Abstract
Adoptive T-cell therapy with anti-CD19 chimeric antigen receptor (CAR)–expressing T cells is a new approach for treating advanced B-cell malignancies. To evaluate anti-CD19–CAR-transduced T cells in a murine model of adoptive T-cell therapy, we developed a CAR that specifically recognized murine CD19. We used T cells that were retrovirally transduced with this CAR to treat mice bearing a syngeneic lymphoma that naturally expressed the self-antigen murine CD19. One infusion of anti-CD19–CAR-transduced T cells completely eliminated normal B cells from mice for at least 143 days. Anti-CD19–CAR-transduced T cells eradicated intraperitoneally injected lymphoma cells and large subcutaneous lymphoma masses. The antilymphoma efficacy of anti-CD19–CAR-transduced T cells was critically dependent on irradiation of mice before anti-CD19–CAR-transduced T-cell infusion. Anti-CD19–CAR-transduced T cells had superior antilymphoma efficacy compared with the anti-CD19 monoclonal antibody from which the anti-CD19 CAR was derived. Our results demonstrated impressive antilymphoma activity and profound destruction of normal B cells caused by anti-CD19–CAR-transduced T cells in a clinically relevant murine model.
Introduction
Adoptive T-cell therapy with T cells expressing chimeric antigen receptors (CARs) is an active area of cancer research. Most CARs that are being evaluated in current clinical and preclinical studies recognize self-antigens that are expressed by normal tissues as well as malignant cells.1-11
CD19 expression is restricted to normal mature B cells, malignant B cells, and B-cell precursors.12,13 Clinical trials in which patients with advanced B-cell malignancies receive T cells expressing anti-CD19 CARs are in early stages, and it is not known whether adoptive transfer of T cells targeting this self-antigen will be an effective therapy for B-cell malignancies.1 In addition, the optimal approach to treating patients with anti-CD19–CAR-expressing T cells is not known. To establish a murine model in which a completely syngeneic lymphoma could be treated by adoptive transfer of syngeneic CAR-transduced T cells, we developed a CAR that could specifically recognize murine CD19.
Most CARs contain an activation domain that is derived from the CD3-ζ molecule.1,2,14 Phosphorylation of tyrosines in immunoreceptor tyrosine-based activation motifs (ITAMs) of CD3-ζ molecules is important for T-cell activation.15 Each CD3-ζ molecule contains 3 ITAMs.15 In addition to promoting T-cell activation, the first and third ITAMs of the CD3-ζ molecule have been shown to cause apoptosis, and inactivation of the first and third ITAMs of the CD3-ζ molecule by converting their tyrosine residues to phenylalanines has been shown to decrease T-cell apoptosis.15 We have recently conducted experiments that demonstrated enhanced in vitro survival of human T cells that were transduced with an ErbB2-specific CAR when the first and third ITAMs of the CD3-ζ domain of the CAR were inactivated.16 We hypothesized that inactivation of the first and third CD3-ζ ITAMs in an antimurine-CD19 CAR would decrease apoptosis of CAR-transduced T cells and cause these T cells to have increased survival in vitro and in vivo.
We constructed and assessed 2 versions of an antimurine-CD19 CAR. One version had the wild-type CD3-ζ molecule with all 3 ITAMs intact, and the other version had the first and third CD3-ζ ITAMs inactivated. We selected the CAR with the first and third CD3-ζ ITAMs inactivated for in vivo antilymphoma efficacy experiments. T cells transduced with this CAR cured mice of established syngeneic lymphoma and caused complete and prolonged eradication of normal B cells.
Methods
Design and construction of CARs
The 1D3 hybridoma from ATCC produces an IgG2aκ antibody that specifically recognizes murine CD19. The variable regions of this hybridoma were cloned in a manner similar to that of Brady et al.17 Details of this process are in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The 1D3-28Z DNA sequence encoded the following components in-frame from the 5′ to the 3′ ends: the signal sequence of the light chain of the 1D3 antibody, the light chain variable region of the 1D3 antibody, a linker peptide18 (GGGGS)3, the heavy chain variable region of the 1D3 antibody, a portion of the murine CD28 molecule from amino acids IEFMY to the 3′ terminus, and the cytoplasmic region of the murine CD3-ζ molecule from amino acids RAKFS to the 3′ terminus. The CD28 sequence in 1D3-28Z had a dileucine motif changed to diglycine because this LL to GG change enhanced CAR expression.19 The 1D3-28Z DNA sequence was synthesized by GeneArt AG. The 1D3-28Z sequence was ligated into the mouse stem cell virus–based splice-gag vector (MSGV) retroviral backbone.20 The retroviral vector encoding 1D3-28Z was designated MSGV-1D3-28Z.
We prepared a version of 1D3-28Z in which the first and third ITAMs of the CD3-ζ molecule were inactivated. This construct, designated 1D3-28Z.1-3 (sequence available at GenBank under accession number HM754222), was prepared by designing a DNA sequence encoding a CD3-ζ moiety that was identical to the CD3-ζ moiety in 1D3-28Z, except that both tyrosines in the first and third ITAMs were changed to phenylalanines as described.15 This DNA sequence was synthesized by GeneArt AG and used to replace the wild-type CD3-ζ moiety in MSGV-1D3-28Z.
A retroviral vector encoding a negative control CAR designated MSGV-SP6-28Z.1-3 was constructed using DNA encoding the SP6 single chain variable region moiety (scFv)21 to replace the 1D3 scFv in MSGV-1D3-28Z.1-3. The SP6-28Z.1-3 CAR recognizes the hapten 2,4,6-trinitrophenyl.
Mice and cell lines
We used female C3H/HeN-MTV-negative (C3H) mice for all experiments (National Cancer Institute Animal Production Area, Frederick, MD). All experiments were carried out with the approval of the National Cancer Institute Animal Use and Care Committee.
The 38c13 lymphoma is a carcinogen-induced, CD19-expressing B-cell lymphoma of C3H/HeN origin.22 Ronald Levy (Stanford University) kindly provided the 38c13 cells, and they were cultured in complete medium.23 CCL12 (ATCC) is a connective tissue fibroblast of C3H origin. Sol8 (ATCC) is a skeletal muscle cell line of C3H origin. Neither CCL12 nor Sol8 expresses CD19. NGFR-K562 are human K562 leukemia cells (ATCC) that were transduced with a retroviral vector, MSGV-NGFR, encoding the gene for low-affinity nerve growth factor receptor.24 We cloned the gene for full-length murine CD19 into the MSGV retroviral backbone to produce the MSGV-murine-CD19 retroviral vector. K562 cells were transduced with retroviruses encoding murine-19 to generate CD19-K562.
