented with 5% (vol/vol) human fresh-frozen plasma (FFP), 107/mL platelets (University of Tübingen blood donor center), 80 IU/mL heparin sulfate, 2mM glutamine (Biochrom), 100 IU/mL penicillin, and 100 mg/mL streptomycin (Biochrom).
Differentiation assays
For osteogenic and adipogenic induction, MSCs were seeded in LG-DMEM (Lonza) containing 5% of FFP, 100 IU/mL of penicillin and 100 mg/mL, 80 IU/mL of heparin sulfate, and 2mM of glutamine. Bone induction medium was supplemented with dexamethasone (10nM; Merck), L-ascorbic acid-2-phosphate (0.1mM, Sigma-Aldrich), beta-glycerol phosphate (10mM, Sigma-Aldrich) and bone morphogenic protein-2 (100 ng/mL; PeproTech EC). After 2 to 3 weeks, osteogenic differentiated MSCs and controls were stained with aqueous 0.5% (vol/vol) AlizarinRed-S (Sigma-Aldrich). For adipogenic induction, the differentiation medium was supplemented with dexamethasone (1μM; Merck), insulin (10mM; Novo Nordisk), isobuthylmethylxanthine (0.5mM; Sigma-Aldrich) and indomethacin (60μM; Sigma-Aldrich). After 2 to 3 weeks, differentiation was verified by Oil-Red-O (Sigma-Aldrich) staining and counterstaining with hematoxilin (Sigma-Aldrich).
Flow cytometry
Flow cytometric analysis was performed on a FACSCalibur (Becton Dickinson) and data were analyzed with CellQuest software (Becton Dickinson). Anti–CD4-PE (RPA-T4), anti–CD8-APC (SK 1), anti–CD34-PE (563), anti–CD56-PE (B159), anti–CD73-PE (A02), and anti–HLA-ABC-PE (G46-2.6) antibodies were obtained from Becton Dickinson. Anti–IgG1-PE (clone MOPC-21), anti–CD45-PE (HI30), and anti–HLA-DR-PE (L243) antibodies were obtained from BioLegend. In addition, the following antibodies were used: anti-CD105 (N1-3A1; Ancell) and anti–CD90-PE (F15-42-1; Serotec). Propidium iodide (Becton Dickinson) was used for staining of dead cells. For detection of galectin-1, cells were fixed with paraformaldehyde (4% in phosphate-buffered saline [PBS]); for intracellular staining, cells were incubated with saponine buffer (0.1% saponine, 1% fetal calf serum [FCS], 0.01% sodium azide in PBS). Afterward, cells were incubated with biotinylated antigalectin-1 antibody (BAF1152; R&D Systems); after washing, the cells were stained with streptavidin-PE (Invitrogen).
Real-time RT-PCR
Total RNA was isolated with TriFast (PeqLab) followed by reverse transcription (RT) with the Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNA was amplified with primers specific for galectins, hAlas, or 18S primers, respectively (40 cycles, 95°C for 10 seconds, 59°C, or 61°C for 30 seconds) with use of SYBR Green chemistry (IQ SYBR Green Supermix Kit from Bio-Rad) on the CFX96 Real-Time PCR Detection System (Bio-Rad). Primer sequences are listed in Table 1; primers were designed to produce exon/intron boundary-spanning products. For quantification in real-time polymerase chain reaction (PCR), the ΔCT method was used.
Galectin primers for quantitative PCR
| . | Forward primer . | Reverse primer . |
|---|---|---|
| Gal-1 | 5′-GGTCTGGTCGCCAGCAACCTGAAT-3′ | 5′-TGAGGCGGTTGGGGAACTTG-3′ |
| Gal-2 | 5′-TGGCACTGATGGCTTTGTAA-3′ | 5′-AAGAGGACATGTTGAACCCG-3′ |
| Gal-3 | 5′-CCAAAGAGGGAATGATGTTGCC-3′ | 5′-TGATTGTACTGCAACAAGTGAGC-3′ |
| Gal-4 | 5′-TGTGCCTCCCACAGGCAAGAG-3′ | 5′-GCCACAGCGAATGGACAGATC-3′ |
| Gal-7 | 5′-GGCTTGGTTCCTCCCAAT-3′ | 5′-GGAAGTGGTGGTACTGGGC-3′ |
| Gal-8 | 5′-CTTTAATGTTGACCTACTAGCAGG-3′ | 5′-TTGTACTCCAGGCTGTGTACGC-3′ |
| Gal-9 | 5′-CAGTGCTCAGAGGTTCCACA-3′ | 5′-TGAGGCAGTGAGCTTCACAC-3′ |
| Gal-10 | 5′-TACCCGTGCCATACACAGAGGCTG-3′ | 5′-CTTATCTGGCAGCACTGAGATGCTC-3′ |
| Gal-12 | 5′-CCTGGGCAGGTCATCATAGT-3′ | 5′-AGGAGCAGCACCTCAAAGAA-3′ |
| Gal-13 | 5′-AATGACCCACAGCTGCAGGTG-3′ | 5′-CGTAAATGCGTATGCCATTGACC-3′ |
| Gal-14 | 5′-CCTTGATGATTGTGGTACCAT-3′ | 5′-GTGGGTCCTTGACAAAAGTG-3′ |
| 18S | 5′-TCGATGCTCTTAGCTGAGTGTCC-3′ | 5′-TGATCGTCTTCGAACCTCCG-3′ |
| hAlas | 5′-AATGAGTCGCCACCCACG-3′ | 5′-CAGCTCCCGCTCTAAGTCCA-3′ |
| . | Forward primer . | Reverse primer . |
|---|---|---|
| Gal-1 | 5′-GGTCTGGTCGCCAGCAACCTGAAT-3′ | 5′-TGAGGCGGTTGGGGAACTTG-3′ |
| Gal-2 | 5′-TGGCACTGATGGCTTTGTAA-3′ | 5′-AAGAGGACATGTTGAACCCG-3′ |
| Gal-3 | 5′-CCAAAGAGGGAATGATGTTGCC-3′ | 5′-TGATTGTACTGCAACAAGTGAGC-3′ |
| Gal-4 | 5′-TGTGCCTCCCACAGGCAAGAG-3′ | 5′-GCCACAGCGAATGGACAGATC-3′ |
| Gal-7 | 5′-GGCTTGGTTCCTCCCAAT-3′ | 5′-GGAAGTGGTGGTACTGGGC-3′ |
| Gal-8 | 5′-CTTTAATGTTGACCTACTAGCAGG-3′ | 5′-TTGTACTCCAGGCTGTGTACGC-3′ |
| Gal-9 | 5′-CAGTGCTCAGAGGTTCCACA-3′ | 5′-TGAGGCAGTGAGCTTCACAC-3′ |
| Gal-10 | 5′-TACCCGTGCCATACACAGAGGCTG-3′ | 5′-CTTATCTGGCAGCACTGAGATGCTC-3′ |
| Gal-12 | 5′-CCTGGGCAGGTCATCATAGT-3′ | 5′-AGGAGCAGCACCTCAAAGAA-3′ |
| Gal-13 | 5′-AATGACCCACAGCTGCAGGTG-3′ | 5′-CGTAAATGCGTATGCCATTGACC-3′ |
| Gal-14 | 5′-CCTTGATGATTGTGGTACCAT-3′ | 5′-GTGGGTCCTTGACAAAAGTG-3′ |
| 18S | 5′-TCGATGCTCTTAGCTGAGTGTCC-3′ | 5′-TGATCGTCTTCGAACCTCCG-3′ |
| hAlas | 5′-AATGAGTCGCCACCCACG-3′ | 5′-CAGCTCCCGCTCTAAGTCCA-3′ |
PCR indicates polymerase chain reaction; and Gal, galectin.
Galectin-1 ELISA
The sandwich enzyme-linked immunosorbent assay (ELISA) for galectin-1 was based on the polyclonal antigalectin-1 antibody from R&D Systems. The antigalectin-1 antibody (1 μg/mL) was used for coating Nunc Maxisorb plates (Nunc). After blocking with 10% FCS (Biochrom) in PBS, plates were washed with PBS containing 0.05% Tween 20 (Roth), followed by an incubation step with cell culture supernatant or standard (recombinant galectin-1; R&D Systems), respectively. After washing, galectin-1 was detected by the biotinylated antigalectin-1 antibody (0.5 μg/mL) in PBS containing 2% goat serum (Dako). Then, the plates were incubated with horseradish peroxidase–conjugated streptavidin (1 μg/mL; Pierce) diluted in PBS with 10% FCS before detection with use of a tetramethylbenzidine peroxidase substrate system (Pierce).
Plasmid construction for RNA silencing
For RNA silencing, retroviral constructs were generated based on pSUPER constructs.39 The knockdown-specific target sequences for RNAi were designed by use of the Dharmacon siRNA design center and obtained from Sigma-Aldrich. Besides galectin-1–specific knockdown sequences, control knockdown constructs were designed targeting luciferase and leukemic fusion protein AML/MTG8, respectively. All oligonucleotides are listed in Table 2, and the contained 19-nt target sequences are indicated in capitals in the sequences. To generate the pSUPER constructs, the pSUPER vector (containing a puromycin resistance cassette) was digested with Bgl II and Sal I, and the annealed oligonucleotides (Table 2) were ligated into the vector. To generate retroviral constructs, the MigR1 plasmid was used, which is based on a self-inactivating murine stem cell virus. EcoR I-digested and XhoI-digested inserts from pSUPER constructs (containing the H1 polymerase III promoter and the targeting knockdown inserts) were cloned into the same sites in the MigR1 retroviral construct. All restriction enzymes were obtained from New England Biolabs.
Oligonucleotides used to generate retroviral plasmids for RNA silencing
| . | Oligonucleotide . |
|---|---|
| Galectin-1 | |
| Forward | 5′-gatccccGCTGCCAGATGGATACGAAttcaagagaTTCGTATCCATCTGGCAGCtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaGCTGCCAGATGGATACGAAtctcttgaaTTCGTATCCATCTGGCAGCggg-3′ |
| Luciferase | |
| Forward | 5′-gatccccCGTACGCGGAATACTTCGAttcaagagaTCGAAGTATTCCGCGTACGtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaCGTACGCGGAATACTTCGAtctcttgaaTCGAAGTATTCCGCGTACGggg-3′ |
| AML/MTG8 fusion protein | |
| Forward | 5′-gatccccCCTCGAAATCGTACTGAGAttcaagagaTCTCAGTACGATTTCGAGGtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaCCTCGAAATCGTACTGAGAtctcttgaaTCTCAGTACGATTTCGAGGggg-3′ |
| . | Oligonucleotide . |
|---|---|
| Galectin-1 | |
| Forward | 5′-gatccccGCTGCCAGATGGATACGAAttcaagagaTTCGTATCCATCTGGCAGCtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaGCTGCCAGATGGATACGAAtctcttgaaTTCGTATCCATCTGGCAGCggg-3′ |
| Luciferase | |
| Forward | 5′-gatccccCGTACGCGGAATACTTCGAttcaagagaTCGAAGTATTCCGCGTACGtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaCGTACGCGGAATACTTCGAtctcttgaaTCGAAGTATTCCGCGTACGggg-3′ |
| AML/MTG8 fusion protein | |
| Forward | 5′-gatccccCCTCGAAATCGTACTGAGAttcaagagaTCTCAGTACGATTTCGAGGtttttggaaa-3′ |
| Reverse | 5′-cgatttccaaaaaCCTCGAAATCGTACTGAGAtctcttgaaTCTCAGTACGATTTCGAGGggg-3′ |
AML/MTG8 indicates fusion protein for acute myeloid leukemia.
Transfections and retroviral infections
293T cells, which are originally referred to as 293tsA1609neo (variant of the human embryonic kidney 293 cells containing the SV40 large T antigen), were used for transient production of viral supernatant, and the HT1080 human fibrosarcoma–derived packaging cell line FLYRD18 (expressing the Moloney murine leukemia virus gag-pol and the cat endogenous virus RD114 env) was used for stable production. Both cell lines were cultured in DMEM medium supplemented with 10% FCS, 2mM of glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. For the generation of FLYRD18 packaging cells, MigR1 constructs and the plasmids psRa-g (encoding for envelope) and PMDoldgag-pol (encoding for gag-pol) were introduced into 293T cells by cotransfection using jetPEI transfection reagent (Polyplus) combined with 50μM chloroquine (Sigma-Aldrich). Tissue culture medium from transfected 293T cells was filtered in a 0.45 μm of polyethersulfone filter (VWR International), and the viral supernatant was used for infection of cells after addition of 6 μg/mL polybrene (Sigma-Aldrich). Infections were carried out for 3 consecutive days. The multiplicity of infections of the generated packing cells FLYRD18 was determined. For infection of MSCs, the ecotropic retroviral supernatant from the FLYRD18 was used and, if necessary, diluted to adapt the multiplicity of infections (3 × 105 TU/mL), filtered again in a 0.45-μm filter, and 6 μg/mL polybrene was added. MSCs were infected for at least 6 hours and were allowed to recover overnight with fresh medium. Infections were carried out for 4 days.