In vitro culture of primary murine T cells
Splenocytes were enriched for T cells with T-cell enrichment columns (R&D Systems). Anti-CD3/anti-CD28 magnetic beads (Dynabeads Mouse CD3/CD28 T Cell Expander; Invitrogen) were added to the splenocytes as recommended by the manufacturer. Cultures contained 30 IU/mL of human interleukin-2 (IL-2; Chiron).
Retroviral transductions
Transductions were conducted as previously described,24 and the details are available in supplemental Methods.
Flow cytometry
Fluorescein isothiocyanate–labeled polyclonal mouse anti–rat-F(ab)2 antibodies (Jackson ImmunoResearch Laboratories) were used to detect the 1D3 scFv; fluorescein isothiocyanate–labeled normal polyclonal mouse IgG antibodies (Jackson ImmunoResearch Laboratories) served as an isotype control. Further details of flow cytometry are in supplemental Methods.
Interferonγ ELISA and intracellular cytokine staining
Standard methods were used for enzyme-linked immunosorbent assay (ELISA) and intracellular cytokine staining. In ex vivo intracellular cytokine staining experiments, the effector cells were splenocytes from 4 mice that were combined and depleted of CD19+ cells with magnetic antibody-labeled beads (Miltenyi). These techniques are described in supplemental Methods.
Intraperitoneal lymphoma experiments
Mice received 5 Gy of total body irradiation (TBI) from a 137Ce source (Gamma Cell 40, MDS; Nordion). Later the same day, the mice were injected intraperitoneally with 100 000 38c13 lymphoma cells. Mice were followed in a blinded manner and killed when they became moribund.
Subcutaneous lymphoma experiments
Mice received 5 Gy of TBI. Later the same day, 0.5 × 106 38c13 cells were injected subcutaneously. Four days later, the mice received intravenous injections of 6 × 106 CAR-transduced T cells. CAR-transduced T cells were infused 1 day after their second transduction. Mice that received IL-2 received a subcutaneous injection of 45 000 IU of IL-2 immediately after the T-cell injections and daily injections of IL-2 for the next 2 days. In some experiments, mice received intraperitoneal injections of 2 mg of the 1D3 monoclonal antibody (National Cell Culture Center, Biovest International) or 2 mg of an isotype-matched control antibody (ChromPure Rat IgG; Jackson ImmunoResearch Laboratories). Tumor measurement and statistics are in supplemental Methods.
Results
Construction of a CAR targeting murine CD19
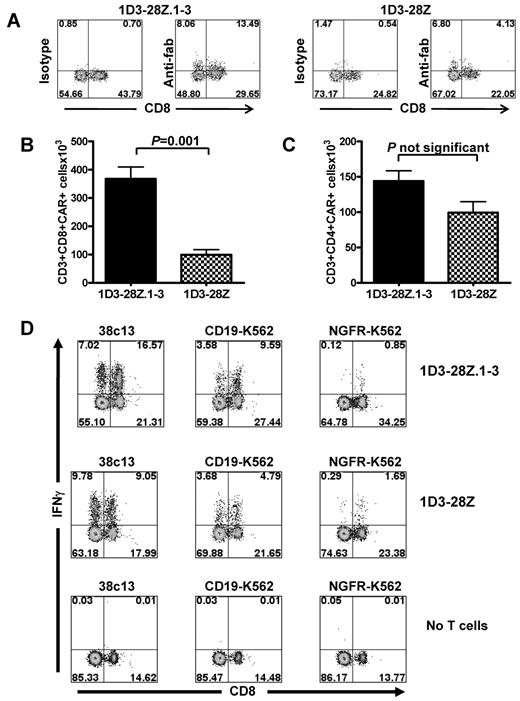
We designed a CAR to specifically recognize murine CD19. This CAR was designated 1D3-28Z. Because inactivation of the first and third ITAMs of CD3-ζ moieties has been shown to decrease apoptosis of T cells,15 we designed a second CAR that was designated 1D3-28Z.1-3 in which the first and third ITAMs of the CD3-ζ molecule were inactivated (Figure 1A-B). We conducted a series of experiments to compare the levels of CAR expression and apoptosis in T cells that were transduced with either 1D3-28Z or 1D3-28Z.1-3. After transduction, the percentage of T cells that expressed 1D3-28Z.1-3 was consistently slightly higher than the percentage of T cells that expressed 1D3-28Z (Figure 1C). In 5 separate experiments in which T cells were transduced with either 1D3-28Z.1-3 or 1D3-28Z, the mean percentage of CAR-expressing T cells 1 day after the second transduction was 64% for 1D3-28Z.1-3 and 53% for 1D3-28Z (P = .001). In 3 separate experiments, viability was assessed by trypan blue staining 5 days after initiation of cultures. The mean percentage of viable cells in cultures of T cells transduced with 1D3-28Z.1-3 was 75%, whereas the mean percentage of viable cells in cultures of T cells transduced with 1D3-28Z was 57% (P = .04). When 1D3-28Z.1-3–expressing T cells were compared with 1D3-28Z–expressing T cells, lower levels of apoptosis were detected by annexin V staining in the 1D3-28Z.1-3–expressing cells (P = .04, Figure 1D).
Anti-CD19 CARs were expressed by T cells and specifically recognized murine CD19. (A) Diagram of the DNA encoding the 1D3-28Z CAR (ψ, retroviral packaging signal). (B) Diagram of the DNA encoding the 1D3-28Z.1-3 CAR. (C) Splenic T cells were placed in culture with anti-CD3/anti-CD28 beads. The cells were transduced 1 day later with retroviruses encoding 1D3-28Z.1-3 or 1D3-28Z. The transduction was repeated the next day. The third day after culture initiation, the cells were analyzed for CAR expression by staining with anti–rat Fab antibodies (top row) or isotype control antibodies (bottom row). Other cells were cultured and analyzed identically but left untransduced. The plots are gated on CD3+ cells, which made up greater than 98% of the total cells in the cultures. The numbers on the plots are the percentages of cells in each quadrant. Data are representative of 5 experiments. (D) T cells from 3 different mice were transduced separately with retroviruses encoding either 1D3-28Z.1-3 or 1D3-28Z. The mean percentages of CAR-expressing CD3+ cells that were apoptotic as indicated by staining with annexin V after 7 days of culture are shown. (E) Splenic T cells were cultured and transduced with 1D3-28Z.1-3 or cultured identically but not transduced. (F) The third day after the cultures were initiated, the T cells were stimulated with either the CD19+ cell line CD19-K562 or the CD19− cell line NGFR-K562. Intracellular cytokine staining for IFN-γ and IL-2 was carried out. The plots are gated on CD3+ cells, and the numbers on the plots are the percentages of cells in each quadrant. Results are representative of 2 experiments.