Immunoblot analysis
Whole-cell lysate of MSCs was generated from confluent cell layers in 6-well plates using lysis buffer made of 50mM Tris-Cl, pH 7.4; 150mM sodium chloride, 1% NP-40, 1% sodium deoxycholate, 1mM EDTA, 0.1% SDS (all from Sigma-Aldrich), and Mini Complete, EDTA-free protease inhibitor cocktail (Roche). Proteins were separated by 12% sulfate-polyacrylamide gel electrophoresis, blotted to nitrocellulose membranes (GE Healthcare), blocked with TBS containing 5% nonfat dry milk and analyzed for galectin-1 with antigalectin-1 antibody (AF1152, R&D Systems), followed by a donkey anti–goat horseradish peroxidase conjugate (Santa Cruz Biotechnology), and visualized with SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Proliferation assays
HLA-mismatched peripheral blood mononuclear cells (PBMCs) were obtained from healthy volunteers (IRB approval 279/2003V). PBMCs were stained with carboxyfluorescein succinimidyl ester (CFSE) according to the manufacturer's protocol (Invitrogen); proliferation was measured by flow cytometry. PBMCs were stimulated with either 100 U/mL interleukin-2 (IL-2; R&D Systems) and 1 μg/mL OKT3 (Janssen Cilag), which is referred to as IL-2/OKT3, or with 1 μg/mL anti-CD28 antibody (eBiosciences) and 1 μg/mL OKT3, which is referred to as αCD28/OKT3. A total of 60 000 PBMCs were added per well of a 96-well plate (Greiner) already containing 5000 to 30 000 MSCs, where indicated. Incubation was carried out in RPMI (Biochrom), supplemented with 20% autologous plasma, 2mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. PBMC proliferation was analyzed after 4 days of incubation by flow cytometry. For mixed lymphocyte reactions (MLR), 100 000 CFSE-labeled PBMCs were cocultured with 100 000 HLA-mismatched and irradiated (30 cGy) PBMCs per well of a 96-well plate already containing 5000 to 30 000 MSCs in X-Vivo 15 medium (Invitrogen) supplemented with 2mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. MLR were analyzed after 6 days of incubation. For flow cytometric analysis of T cells, PBMC subpopulations were stained with anti-CD4–PE and anti-CD8–APC; dead cells were excluded by propidium iodide staining. For proliferation assays with pure T cells CD4+ T cells, cells were separated by magnetic-activated cell sorting (MACS) technology (Miltenyi Biotec) with use of an indirect magnetic labeling system according to the manufacturer's recommendation. Cell separation was performed with the autoMACS device (Miltenyi Biotec). In the absence of accessory cells, sufficient proliferation could not be induced by CD3/IL-2; thus, CD4+ T cells were stimulated with 7.5 μg/mL phytohemagglutinin (PHA; Boehringer) and incubated in X-Vivo culture medium.
NK cells were separated by MACS technology; cells were labeled with anti-CD56 beads and separated with the autoMACS system. NK cells were stimulated with 200 U/mL of IL-2 and incubated in RPMI supplemented with 20% autologous plasma, 2mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. NK cell proliferation was analyzed by flow cytometry after 5 days of incubation.
Generation of DC and cocultures of DC with MSCs
Monocytes were isolated from peripheral blood by MACS technology. Briefly, cells were positively labeled with anti-CD14 MicroBeads (Miltenyi) and separated with the autoMACS device. For differentiation to immature DC (iDC), 1000 IU/mL granulocyte-macrophage colony-stimulating factor (Amgen) and 500 IU/mL IL-4 (R&D Systems) were added every 2 days. To analyze the effect of MSCs on monocyte to DC differentiation, 1 × 106 monocytes were plated in the lower chamber of 6-well transwell plates (Corning), and HLA-mismatched MSCs (ratio 1:10 MSC/monocytes) were seeded in the upper chamber in RPMI supplemented with 5% autologous plasma of the DC donor, 2mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. After 5 days, MSCs were removed for DC maturation with 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich). Supernatants were collected 20 hours after LPS stimulation for quantification of IL-12 (IL-12p70).
Cytokine assays
Interferon-γ (IFNγ) release of IL-2/OKT3 stimulated PBMCs in the presence of MSCs was measured after 48 hours of incubation by sandwich ELISA. The IFNγ ELISA was performed according to the galectin-1 ELISA described in “Galectin-1 ELISA.” For coating of an anti-IFNγ antibody (MAB2852; 2 μg/mL, R&D Systems) and for detection, a biotinylated anti-IFNγ antibody (BAF2852, 0.175 μg/mL, R&D Systems) was used.
Production of IL-12p70 by mDCs was evaluated in supernatants 20 hours after LPS stimulation, and measurement was performed with the IL-12p70 ELISA kit from eBiosciences according to the manu-facturer's protocol.
Cocultures of MSCs with stimulated PBMCs were analyzed additionally with use of a cytokine bead array. Supernatants from IL-2/OKT3 and αCD28/OKT3 stimulated cocultures were collected 48 hours and 72 hours after stimulation, respectively. Optimal MSC:PBMC ratios were determined for each cytokine and for the different stimulations according to the detection limits and linear range of the standard curve. For stimulation with αCD28/OKT3, the MSC:PBMC ratio was 1:12, except for IL-5 and IL-10, where the ratio was 1:6. For stimulation with IL-2/OKT3, the MSC:PBMC ratio was 1:6, except for IFNγ and TNFα, where the ratios were 1:3 and 1:12, respectively. The supernatants were analyzed with Bio-Plex Pro human cytokine Th1/Th2 panel (Bio-Rad) according to the manu-facturer's protocol.
Statistical analysis
Analysis of significance was performed with an unpaired, 2-tailed Student t test with use of Microsoft Excel software. P ≤ .05 was considered statistically significant and is indicated by single asterisks.
Results
Galectin-1 is highly expressed in human MSCs
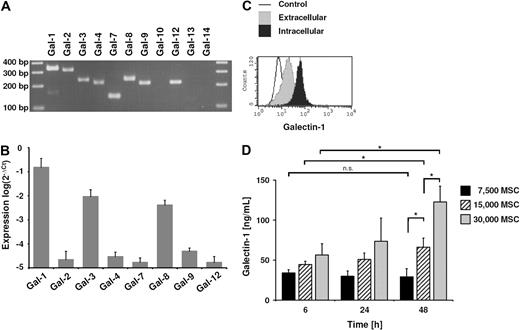
Human MSCs were isolated from bone-marrow aspirates by plastic adherence, and expression of typical surface markers as well as trilineage differentiation potential was confirmed (data not shown). Subsequently, mRNA expression profiling was performed to identify further candidate molecules involved in the immunomodulatory properties of MSCs. One of the most abundant mRNAs detected was galectin-1 (data not shown), which is known to have immunoregulatory properties. As not all galectins were included in the microarray, we performed a reverse transcription PCR for all galectins known to be expressed in humans. In addition to galectin-1 mRNA, mRNA of galectin-2, to galectin-4, galectin-7 to galectin-9, and galectin-12 was detected, whereas mRNA of galectin-10, galectin-13, and galectin-14 was absent (Figure 1A). Expression of these galectins was further analyzed by qPCR. On confirmation of the array data, galectin-1 was found most abundantly in MSCs. mRNA of galectin-3 and galectin-8 was detected to a lesser extent than galectin-1, and mRNA of galectin-2, galectin-4, galectin-7, galectin-9, and galectin-12 was only weakly expressed (Figure 1B). Because of the abundance of galectin-1 mRNA and the immunomodulatory function of this lectin, we next analyzed the protein expression of galectin-1 in more detail. Flow cytometric analysis revealed vigorous intracellular translation of galectin-1. Smaller amounts of galectin-1 were located at the outer cell surface of MSCs (Figure 1C). This finding suggested that galectin-1 may be released by MSCs, and cell culture supernatant was analyzed by ELISA. MSCs were seeded in 96-well plates: 7500, 15 000, and 30 000 cells per well; the latter cell number resulted in a highly confluent cell layer. After 6 hours of incubation, galectin-1 was detectable in all supernatants (Figure 1D). Galectin-1 was released by MSCs in a cell-number–dependent way. Within 48 hours, there was a significant increase in galectin-1 secretion in the supernatant of confluent cell layers (30 000 cells per well), which was not seen when MSCs were subconfluent (7500 cells per well). This suggests an influence of cell-cell contact on the release of galectin-1 by MSCs.
Galectin-1 expression in human MSCs. (A) Reverse transcription PCR of galectin family members in MSCs analyzed by agarose gel electrophoresis. mRNA of galectin-1 to galectin-4, galectin-7 to galectin-9, and galectin-12 were detected, whereas mRNA of galectin-10, galectin-13, and galectin-14 were not detectable. (B) Comparison of the galectin expression by qPCR in MSCs. Besides the abundant expression of galectin-1, galectin-3 and galectin-8 were also highly expressed. The relative expression level was normalized to expression of 18S RNA. The qPCR data are representative of 3 independent experiments representing 3 different MSC donors (mean ± SD). (C) Flow cytometric analysis of galectin-1 by intracellular and cell-surface staining of MSCs. (D) Detection of galectin-1 in the supernatant of MSCs by ELISA. MSCs released galectin-1 in high amounts into the supernatant in a cell-number–dependent manner. The correlation between cell number and galectin-1 release was not linear, as evidenced by the significant difference in the galectin-1 concentration between 6 hours and 48 hours of incubation, when 30 000 MSCs were seeded (confluent cell layer). However, there was no significant difference, when only 7500 (subconfluent layer) were seeded. This suggests a cell-contact–dependent increase in the confluent culture (30 000 cells per 96-well plate). Data are shown as means (± SD) of triplicates (n = 3); n.s., not significant.
Galectin-1 expression in human MSCs. (A) Reverse transcription PCR of galectin family members in MSCs analyzed by agarose gel electrophoresis. mRNA of galectin-1 to galectin-4, galectin-7 to galectin-9, and galectin-12 were detected, whereas mRNA of galectin-10, galectin-13, and galectin-14 were not detectable. (B) Comparison of the galectin expression by qPCR in MSCs. Besides the abundant expression of galectin-1, galectin-3 and galectin-8 were also highly expressed. The relative expression level was normalized to expression of 18S RNA. The qPCR data are representative of 3 independent experiments representing 3 different MSC donors (mean ± SD). (C) Flow cytometric analysis of galectin-1 by intracellular and cell-surface staining of MSCs. (D) Detection of galectin-1 in the supernatant of MSCs by ELISA. MSCs released galectin-1 in high amounts into the supernatant in a cell-number–dependent manner. The correlation between cell number and galectin-1 release was not linear, as evidenced by the significant difference in the galectin-1 concentration between 6 hours and 48 hours of incubation, when 30 000 MSCs were seeded (confluent cell layer). However, there was no significant difference, when only 7500 (subconfluent layer) were seeded. This suggests a cell-contact–dependent increase in the confluent culture (30 000 cells per 96-well plate). Data are shown as means (± SD) of triplicates (n = 3); n.s., not significant.
Generation of an efficient galectin-1 knockdown in MSCs
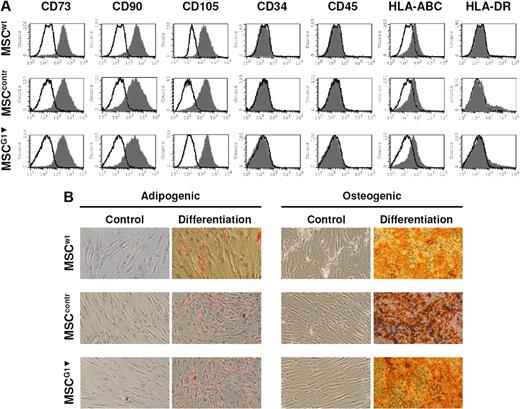
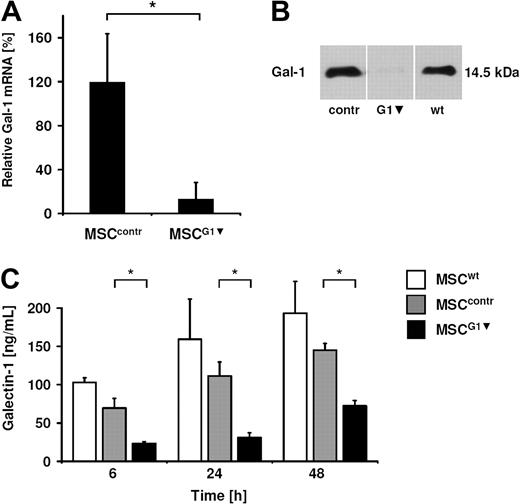
To analyze whether galectin-1 derived from MSCs contributes to their immunomodulatory properties, we generated a knockdown of galectin-1 in MSCs by RNA interference. We chose this approach instead of using a neutralizing antibody, because activated effector cells in the MLR secrete galectin-1 as well. We tested several transient transfection strategies and failed to significantly reduce galectin-1 protein content in MSCs because of the extremely high expression of galectin-1 (data not shown). Finally, MSCs were infected with a retroviral construct containing the H1 polymerase III promoter and the targeting knockdown sequences against galectin-1 based on small hairpins. We generated 2 control constructs using sequences against luciferase and AML/MTG8, which are not expressed in MSCs. The retroviral infection and also the galectin-1 knockdown did not alter the immune phenotype of MSCs, which was verified by positive staining for CD73, CD90, CD105, and the absence of HLA-DR, CD45, and CD34 on the cell surface of MSCs (Figure 2A). Furthermore, the differentiation potential was not affected by the retroviral transfection or galectin-1 knockdown, as MSCG1▾ could be induced toward the adipogenic and osteogenic lineage (Figure 2B). The knockdown of galectin-1 was analyzed by qPCR and showed a reduction of galectin-1 mRNA to 13% compared with untreated MSCs (MSCwt; Figure 3A). Subsequently, the effect of RNAi was determined on protein level. By immunoblot analysis, galectin-1 was virtually undetectable in whole-cell lysate of MSCG1▾ (Figure 3B). Moreover, the release of galectin-1 by MSCs was strongly reduced as assessed by ELISA of MSC supernatants. MSCs transfected with control sequences (MSCcontr) showed no significant difference in the release of galectin-1 into the cell culture supernatant compared with MSCwt. However, the concentration of galectin-1 in the supernatant of MSCG1▾ was significantly reduced compared with both MSCwt and MSCcontr, which was detected after 6 hours, 24 hours, and 48 hours of incubation, respectively (Figure 3C).
Characterization of retrovirally transfected MSCs. (A) Flow cytometric analysis of cell-surface markers on untransfected MSCs (MSCwt), MSCs transfected to express nontargeting control RNAi (MSCcontr), and MSCs expressing RNAi for galectin-1 (MSCG1▾). The immunophenotype of MSCG1▾ was not altered compared with MSCwt or MSCcontr. (B) Adipogenic and osteogenic differentiation of MSCwt, MSCcontr, and MSCG1▾. MSCG1▾ showed normal differentiation into adipocytes and osteoblasts.