Anti-CD19 CARs were expressed by T cells and specifically recognized murine CD19. (A) Diagram of the DNA encoding the 1D3-28Z CAR (ψ, retroviral packaging signal). (B) Diagram of the DNA encoding the 1D3-28Z.1-3 CAR. (C) Splenic T cells were placed in culture with anti-CD3/anti-CD28 beads. The cells were transduced 1 day later with retroviruses encoding 1D3-28Z.1-3 or 1D3-28Z. The transduction was repeated the next day. The third day after culture initiation, the cells were analyzed for CAR expression by staining with anti–rat Fab antibodies (top row) or isotype control antibodies (bottom row). Other cells were cultured and analyzed identically but left untransduced. The plots are gated on CD3+ cells, which made up greater than 98% of the total cells in the cultures. The numbers on the plots are the percentages of cells in each quadrant. Data are representative of 5 experiments. (D) T cells from 3 different mice were transduced separately with retroviruses encoding either 1D3-28Z.1-3 or 1D3-28Z. The mean percentages of CAR-expressing CD3+ cells that were apoptotic as indicated by staining with annexin V after 7 days of culture are shown. (E) Splenic T cells were cultured and transduced with 1D3-28Z.1-3 or cultured identically but not transduced. (F) The third day after the cultures were initiated, the T cells were stimulated with either the CD19+ cell line CD19-K562 or the CD19− cell line NGFR-K562. Intracellular cytokine staining for IFN-γ and IL-2 was carried out. The plots are gated on CD3+ cells, and the numbers on the plots are the percentages of cells in each quadrant. Results are representative of 2 experiments.
Anti-CD19–CAR-transduced T cells specifically recognized CD19
In preliminary experiments, we noted a high level of nonspecific interferon-γ (IFN-γ) production by antimurine-CD19–CAR-transduced T cells. We hypothesized that inactivation of the first and third CD3-ζ ITAMs would decrease this nonspecific IFN-γ production. T cells that were transduced with either 1D3-28Z.1-3 or 1D3-28Z produced higher amounts of IFN-γ when they were cultured with CD19+ target cells than when they were cultured with CD19-negative targets (Table 1). Compared with 1D3-28Z–transduced T cells, T cells that were transduced with 1D3-28Z.1-3 produced much less IFN-γ when they were cultured with CD19− targets and exhibited substantially less spontaneous IFN-γ production when they were cultured in medium alone (Table 1). Decreased nonspecific IFN-γ production by 1D3-28Z.1-3–transduced T cells was a consistent finding in multiple experiments. We also demonstrated CD19-specific cytokine production by 1D3-28Z.1-3–transduced T cells by intracellular cytokine staining (Figure 1E). A larger fraction of CD8+ T cells than CD4+ T cells produced IFN-γ. In contrast, more CD4+ T cells than CD8+ T cells produced IL-2. When nontransduced T cells were cultured with CD19+ target cells, little to no cytokine production occurred (Figure 1F). In addition, little to no cytokine production occurred when T cells transduced with the negative control CAR SP6-28Z.1-3 were cultured with CD19-expressing target cells (data not shown). 1D3-28Z.1-3–transduced T cells produced more IL-2 than 1D3-28Z–transduced T cells (Figure 2A). 1D3-28Z.1-3–transduced T cells proliferated in response to CD19 and persisted in vitro better than 1D3-28Z–transduced T cells after stimulation with CD19-expressing target cells (Figure 2B-C).
Anti-CD19 CAR-transduced T cells produced IFN-γ specifically in response to CD19
| Effector T cells* . | CD19+ targets . | CD19− targets . | Effectors alone . | ||||
|---|---|---|---|---|---|---|---|
| 38c13 . | CD19-K562 . | Splenocytes . | Sol8 . | CCL12 . | NGFR-K562 . | ||
| 1D3–28Z.1-3 | 286 480 | 234 252 | 16 378 | 1224 | 969 | 1531 | 1126 |
| 1D3–28Z | 294 150 | 297 100 | 18 085 | 5892 | 4480 | 6055 | 6267 |
| Not transduced | 211 | 123 | 292 | 178 | 129 | 124 | 122 |
| Effector T cells* . | CD19+ targets . | CD19− targets . | Effectors alone . | ||||
|---|---|---|---|---|---|---|---|
| 38c13 . | CD19-K562 . | Splenocytes . | Sol8 . | CCL12 . | NGFR-K562 . | ||
| 1D3–28Z.1-3 | 286 480 | 234 252 | 16 378 | 1224 | 969 | 1531 | 1126 |
| 1D3–28Z | 294 150 | 297 100 | 18 085 | 5892 | 4480 | 6055 | 6267 |
| Not transduced | 211 | 123 | 292 | 178 | 129 | 124 | 122 |
Values are the means of duplicate wells. The units are in picograms per milliliter of IFN-γ. All target cells cultured alone produced undetectable levels of IFN-γ.
Effector T cells were transduced with the indicated CAR as described in Figure 1C. Cultures containing the indicated effector cells and target cells were set up 1 day after transductions were completed.
Anti-CD19–CAR-expressing T cells produce IL-2 and proliferate in response to CD19. T cells were cultured with anti-CD3/anti-CD28 beads and transduced with retroviruses encoding 1D3-28Z.1-3, 1D3-28Z, or SP6-28Z.1-3. On day 5 of culture, 46% of 1D3-28Z.1-3-transduced cells expressed detectable levels of CAR protein and 19% of 1D3-28Z–transduced cells expressed detectable levels of CAR protein. On day 5 of culture, CAR-transduced cells were used in an IL-2 ELISA and a carboxyfluorescein succinimidyl ester (CFSE) proliferation assay. (A) 1D3-28Z.1-3–transduced T cells, 1D3-28Z–transduced T cells, or SP6-28Z.1-3–transduced T cells were cultured overnight alone or with the indicated target cells, and an IL-2 ELISA was performed. The 38c13 and CD19-K562 cells were CD19+ and NGFR-K562 cells were CD19−. The units are in picograms per milliliter. Error bars represent the SEM of duplicate wells. 1D3-28Z.1-3–transduced T cells and 1D3-28Z–transduced T cells produced IL-2 in response to CD19. Minimal IL-2 production occurred when T cells were cultured alone or with NGFR-K562 cells. This is 1 of 2 experiments with similar results. (B) CFSE-labeled 1D3-28Z.1-3–transduced T cells were cultured for 4 days with either irradiated CD19-K562 cells or irradiated NGFR-K562 cells as indicated above each plot. The cells were stained with anti-Fab antibodies to detect the CAR. Almost all 1D3-28Z.1-3–expressing T cells that were cultured with CD19-K562 cells proliferated as indicated by CFSE dilution. Only a minority of 1D3-28Z.1-3–expressing T cells that were cultured with NGFR-K562 cells proliferated. The plots are gated on CD3+ lymphocytes, and the numbers on the plots are the percentages of cells in each quadrant. Results are representative of 2 experiments. (C) Only a small fraction of T cells expressed 1D3-28Z after 4 days of culture with either CD19-K562 cells or NGFR-K562 cells. More of the persisting cells underwent proliferation when they were cultured with CD19-K562 cells than when they were cultured with NGFR-K562 cells. The plots are gated on CD3+ lymphocytes, and the numbers on the plots are the percentages of cells in each quadrant.