Characterization of retrovirally transfected MSCs. (A) Flow cytometric analysis of cell-surface markers on untransfected MSCs (MSCwt), MSCs transfected to express nontargeting control RNAi (MSCcontr), and MSCs expressing RNAi for galectin-1 (MSCG1▾). The immunophenotype of MSCG1▾ was not altered compared with MSCwt or MSCcontr. (B) Adipogenic and osteogenic differentiation of MSCwt, MSCcontr, and MSCG1▾. MSCG1▾ showed normal differentiation into adipocytes and osteoblasts.
Knockdown of galectin-1 in MSCs by retroviral transfection. (A) qPCR analysis of the galectin-1 knockdown in MSCs. After normalization with hAlas mRNA, the relative copy numbers of galectin-1 in retroviral transfected MSCs were compared with the copy number in untreated MSCs (100%). The data are shown as means ± SD of 5 independent experiments. (B) Western blot analysis of the galectin-1 knockdown in whole-cell lysates of MSCs (20 μg protein was loaded per lane). (C) MSCG1▾ released less galectin-1 than MSCwt or MSCcontr into the supernatant measured by ELISA, 15 000 MSCs were seeded (representative of n = 3). Data are shown as means of quadruplicates (± SD).
Knockdown of galectin-1 in MSCs by retroviral transfection. (A) qPCR analysis of the galectin-1 knockdown in MSCs. After normalization with hAlas mRNA, the relative copy numbers of galectin-1 in retroviral transfected MSCs were compared with the copy number in untreated MSCs (100%). The data are shown as means ± SD of 5 independent experiments. (B) Western blot analysis of the galectin-1 knockdown in whole-cell lysates of MSCs (20 μg protein was loaded per lane). (C) MSCG1▾ released less galectin-1 than MSCwt or MSCcontr into the supernatant measured by ELISA, 15 000 MSCs were seeded (representative of n = 3). Data are shown as means of quadruplicates (± SD).
Galectin-1 knockdown in MSCs restores proliferation and IFNγ production of PBMC
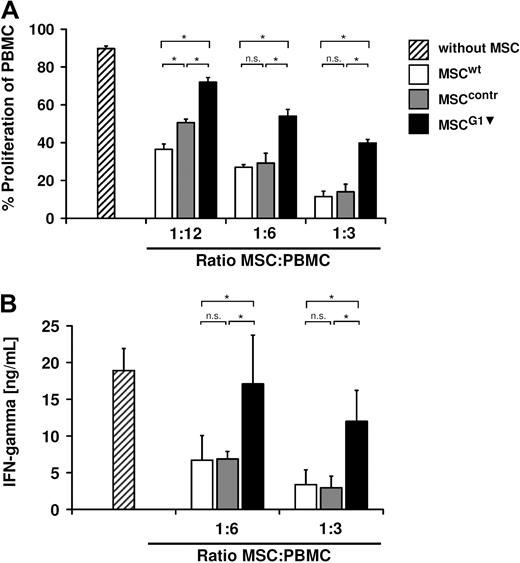
In a first approach to characterize galectin-1 contribution to the immunomodulatory properties of MSCs, the antiproliferative effects of MSCG1▾ on PBMCs were analyzed. CFSE-labeled PBMCs were stimulated with 100 U/mL IL-2 and 1 μg/mL OKT3 and cocultured with MSCs for 4 more days, before proliferation was analyzed by flow cytometry. MSCcontr showed the same antiproliferative properties as MSCwt. By contrast, inhibition of PBMC proliferation by MSCG1▾ was significantly reduced compared with both MSCcontr and MSCwt (Figure 4A). MSCs are known to decrease the release of proinflammatory cytokines by PBMCs. Therefore, we analyzed the IFNγ concentrations in supernatants of MSCs cocultured with IL-2/OKT3–stimulated PBMCs (Figure 4B). After 48 hours, a significantly increased concentration of IFNγ was detectable in the presence of MSCG1▾, indicating that galectin-1 not only contributes to the antiproliferative functions of MSCs but is also involved in the modulation of the cytokine production of PBMCs.
Galectin-1 knockdown in MSCs resulted in a strongly reduced inhibition of IL-2/OKT3–stimulated PBMCs. (A) Proliferation of HLA-mismatched PBMCs in the absence or presence of MSCs. MSCwt and MSCcontr showed comparable inhibition of PBMC proliferation, whereas MSCG1▾ showed significantly reduced antiproliferative effects. (B) IFNγ-secretion by PBMCs measured 48 hours after stimulation with IL-2/OKT3 in the presence of HLA-mismatched MSCs. MSCG1▾ showed a significantly reduced inhibition of IFNγ secretion by PBMCs compared with MSCwt and MSCcontr. Data are shown as means (± SD) of quadruplicates (n = 3).
Galectin-1 knockdown in MSCs resulted in a strongly reduced inhibition of IL-2/OKT3–stimulated PBMCs. (A) Proliferation of HLA-mismatched PBMCs in the absence or presence of MSCs. MSCwt and MSCcontr showed comparable inhibition of PBMC proliferation, whereas MSCG1▾ showed significantly reduced antiproliferative effects. (B) IFNγ-secretion by PBMCs measured 48 hours after stimulation with IL-2/OKT3 in the presence of HLA-mismatched MSCs. MSCG1▾ showed a significantly reduced inhibition of IFNγ secretion by PBMCs compared with MSCwt and MSCcontr. Data are shown as means (± SD) of quadruplicates (n = 3).
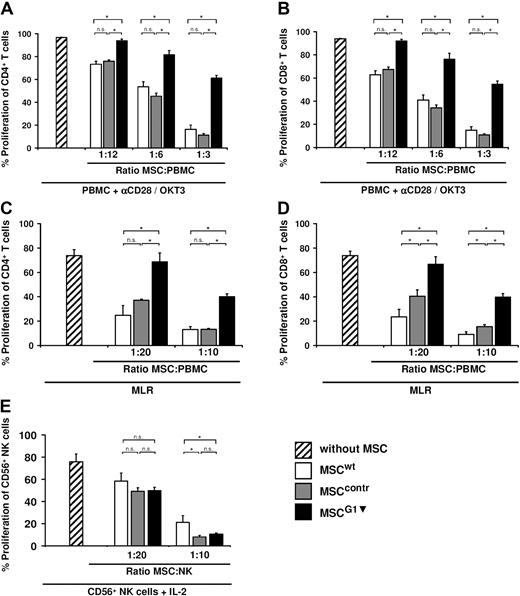
MSC-derived galectin-1 inhibits alloreactive CD4+ and CD8+ T cells
As PBMCs are a heterogeneous cell population, the antiproliferative properties of MSCs were analyzed in more detail. Therefore, CD4+ and CD8+ T cells were stimulated with IL-2/OKT-3 in one set of experiments and with αCD28/OKT3 in another. The proliferation of both populations, CD4+ and CD8+ T cells, was restored to the same extent when galectin-1 expression was silenced in MSCs. This was also true for both stimulation methods, IL-2/OKT3 (data not shown) and αCD28/OKT3 (Figure 5A-B). In addition, the effect of MSCG1▾ in mixed lymphocyte reactions (MLR) was assessed. Comparable with the experiments with cytokine and antibody-stimulated T cells, MSCG1▾ were also significantly less potent in suppressing CD4+ and CD8+ T cells in MLRs (Figure 5C-D).
Galectin-1 contributes to the antiproliferative effects of MSCs against alloreactive T cells but not against NK cells. (A-D) Analysis of proliferation of CD4+ T cells (A,C) and CD8+ T cells (B,D) in the presence of MSCs; MSCG1▾ showed strongly reduced antiproliferative effects compared with MSCwt or MSCcontr. This was independent of the T-cell stimulus; the T cells were either simulated by αCD28/OKT3 (A,B), or they were stimulated by a mixed lymphocyte reaction (C-D). (E) Proliferation of CD56+ NK cells in the absence or presence of MSCs. Suppression of NK cell proliferation in response to IL-2 was comparable when MSCG1▾, MSCwt, or MSCcontr were present. Data in all panels are shown as means (± SD) of quadruplicates (n = 3).
Galectin-1 contributes to the antiproliferative effects of MSCs against alloreactive T cells but not against NK cells. (A-D) Analysis of proliferation of CD4+ T cells (A,C) and CD8+ T cells (B,D) in the presence of MSCs; MSCG1▾ showed strongly reduced antiproliferative effects compared with MSCwt or MSCcontr. This was independent of the T-cell stimulus; the T cells were either simulated by αCD28/OKT3 (A,B), or they were stimulated by a mixed lymphocyte reaction (C-D). (E) Proliferation of CD56+ NK cells in the absence or presence of MSCs. Suppression of NK cell proliferation in response to IL-2 was comparable when MSCG1▾, MSCwt, or MSCcontr were present. Data in all panels are shown as means (± SD) of quadruplicates (n = 3).
MSC-derived galectin-1 is negligible in MSC-mediated inhibition of NK cells
Galectin-1 played an important role in the inhibition of alloreactive T cells by MSCs. NK cells are another important cytotoxic effector cell population in the immune system. NK cells are not alloreactive in a classic MHC-restricted sense but are indirectly involved in GVHD (eg, by cytokine secretion). MSCs are known to inhibit proliferation and several other functions of NK cells. To analyze a possible role of MSC-derived galectin-1 in suppressing NK cells, CD56+ NK cells were isolated by MACS technology, stimulated with 200 U/mL IL-2, and then cocultured with MSCs. Expectedly, MSCcontr and MSCwt inhibited proliferation of NK cells. However, the galectin-1 knockdown in MSCs had no significant effect on NK cell proliferation (Figure 5E). This indicates that MSCs use different signals to inhibit T cells and NK cells.
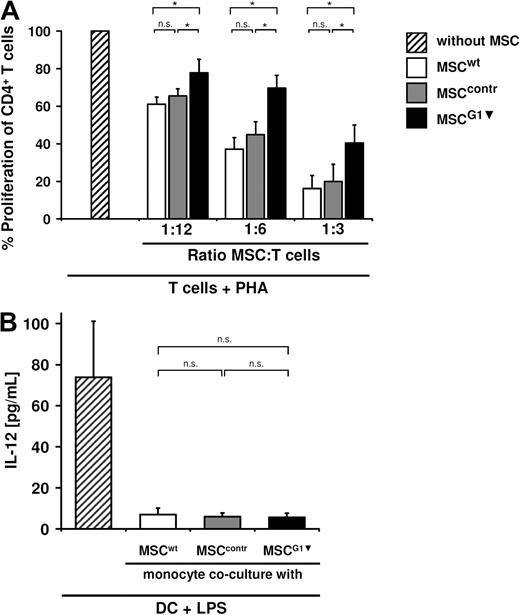
MSCs inhibit T-cell proliferation in the absence of other cell types and in part by galectin-1
On the basis of the literature, we asked whether the galectin-1–dependent inhibition of T-cell proliferation was a direct signal of galectin-1 to the T cells themselves or mediated by galectin-1 effects on other PBMCs (eg, DCs). To this end, CD4+ T cells were purified, stimulated, and cocultured with MSCwt, MSCcontr, and MSCG1▾, respectively. MSCwt and MSCcontr suppressed proliferation of CD4+-T cells to a comparable extent, indicating that MSCs can act directly on T cells without the help of accessory PBMCs. Again, proliferation of purified T cells was significantly less affected when cocultured with MSCG1▾ (Figure 6A). This shows that galectin-1 expressed by MSCs causes antiproliferative effects on T cells directly.
MSC-derived galectin-1 inhibits T-cell proliferation directly. (A) Purified CD4+ T cells were stimulated with PHA and cocultured with MSCwt, MSCcontr, or MSCG1▾ as indicated. Proliferation of purified CD4+ T cells was inhibited by MSCwt or MSCcontr in the absence of any other cell type. Proliferation of purified CD4+ T cells was less affected by the presence of MSCG1▾ compared with MSCwt or MSCcontr. These experiments show that MSC-derived galectin-1 suppresses T cells directly in the absence of other cell types. (B) IL-12 secretion of monocyte-derived DC after coculture with MSCs. Monocytes were incubated with IL-4 and granulocyte-macrophage colony-stimulating factor alone and in the presence of MSCwt, MSCcontr, or MSCG1▾ (ratio 1:10 MSC/monocytes) for 5 days. The proinflammatory cytokine IL-12 was measured after maturation with LPS for 20 hours in the supernatant. IL-12 secretion of DC was suppressed to the same extent by MSCwt, MSCcontr, and MSCG1▾. All data are shown as means (± SE) of 6 independent experiments.
MSC-derived galectin-1 inhibits T-cell proliferation directly. (A) Purified CD4+ T cells were stimulated with PHA and cocultured with MSCwt, MSCcontr, or MSCG1▾ as indicated. Proliferation of purified CD4+ T cells was inhibited by MSCwt or MSCcontr in the absence of any other cell type. Proliferation of purified CD4+ T cells was less affected by the presence of MSCG1▾ compared with MSCwt or MSCcontr. These experiments show that MSC-derived galectin-1 suppresses T cells directly in the absence of other cell types. (B) IL-12 secretion of monocyte-derived DC after coculture with MSCs. Monocytes were incubated with IL-4 and granulocyte-macrophage colony-stimulating factor alone and in the presence of MSCwt, MSCcontr, or MSCG1▾ (ratio 1:10 MSC/monocytes) for 5 days. The proinflammatory cytokine IL-12 was measured after maturation with LPS for 20 hours in the supernatant. IL-12 secretion of DC was suppressed to the same extent by MSCwt, MSCcontr, and MSCG1▾. All data are shown as means (± SE) of 6 independent experiments.
These experiments do not rule out that MSCs inhibit T-cell proliferation and effector functions additionally by interfering with DC function using galectin-1. Thus, to further analyze the effects of MSC-derived galectin-1 on DC, monocytes were differentiated to immature DC in the absence or presence of MSCwt, MSCcontr, and MSCG1▾ (ratio 1:10 MSC/monocyte). To determine the function of these iDC, they were then stimulated with 1 μg/mL of LPS. As a readout, we chose to measure the proinflammatory cytokine IL-12 released by DC, which is known to play a key role in DC interaction with T cells. DCs, which were cocultured with MSCs, showed a strong reduction of IL-12 secretion, as was to be expected based on earlier reports. Interestingly, this interference of MSCs with DC differentiation and maturation was independent of galectin-1, indicating that other molecules released by MSCs may be more potent inhibitors of DC differentiation under these conditions (Figure 6B).