Anti-CD19–CAR-expressing T cells produce IL-2 and proliferate in response to CD19. T cells were cultured with anti-CD3/anti-CD28 beads and transduced with retroviruses encoding 1D3-28Z.1-3, 1D3-28Z, or SP6-28Z.1-3. On day 5 of culture, 46% of 1D3-28Z.1-3-transduced cells expressed detectable levels of CAR protein and 19% of 1D3-28Z–transduced cells expressed detectable levels of CAR protein. On day 5 of culture, CAR-transduced cells were used in an IL-2 ELISA and a carboxyfluorescein succinimidyl ester (CFSE) proliferation assay. (A) 1D3-28Z.1-3–transduced T cells, 1D3-28Z–transduced T cells, or SP6-28Z.1-3–transduced T cells were cultured overnight alone or with the indicated target cells, and an IL-2 ELISA was performed. The 38c13 and CD19-K562 cells were CD19+ and NGFR-K562 cells were CD19−. The units are in picograms per milliliter. Error bars represent the SEM of duplicate wells. 1D3-28Z.1-3–transduced T cells and 1D3-28Z–transduced T cells produced IL-2 in response to CD19. Minimal IL-2 production occurred when T cells were cultured alone or with NGFR-K562 cells. This is 1 of 2 experiments with similar results. (B) CFSE-labeled 1D3-28Z.1-3–transduced T cells were cultured for 4 days with either irradiated CD19-K562 cells or irradiated NGFR-K562 cells as indicated above each plot. The cells were stained with anti-Fab antibodies to detect the CAR. Almost all 1D3-28Z.1-3–expressing T cells that were cultured with CD19-K562 cells proliferated as indicated by CFSE dilution. Only a minority of 1D3-28Z.1-3–expressing T cells that were cultured with NGFR-K562 cells proliferated. The plots are gated on CD3+ lymphocytes, and the numbers on the plots are the percentages of cells in each quadrant. Results are representative of 2 experiments. (C) Only a small fraction of T cells expressed 1D3-28Z after 4 days of culture with either CD19-K562 cells or NGFR-K562 cells. More of the persisting cells underwent proliferation when they were cultured with CD19-K562 cells than when they were cultured with NGFR-K562 cells. The plots are gated on CD3+ lymphocytes, and the numbers on the plots are the percentages of cells in each quadrant.
Functional anti-CD19–CAR-transduced T cells were detected ex vivo
Our in vitro characterization of CAR-transduced T cells demonstrated increased CAR expression and decreased apoptosis with 1D3-28Z.1-3–transduced T cells compared with 1D3-28Z–transduced T cells. In our next series of experiments, we adoptively transferred T cells transduced with these CARs to mice to learn if the in vitro benefits of inactivating the CD3-ζ ITAMs would lead to greater numbers of CAR-transduced T cells in vivo. Mice received 5 Gy of TBI. Later the same day, we injected the mice intraperitoneally with 38c13 lymphoma cells. The next day, T cells that were transduced with either 1D3-28Z.1-3 or 1D3-28Z were transferred intravenously to the mice. Eight days after the T cells were transferred to the mice, CAR-expressing CD8+ and CD4+ T cells could be detected ex vivo. An example of the anti–rat-Fab staining used to detect CAR-transduced T cells is shown in Figure 3A. We consistently detected a higher number of CAR-expressing CD3+CD8+ cells in mice that received 1D3-28Z.1-3–transduced T cells than in mice that received 1D3-28Z–transduced T cells (Figure 3B). This difference cannot be completely attributed to in vivo events because the number of CAR-expressing T cells that were injected into mice was consistently slightly higher for T cells that underwent transduction with 1D3-28Z.1-3 compared with cells that underwent transduction with 1D3-28Z. At the time of injection in the experiment reported in Figure 3B, 65% of T cells that underwent transduction with 1D3-28Z.1-3 expressed the CAR, and 55% of T cells that underwent transduction with 1D3-28Z expressed the CAR. Because 6 × 106 live T cells were injected into each mouse in this experiment, the number of 1D3-28Z.1-3–expressing cells injected was 3.9 × 106, and the number of 1D3-28Z–expressing cells injected was 3.3 × 106. In contrast to CD3+CD8+ cells, we did not detect a difference in the number of CD3+CD4+ CAR-expressing cells between mice that received 1D3-28Z.1-3–transduced T cells and mice that received 1D3-28Z–transduced T cells (Figure 3C).
Functional anti-CD19–CAR-transduced T cells were detected in mice after adoptive transfer. (A) T cells were cultured and transduced as described in Figure 1C. Two groups of mice received 5 Gy of TBI. After the TBI, the mice were injected intraperitoneally with 38c13 lymphoma cells. The next day, one group of mice received T cells transduced 1D3-28Z.1-3, and the other group received T cells transduced with 1D3-28Z. Both groups received IL-2 on the day of T-cell infusion and the next day. Eight days after the T-cell transfer, the mice were killed and splenocytes were stained with mouse anti–rat Fab antibodies to detect CAR-transduced cells. Splenocytes were also stained with isotype control antibodies. The plots are gated on CD3+ cells, and the numbers on the plots represent the percentage of total CD3+ cells. The results are representative of 3 experiments. (B) Mean absolute numbers of CAR-expressing CD3+CD8+ splenocytes in mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. Mice were treated as described in Figure 3A. (C) Mean absolute numbers of CAR-expressing CD3+CD4+ splenocytes in mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. Mice were treated as described in Figure 3A. Results are representative of 3 experiments. (D) Three groups of mice received radiation and intraperitoneal injections of 38c13 lymphoma cells as described in Figure 3A. The mice were then injected with 1D3-28Z.1-3–transduced T cells (top row), 1D3-28Z–transduced T cells (middle row), or no T cells (bottom row). Eight days after T-cell transfer, the mice were killed, and splenocytes were cultured with 38c13, CD19-K562, or NGFR-K562. 38c13 and CD19-K562 were CD19+. NGFR-K562 was CD19−. Intracellular cytokine staining was performed for IFN-γ. The numbers on the plots represent the percentage of CD3+ cells. This is 1 of 2 representative experiments.