MSCs regulate cytokine expression of PBMCs by galectin-1
To analyze effects of MSC-derived galectin-1 on the cytokine profile of PBMCs, cytokine levels were determined in supernatants of cocultures stimulated with IL-2/OKT3 and αCD28/OKT3, respectively. As shown in Table 3, IFNγ and TNFα levels were strongly reduced in the presence of MSCwt or MSCcontr, whereas IFNγ and TNFα secretion were partially restored in the presence of MSCG1▾. This was true for both IL-2/OKT3 and αCD28/OKT3 stimulation. In addition, the suppression of IL-12 and IL-5 by MSCwt was partially restored in the presence of MSCG1▾. Unexpectedly, the IL-2 production in PBMCs, which was reduced by MSCwt, was further decreased by the knockdown of galectin-1 in MSCs (Table 3B). Another important cytokine reportedly involved in immune suppression is IL-10. The IL-10 concentration was decreased by MSCwt in IL-2/OKT3–stimulated cultures of PBMCs. However, in αCD28/OKT3–stimulated cultures, IL-10 levels were increased in the presence of MSCwt compared with PBMC alone. In both stimulation methods, the IL-10 release was increased by MSCG1▾ compared with MSCwt and MSCcontr. IL-4 production was slightly increased by MSCs in αCD28/OKT3–stimulated cultures, which was reduced again in the presence of MSCG1▾; on the other hand, in IL-2/OKT3–stimulated cultures, IL-4 release was suppressed by MSCs, which was not influenced by galectin-1. In addition, IL-13 production was clearly not influenced by the knockdown of galectin-1 in MSCs. In summary, the effects of the galectin-1 knockdown in MSCs on cytokine production in PBMCs depends on the stimulation method; however, the partial recovery of IFNγ and TNFα secretion, which are important proinflammatory cytokines, confirms that galectin-1 plays an important role in the immunomodulatory effects of MSCs.
Knockdown of galectin-1 in MSCs resulted in an altered cytokine levels in a coculture with stimulated PBMCs
| . | IFNγ . | TNFα . | IL-2 . | IL-4 . | IL-5 . | IL-10 . | IL-12 . | IL-13 . |
|---|---|---|---|---|---|---|---|---|
| IL-2/OKT3 | ||||||||
| w/o MSC | 13656 ± 1673 | 3533 ± 524 | 18.5 ± 1.8 | 684 ± 61 | 1353 ± 328 | 9.0 ± 1.0 | 368 ± 35 | |
| MSCwt | 2191 ± 1396 | 162 ± 2 | 6.2 ± 1.3 | 176 ± 29 | 651 ± 104 | 1.9 ± 1.6 | 250 ± 25 | |
| MSCcontr | 2998 ± 2010 | 182 ± 2 | 9.2 ± 1.9 | 263 ± 33 | 726 ± 64 | 2.9 ± 1.1 | 259 ± 28 | |
| MSCG1▾ | 10868* ± 2504 | 647* ± 9 | 10.8 ± 1.4 | 433 ± 117 | 1173* ± 108 | 5.7 ± 1.5 | 334 ± 54 | |
| aCD28/OKT3 | ||||||||
| w/o MSC | 8487 ± 1381 | 946 ± 228 | 42.0 ± 32.7 | 10.0 ± 1.6 | 1463 ± 176 | 235 ± 33 | 390 ± 2 | |
| MSCwt | 3305 ± 2351 | 63 ± 13 | 29.1 ± 2.5 | 14.8 ± 2.2 | 628 ± 81 | 353 ± 48 | 1469 ± 90 | |
| MSCcontr | 3655 ± 1382 | 84 ± 5 | 37.8 ± 0.6 | 13.7 ± 0.8 | 590 ± 57 | 357 ± 51 | 1051 ± 71 | |
| MSCG1▾ | 7579 ± 2770 | 180* ± 26 | 14.5* ± 2.0 | 10.7* ± 1.0 | 1300* ± 248 | 495* ± 64 | 1050 ± 124 |
| . | IFNγ . | TNFα . | IL-2 . | IL-4 . | IL-5 . | IL-10 . | IL-12 . | IL-13 . |
|---|---|---|---|---|---|---|---|---|
| IL-2/OKT3 | ||||||||
| w/o MSC | 13656 ± 1673 | 3533 ± 524 | 18.5 ± 1.8 | 684 ± 61 | 1353 ± 328 | 9.0 ± 1.0 | 368 ± 35 | |
| MSCwt | 2191 ± 1396 | 162 ± 2 | 6.2 ± 1.3 | 176 ± 29 | 651 ± 104 | 1.9 ± 1.6 | 250 ± 25 | |
| MSCcontr | 2998 ± 2010 | 182 ± 2 | 9.2 ± 1.9 | 263 ± 33 | 726 ± 64 | 2.9 ± 1.1 | 259 ± 28 | |
| MSCG1▾ | 10868* ± 2504 | 647* ± 9 | 10.8 ± 1.4 | 433 ± 117 | 1173* ± 108 | 5.7 ± 1.5 | 334 ± 54 | |
| aCD28/OKT3 | ||||||||
| w/o MSC | 8487 ± 1381 | 946 ± 228 | 42.0 ± 32.7 | 10.0 ± 1.6 | 1463 ± 176 | 235 ± 33 | 390 ± 2 | |
| MSCwt | 3305 ± 2351 | 63 ± 13 | 29.1 ± 2.5 | 14.8 ± 2.2 | 628 ± 81 | 353 ± 48 | 1469 ± 90 | |
| MSCcontr | 3655 ± 1382 | 84 ± 5 | 37.8 ± 0.6 | 13.7 ± 0.8 | 590 ± 57 | 357 ± 51 | 1051 ± 71 | |
| MSCG1▾ | 7579 ± 2770 | 180* ± 26 | 14.5* ± 2.0 | 10.7* ± 1.0 | 1300* ± 248 | 495* ± 64 | 1050 ± 124 |
MSCwt, MSCcontr, and MSCG1▾ were cocultured with PBMC either stimulated with IL-2/OKT3 or αCD28/OKT3. The cytokine levels were determined by Bio-Plex Pro Assays and are shown in picograms per milliliter. Knockdown of galectin-1 resulted in an increase of the proinflammatory cytokines IFNγ and TNFα. Data are shown as means of triplicates (± SD).
MSC indicates mesenchymal stromal cell; and PBMC, peripheral blood mononuclear cell.
Significant difference between MSCcontr and MSCG1▾ (P < .05).
Taken together, galectin-1 significantly contributes to the mechanism of immune modulation by MSCs, as shown by restored proliferation and effector functions (eg, cytokine production), in T cells after down-regulation of galectin-1 in MSCs.