Functional anti-CD19–CAR-transduced T cells were detected in mice after adoptive transfer. (A) T cells were cultured and transduced as described in Figure 1C. Two groups of mice received 5 Gy of TBI. After the TBI, the mice were injected intraperitoneally with 38c13 lymphoma cells. The next day, one group of mice received T cells transduced 1D3-28Z.1-3, and the other group received T cells transduced with 1D3-28Z. Both groups received IL-2 on the day of T-cell infusion and the next day. Eight days after the T-cell transfer, the mice were killed and splenocytes were stained with mouse anti–rat Fab antibodies to detect CAR-transduced cells. Splenocytes were also stained with isotype control antibodies. The plots are gated on CD3+ cells, and the numbers on the plots represent the percentage of total CD3+ cells. The results are representative of 3 experiments. (B) Mean absolute numbers of CAR-expressing CD3+CD8+ splenocytes in mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. Mice were treated as described in Figure 3A. (C) Mean absolute numbers of CAR-expressing CD3+CD4+ splenocytes in mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. Mice were treated as described in Figure 3A. Results are representative of 3 experiments. (D) Three groups of mice received radiation and intraperitoneal injections of 38c13 lymphoma cells as described in Figure 3A. The mice were then injected with 1D3-28Z.1-3–transduced T cells (top row), 1D3-28Z–transduced T cells (middle row), or no T cells (bottom row). Eight days after T-cell transfer, the mice were killed, and splenocytes were cultured with 38c13, CD19-K562, or NGFR-K562. 38c13 and CD19-K562 were CD19+. NGFR-K562 was CD19−. Intracellular cytokine staining was performed for IFN-γ. The numbers on the plots represent the percentage of CD3+ cells. This is 1 of 2 representative experiments.
The spleen is a major site of 38c13 lymphoma. Large numbers of CD3+CD8+ and CD3+CD4+ cells that produced IFN-γ in a CD19-specific manner could be detected in the spleens of mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells 8 days after T-cell injection into lymphoma-bearing mice (Figure 3D). After demonstrating that highly functional anti-CD19–CAR-transduced T cells could be generated and adoptively transferred, we reasoned that these T cells would be able to destroy both normal B cells and lymphoma cells in syngeneic mice.
Anti-CD19–CAR-transduced T cells eradicated both lymphoma cells and normal B cells in vivo
To assess the ability of antimurine–CD19-transduced T cells to eradicate lymphoma in vivo, we carried out a series of experiments in which mice were challenged with the rapidly proliferating and widely metastatic 38c13 lymphoma line that naturally expresses murine CD19. The 38c13 lymphoma had an aberrant phenotype that allowed it to be distinguished from normal B cells. The 38c13 cells expressed κ light chain and CD19 but not B220 (Figure 4A-B). A total of 95% to 98% of normal murine B cells expressed κ light chain as shown previously.25 Normal B cells also expressed CD19, and in contrast to 38c13 they also expressed B220.26
Anti-CD19 CARs eradicated lymphoma cells and normal B cells. (A) The 38c13 lymphoma expressed κ light chain but not B220. In contrast, normal splenocytes did not have a substantial population of cells that expressed κ light chain but not B220. Almost all normal B cells expressed both κ light chain and B220. The numbers on the plots are the percentages of live cells in each quadrant. (B) The 38c13 lymphoma expressed CD19 but not B220. Normal splenocytes did not have a substantial population of CD19-expressing B220− cells. Normal B cells are CD19+B220+. The numbers on the plots are the percentages of live cells in each quadrant. (C) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 cells. One day after the lymphoma injection, groups of mice were injected intravenously with either 1D3-28Z.1-3-transduced T cells or 1D3-28Z–transduced T cells. Each mouse was injected with 6 × 106 total T cells. The mice received IL-2 once daily on the day of T-cell transfer and the next day. A control group that did not receive any T cells was also included. Eight days later, the mice were killed and splenocytes were analyzed by flow cytometry. The plots are gated on live lymphocytes. The numbers on the plots are the percentages of live cells in each quadrant. Nearly identical results were obtained in 2 separate experiments in which 5 mice were injected with either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. (D) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 cells. The next day, groups of mice were injected intravenously with T cells that were transduced with either the anti-CD19 CAR 1D3-28Z.1-3 or the negative-control CAR SP6-28Z.1-3. The mice received IL-2 once daily on the day of T-cell transfer and the next day. The mice were killed 8 days after T-cell transfer. Splenocytes were stained with anti-κ light chain and B220 to detect normal B cells. The graph summarizes the numbers of splenic B cells from mice in a single experiment in which 3 mice received 1D3-28Z.1-3–transduced T cells and 4 mice received SP6-28Z.1-3–transduced T cells. Nearly identical results were obtained in 2 separate experiments. (E) Mice were irradiated and then injected intraperitoneally with 38c13 lymphoma. The next day, the mice received 1D3-28Z.1-3–transduced T cells. The mice received IL-2 on the day of T-cell transfer and the next day. One hundred forty-three days later, the mice were killed and splenocytes were analyzed for B cells and T cells by flow cytometry. A representative example of 5 mice tested is shown. The CD19 versus B220 plot and the κ light chain versus B220 plot are gated on live lymphocytes. The CD8 versus CD4 plot is gated on CD3+ cells. The numbers on the plots are the percentages of cells in each quadrant.
Anti-CD19 CARs eradicated lymphoma cells and normal B cells. (A) The 38c13 lymphoma expressed κ light chain but not B220. In contrast, normal splenocytes did not have a substantial population of cells that expressed κ light chain but not B220. Almost all normal B cells expressed both κ light chain and B220. The numbers on the plots are the percentages of live cells in each quadrant. (B) The 38c13 lymphoma expressed CD19 but not B220. Normal splenocytes did not have a substantial population of CD19-expressing B220− cells. Normal B cells are CD19+B220+. The numbers on the plots are the percentages of live cells in each quadrant. (C) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 cells. One day after the lymphoma injection, groups of mice were injected intravenously with either 1D3-28Z.1-3-transduced T cells or 1D3-28Z–transduced T cells. Each mouse was injected with 6 × 106 total T cells. The mice received IL-2 once daily on the day of T-cell transfer and the next day. A control group that did not receive any T cells was also included. Eight days later, the mice were killed and splenocytes were analyzed by flow cytometry. The plots are gated on live lymphocytes. The numbers on the plots are the percentages of live cells in each quadrant. Nearly identical results were obtained in 2 separate experiments in which 5 mice were injected with either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. (D) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 cells. The next day, groups of mice were injected intravenously with T cells that were transduced with either the anti-CD19 CAR 1D3-28Z.1-3 or the negative-control CAR SP6-28Z.1-3. The mice received IL-2 once daily on the day of T-cell transfer and the next day. The mice were killed 8 days after T-cell transfer. Splenocytes were stained with anti-κ light chain and B220 to detect normal B cells. The graph summarizes the numbers of splenic B cells from mice in a single experiment in which 3 mice received 1D3-28Z.1-3–transduced T cells and 4 mice received SP6-28Z.1-3–transduced T cells. Nearly identical results were obtained in 2 separate experiments. (E) Mice were irradiated and then injected intraperitoneally with 38c13 lymphoma. The next day, the mice received 1D3-28Z.1-3–transduced T cells. The mice received IL-2 on the day of T-cell transfer and the next day. One hundred forty-three days later, the mice were killed and splenocytes were analyzed for B cells and T cells by flow cytometry. A representative example of 5 mice tested is shown. The CD19 versus B220 plot and the κ light chain versus B220 plot are gated on live lymphocytes. The CD8 versus CD4 plot is gated on CD3+ cells. The numbers on the plots are the percentages of cells in each quadrant.
Mice were irradiated and injected intraperitoneally with lymphoma cells. The next day, mice were injected intravenously with T cells that were transduced with either 1D3-28Z.1-3 or 1D3-28Z. Eight days after T-cell injection, both 38c13 lymphoma cells and normal B cells were absent from the spleens of mice that received either 1D3-28Z.1-3–transduced T cells or 1D3-28Z–transduced T cells. In contrast, mice that did not receive a T-cell infusion had prominent splenic populations of both 38c13 lymphoma cells and normal B cells (Figure 4C). In these experiments, all mice that received infusions of T cells transduced with either 1D3-28Z.1-3 or 1D3-28Z were alive without any signs of lymphoma or toxicity 8 to 9 days after T-cell infusion. We have observed mice that received 1D3-28Z.1-3–transduced T cells for up to 8 months. The only toxicity ever noted in the treated mice was a lack of B cells. Although we did not detect any difference in the in vivo antilymphoma efficacy of T cells that expressed either 1D3-28Z.1-3 or 1D3-28Z, we carried out further in vivo studies with 1D3-28Z.1-3–transduced T cells because, compared with cultures of T cells that were transduced with 1D3-28Z, cultures of T cells that were transduced with 1D3-28Z.1-3 exhibited higher viability and a higher level of in vitro CAR expression. In addition, 1D3-28Z.1-3–expressing T cells underwent less apoptosis than 1D3-28Z–transduced T cells (Figure 1D), and higher numbers of CD3+CD8+ CAR-expressing cells were detected ex vivo from mice that received 1D3-28Z.1-3–transduced T cells compared with mice that received 1D3-28Z–transduced T cells (Figure 3B).
To rigorously test whether eradication of B cells was antigen-specific, we conducted experiments in which one group of mice received T cells that were transduced with 1D3-28Z.1-3 and another group of mice received T cells that were transduced with the negative control CAR SP6-28Z.1-3. Both groups of mice were irradiated and then injected intraperitoneally with 38c13 before infusion of the T cells. When mice received 1D3-28Z.1-3–transduced T cells, normal B cells were eliminated, but when mice received SP6-28Z.1-3, normal B cells could be detected 8 days after T-cell infusion (Figure 4D). 38c13 lymphoma cells were also eliminated in an antigen-specific manner in these experiments (supplemental Figure 1).
Anti-CD19–CAR-transduced T cells caused a prolonged absence of normal B cells
We carried out a series of experiments to assess long-term B-cell recovery in mice treated with 1D3-28Z.1-3–transduced T cells. Mice were irradiated and injected intraperitoneally with 38c13 cells. The next day, the mice were injected with 1D3-28Z.1-3–transduced T cells. When a cohort of 5 mice was killed 63 days later, none of the mice had detectable splenic B cells or evidence of lymphoma, and 1D3-28Z.1-3–transduced T cells were not detected by flow cytometry with staining for the rat Fab component of the CAR (supplemental Figure 2). T-cell responses against T cells genetically engineered to express foreign proteins have been demonstrated previously.27 One explanation that we considered for the lack of long-term persistence of large numbers of CAR-transduced T cells in mice that received infusions of 1D3-28Z.1-3–transduced T cells was that a T-cell response was generated against peptides from the CAR protein. However, we did not detect any T cells that specifically recognized CAR-transduced T cells when we used a sensitive intracellular cytokine staining assay that is capable of detecting weak vaccine responses28 to test for CAR-specific, IFN-γ-producing T cells among splenocytes of mice that had received 1D3-28Z.1-3–transduced T cells 4 months previously (supplemental Figure 3).
A total of 143 days after 1D3-28Z.1-3–transduced T cells were administered, all mice in a cohort of 5 mice lacked splenic B cells. T cells had recovered to normal levels in these mice (Figure 4E). B cells were also absent from the lymph nodes and bone marrow of mice receiving 1D3-28Z.1-3–transduced T cells 143 days after 1D3-28Z.1-3–transduced T cells were administered (data not shown). This complete and prolonged absence of B cells was not accompanied by any evident toxicity. B cells were absent for up to 209 days after 1D3-28Z.1-3–transduced T-cell infusion (supplemental Figure 4). Prolonged absence of B cells was unique to mice that were treated with anti-CD19–CAR-transduced T cells. Mice that received either 5 Gy of TBI alone or 5 Gy of TBI followed by infusions of T cells expressing a control CAR had detectable splenic B cells 4 to 8 days after irradiation (Figure 4C-D; supplemental Figure 4B).
Treatment with anti-CD19–CAR-transduced T cells eradicated intraperitoneally injected lymphoma cells and led to long-term lymphoma-free survival
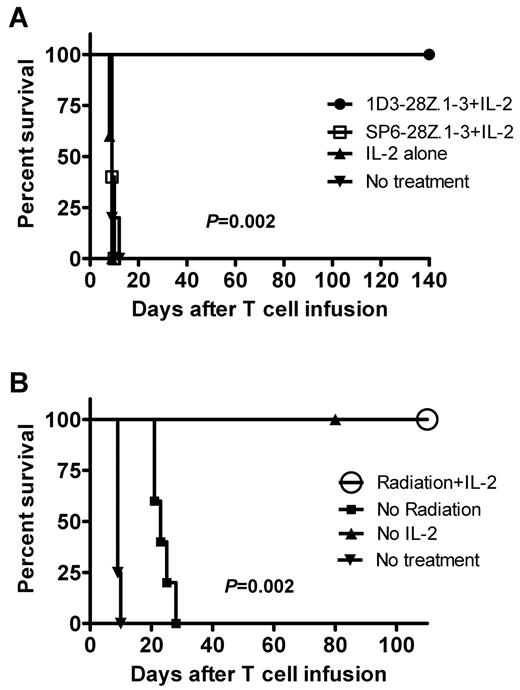
After demonstrating that 1D3-28Z.1-3–transduced T cells can eliminate CD19+ cells in an antigen-specific manner in vivo, we conducted a series of antilymphoma efficacy experiments with an intraperitoneal lymphoma model. All of the mice that were treated with 1D3-28Z.1-3–transduced T cells remained healthy long-term. The mice in all of the control groups, including mice that received a CAR, which was specific for an irrelevant antigen, rapidly died of lymphoma (Figure 5A). These experiments demonstrated an antigen-specific elimination of lymphoma by 1D3-28Z.1-3–transduced T cells.
Complete eradication of lymphoma by anti-CD19–CAR-transduced T cells only occurred when mice were irradiated before adoptive T-cell transfer. (A) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 lymphoma cells. The next day, the mice were divided into 4 treatment groups. One group was injected intravenously with 1D3-28Z.1-3–transduced T cells. Mice in this group received IL-2 on the day of T-cell transfer and the next day. A second group received T cells that were transduced with the control CAR SP6-28Z.1-3 plus IL-2. A third group received IL-2 alone, and a fourth group was left untreated. Each group included 5 mice. The P value of .002 refers to the comparison of the group that received 1D3-28Z.1-3–transduced T cells plus IL-2 to the group that received SP6-28Z.1-3–transduced T cells plus IL-2. The results from 1 of 2 experiments with nearly identical results are shown. (B) Mice were separated into 4 groups. The Radiation + IL-2 group received 5 Gy of TBI followed by an intraperitoneal injection of 38c13 cells. The Radiation + IL-2 group was injected intravenously with 1D3-28Z.1-3–transduced T cells the day after lymphoma injection. A dose of IL-2 was administered immediately after the T-cell injection, and a second dose of IL-2 was administered 1 day later. The No radiation group was treated identically to the Radiation + IL-2 group, except that TBI was not administered. The No IL-2 group was treated identically to the Radiation + IL-2 group, except that phosphate-buffered saline injections were administered in place of IL-2 injections. The No treatment group was left untreated. There were 5 mice in each group, except the No treatment group that included 4 mice. There was 100% survival of both the Radiation + IL-2 group and the No IL-2 group. The results are from 1 of 2 nearly identical experiments. The P value of .002 is for the comparison of the Radiation + IL-2 group to the No radiation group.
Complete eradication of lymphoma by anti-CD19–CAR-transduced T cells only occurred when mice were irradiated before adoptive T-cell transfer. (A) Mice received 5 Gy of TBI, and later the same day the mice were injected intraperitoneally with 38c13 lymphoma cells. The next day, the mice were divided into 4 treatment groups. One group was injected intravenously with 1D3-28Z.1-3–transduced T cells. Mice in this group received IL-2 on the day of T-cell transfer and the next day. A second group received T cells that were transduced with the control CAR SP6-28Z.1-3 plus IL-2. A third group received IL-2 alone, and a fourth group was left untreated. Each group included 5 mice. The P value of .002 refers to the comparison of the group that received 1D3-28Z.1-3–transduced T cells plus IL-2 to the group that received SP6-28Z.1-3–transduced T cells plus IL-2. The results from 1 of 2 experiments with nearly identical results are shown. (B) Mice were separated into 4 groups. The Radiation + IL-2 group received 5 Gy of TBI followed by an intraperitoneal injection of 38c13 cells. The Radiation + IL-2 group was injected intravenously with 1D3-28Z.1-3–transduced T cells the day after lymphoma injection. A dose of IL-2 was administered immediately after the T-cell injection, and a second dose of IL-2 was administered 1 day later. The No radiation group was treated identically to the Radiation + IL-2 group, except that TBI was not administered. The No IL-2 group was treated identically to the Radiation + IL-2 group, except that phosphate-buffered saline injections were administered in place of IL-2 injections. The No treatment group was left untreated. There were 5 mice in each group, except the No treatment group that included 4 mice. There was 100% survival of both the Radiation + IL-2 group and the No IL-2 group. The results are from 1 of 2 nearly identical experiments. The P value of .002 is for the comparison of the Radiation + IL-2 group to the No radiation group.
Irradiation before CAR-transduced T-cell transfer was a critical factor for antilymphoma efficacy
Lymphocyte depletion29-31 and exogenous IL-232 have been shown to enhance the anticancer efficacy of adoptively transferred T cells. We conducted a series of experiments to assess the impact of irradiation before T-cell transfer and IL-2 after T-cell transfer on the antilymphoma efficacy of CAR-transduced T cells targeting the self-antigen CD19.
Whether or not IL-2 was administered, all mice that were irradiated before infusion of lymphoma cells and 1D3-28Z.1-3–transduced T cells survived long-term. In contrast, all mice that received lymphoma cells followed by 1D3-28Z.1-3-transduced T cells and IL-2 without irradiation died of widespread lymphoma (Figure 5B). The presence of lymphoma was confirmed by flow cytometry in the spleens and lymph nodes of moribund mice.
Anti-CD19–CAR-transduced T cells eradicated established subcutaneous lymphoma masses
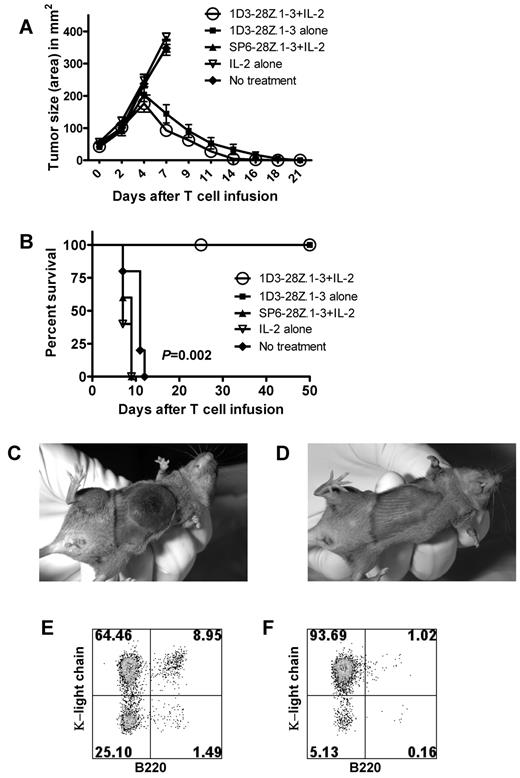
To assess the ability of anti-CD19–CAR-transduced T cells to eliminate large lymphoma masses, we conducted experiments with a subcutaneous 38c13 model. Mice received 5 Gy of TBI and were subcutaneously injected later the same day with 38c13 cells. Four days later when the mice had visible tumors with largest diameters of 6 to 7 mm, the mice were divided into 5 treatment groups. The first group received 6 × 106 1D3-28Z.1-3–transduced T cells plus IL-2. The second group received 1D3-28Z.1-3–transduced T cells alone. The third group received T cells that were transduced with the SP6-28Z.1-3 control CAR plus IL-2. The fourth group received IL-2 alone, and the fifth group was left untreated. T cells that were transduced with 1D3-28Z.1-3 were highly effective at eradicating established lymphoma masses (Figure 6A,D). Treatment with 1D3-28Z.1-3–transduced T cells with or without IL-2 led to long-term survival of all mice (Figure 6B). Mice that did not receive 1D3-28Z.1-3–transduced T cells rapidly developed large lymphoma masses (Figures 6A,C) as well as extensive metastatic lymphoma in their spleens (Figure 6E) and lymph nodes (Figure 6F).
Anti-CD19–CAR-transduced T cells eradicated large lymphoma masses. (A) Mice received 5 Gy of TBI, and later the same day the mice were injected subcutaneously with 38c13 lymphoma cells. Four days later when visible tumors had formed, the mice received 1 of 4 treatments, or they were left untreated. The 1D3-28Z.1-3 + IL-2 group received an intravenous infusion of 1D3-28Z.1-3–transduced T cells. These mice also received IL-2 daily for 3 days. The first IL-2 injection was administered immediately after the T-cell infusion. The 1D3-28Z.1-3 alone group received 1D3-28Z.1-3–transduced T cells with phosphate-buffered saline injections substituted for IL-2. The SP6-28Z.1-3 + IL-2 group received infusions of the negative control CAR SP6-28Z.1-3 and IL-2. A group of mice received IL-2 alone. The mean tumor sizes of each group are shown. The tumor size curves of the control groups end when the first mouse from a group was killed. There were 5 mice in each group, and these results are representative of 2 experiments with nearly identical results. (B) The survival of the same groups of mice described in panel A is shown. The P value refers to the comparison of the 1D3-28Z.1-3 + IL-2 group and the No treatment group. The 1D3-28Z.1-3 + IL-2 group and the 1D3-28Z.1-3 alone group both had 100% survival. (C) Representative example of a lymphoma mass of a mouse from the SP6-28Z.1-3 + IL-2 group 7 days after T-cell infusion. (D) Representative example of a lymphoma site of a mouse from the 1D3-28Z.1-3 + IL-2 group 7 days after T-cell infusion. (E) Representative examples of κ light chain versus B220 staining of spleen and (F) inguinal lymph nodes demonstrate that subcutaneously injected 38c13 lymphoma cells have metastasized widely in mice from the SP6-28Z.1-3 + IL-2 group 8 days after T-cell infusion. The 38c13 lymphoma cells express kappa light chain but not B220. The plots are gated on live lymphocytes. The numbers on the plots are the percentages of cells in each quadrant.
Anti-CD19–CAR-transduced T cells eradicated large lymphoma masses. (A) Mice received 5 Gy of TBI, and later the same day the mice were injected subcutaneously with 38c13 lymphoma cells. Four days later when visible tumors had formed, the mice received 1 of 4 treatments, or they were left untreated. The 1D3-28Z.1-3 + IL-2 group received an intravenous infusion of 1D3-28Z.1-3–transduced T cells. These mice also received IL-2 daily for 3 days. The first IL-2 injection was administered immediately after the T-cell infusion. The 1D3-28Z.1-3 alone group received 1D3-28Z.1-3–transduced T cells with phosphate-buffered saline injections substituted for IL-2. The SP6-28Z.1-3 + IL-2 group received infusions of the negative control CAR SP6-28Z.1-3 and IL-2. A group of mice received IL-2 alone. The mean tumor sizes of each group are shown. The tumor size curves of the control groups end when the first mouse from a group was killed. There were 5 mice in each group, and these results are representative of 2 experiments with nearly identical results. (B) The survival of the same groups of mice described in panel A is shown. The P value refers to the comparison of the 1D3-28Z.1-3 + IL-2 group and the No treatment group. The 1D3-28Z.1-3 + IL-2 group and the 1D3-28Z.1-3 alone group both had 100% survival. (C) Representative example of a lymphoma mass of a mouse from the SP6-28Z.1-3 + IL-2 group 7 days after T-cell infusion. (D) Representative example of a lymphoma site of a mouse from the 1D3-28Z.1-3 + IL-2 group 7 days after T-cell infusion. (E) Representative examples of κ light chain versus B220 staining of spleen and (F) inguinal lymph nodes demonstrate that subcutaneously injected 38c13 lymphoma cells have metastasized widely in mice from the SP6-28Z.1-3 + IL-2 group 8 days after T-cell infusion. The 38c13 lymphoma cells express kappa light chain but not B220. The plots are gated on live lymphocytes. The numbers on the plots are the percentages of cells in each quadrant.
The antilymphoma efficacy of 1D3-28Z.1-3–transduced T cells was superior to the antilymphoma efficacy of the 1D3 monoclonal antibody
The antigen recognition moiety of the 1D3-28Z.1-3 CAR is derived from the 1D3 monoclonal antibody. The 1D3 monoclonal antibody has been shown to mediate a noncurative in vivo antilymphoma effect against a different murine lymphoma cell line than the 38c13 lymphoma cell line used in our experiments.33 The 1D3 monoclonal antibody has also been shown to kill lymphoma cells in vitro by antibody-dependent cell-mediated cytotoxicity.33 We conducted a series of experiments to compare the antilymphoma efficacy of 1D3-28Z.1-3–transduced T cells and the 1D3 monoclonal antibody. We irradiated mice, and later the same day we injected them subcutaneously with 38c13. Four days later when the mice had visible lymphoma masses, the mice were treated with 1D3-28Z.1-3–transduced T cells, the 1D3 monoclonal antibody, or an isotype-matched control antibody. Only the mice treated with 1D3-28Z.1-