Discussion
Human MSCs interfere with immune effector cell function at different levels using soluble as well as membrane-bound signaling molecules, which have not yet been entirely identified. TGF-β, hepatocyte growth factor, IDO, PGE2, and HLA-G account for some, but not all, of MSC-mediated effects in the immune system.5,11,16,21 Their individual contribution is dependent on the experimental setting and on the species studied. Furthermore, inhibition of any single one of these molecules did not result in a total loss of the immunosuppressive activity of MSCs, suggesting that MSCs use a network of several molecules to accomplish this effect.
In past years, galectins emerged as important regulators of immune tolerance and inflammation. Galectin-1 plays an important role in fetomaternal tolerance and in the human thymus during negative and positive selection of T cells.36,37,40 Therapeutic administration of galectin-1 in mouse models was effective in several autoimmune diseases.27 Similarly, GVHD was ameliorated by galectin-1 in a mouse model.41 On the other hand, in solid tumors galectin-1 helps malignant cells escape from immune surveillance.42 Recently, galectins attracted considerable interest as key messenger molecules in regulatory T cells. Human galectin-1 and galectin-10 as well as murine galectin-9 were found to be crucial for suppressive activities of regulatory T cells.43-45 In this context, a critical observation in our report was that galectin-1 was generated and released by human MSCs, which are used in clinical settings to dampen down overreacting immune effector cells in autoimmunity and GVHD.7 Importantly, galectin-1 predominantly mediated antiproliferative effects of MSCs against alloreactive T cells, whereas NK cells were inhibited by different mechanisms.
The amount of galectin-1 released by MSCs was dependent on cell culture density (Figure 1D). The influence of cell-cell contact during expansion of MSCs for clinical application has not yet been investigated sufficiently regarding their immunomodulatory potency, both in vitro and in vivo. Moreover, homotypic cell-cell contact in vitro may have different effects than contact with cells of the patient after transfusion. This important aspect will have to be addressed in future experiments and clinical trials in more detail. Previous reports have shown in a murine model of acute GVHD that application of purified galectin-1 increased survival from 3% to 68%.41 Meaningful, so-called humanized animal models for GVHD are needed to elucidate the role of galectin-1 released by human MSCs in vivo.
The contribution of galectin-1 to the immunosuppressive effects of regulatory CD4+ and CD25+ T cells was shown by neutralizing antibodies.43 However, there are several reasons why silencing galectin-1 by RNA interference in MSCs is preferable. Most importantly, galectin-1 is not only produced by MSCs but also by activated T cells.30 Thus, only a knockdown of galectin-1 in MSCs can definitely elucidate the functional role of galectin-1 originating from MSCs. Furthermore, the stable knockdown by the retroviral system allowed for functional assays for a longer period.
The inhibition of T cells by galectin-1 was independent of very different stimuli studied (ie, proliferation was partially restored by galectin-1 knockdown when either IL-2/OKT3, αCD28/OKT3, or PHA were used, or a classic mixed lymphocyte reaction was performed). Both CD4+ and CD8+ T cells were suppressed to the same extent (Figure 5). These results are in agreement with the fact that MSCs mediate T-cell suppressive effects independently of MHC and any antigen specificity.10 The latter is also true for the suppressive activity of galectin-1 itself, which binds to glycoproteins such as CD2, CD7, CD43, and CD45 on T-cell surfaces.46 The suppression mediated by MSCs is dependent on soluble factors, but cell-cell contact may enhance the inhibition. A reasonable explanation is that cell-surface–bound galectin-1 is much more efficient than soluble galectin-1 in the inhibition of T cells.47 Interestingly, proliferation of NK cells was not affected by the galectin-1–mediated MSC immunosuppressive activity. Inhibition of NK cell proliferation was reported to be mediated by PGE2 and IDO expressed in MSCs.17,18 The contribution of other galectins besides galectin-1, which have redundant functions, may also explain why IL-12 production by DC was suppressed independently of galectin-1 (Figure 6B). In this context, it is important to consider that direct effects of MSCs on T cells (Figure 6A) may be complemented by effects of MSCs and galectin-1 on antigen-presenting cells such as DC and on several other cells of the immune system.2,11,13,18,48 Nevertheless, galectin-1 may even have influenced DC in our experimental setup, which was not detectable because of pleiotropic effects of MSC-derived signals. In fact, galectin-1 is well known to affect dendritic cells. On the one hand, galectin-1 can induce a maturation and migration in monocyte-derived DC.49 On the other hand, galectin-1 has been shown to drive the differentiation of DC with regulatory function involving IL-17 and IL-10, which promote T-cell tolerance in another experimental setup.38
MSCs promote a shift from a proinflammatory Th1 toward a more anti-inflammatory Th2 T-cell response.11,50 This was also indicated by our cytokine array analysis; the proinflammatory cytokines IFNγ, TNFα, IL-2, and IL-12 were suppressed by MSCs. Moreover, in the case of αCD28/OKT3 stimulation, the anti-inflammatory cytokines IL-4, IL-10, and IL-13 were up-regulated. Similar observations have been made with galectin-1 itself, which suppresses Th1 and favors Th2 T-cell responses.34,51 Interestingly, knockdown of galectin-1 in MSCs impaired the transition from a Th1 to a Th2 profile, as secretion of the important proinflammatory cytokines IFNγ, TNFα, and IL-12 was increased.
The question of how the inhibitory effect of MSC-derived galectin-1 is mediated cannot yet be answered definitively. Some effects of galectin-1 depend on the distribution between the monomeric and dimeric forms.52 The monomeric form of galectin-1 inhibits T-cell adhesion to the extracellular matrix and abrogates secretion of proinflammatory cytokines such as TNFα and IFNγ.35 Dimeric galectin-1, however, led to an increase in IL-10 secretion by T cells and apoptosis in many settings.53
In summary, galectin-1 is strongly expressed in human MSCs. A knockdown of galectin-1 has established galectin-1 as the first lectin involved in MSC-mediated suppression of alloreactive T cells. As galectin-1 has been used successfully as treatment in an animal model of GVHD, it is tempting to hypothesize that human galectin-1 may be important for clinical effects of MSCs in studies of GVHD. Further experiments are needed to prove the relevance of galectin-1 (eg, in mouse models of GVHD) with a humanized immune system.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maria Pechan and Claudia Treuner for continuous technical support, Dr Jörg Wischhusen for providing the pSUPERpuro plasmid, and Andreas Wirth for technical assistance with the Bio-Plex Pro Assays.
This study was supported by the German Ministry of Education and Research and by the Förderverein für krebskranke Kinder Tübingene e.V. F.G. is a scholar of the Deutsche José Carreras Leukämie-Stiftung e.V. (F 07/06). M.D. is supported in part by a grant from the Ministero Istruzione Università e Ricerca–PRIN 2006 and by Fondazione Cassa di Risparmio di Modena.
Authorship
Contribution: F.G. designed and performed research and wrote the paper; J.B. and R.B. performed research; M.D. designed research; R.H. wrote the paper; and I.M. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ingo Müller, Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Martinistr 52, 20251 Hamburg, Germany; e-mail: i.mueller@uke.uni-hamburg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal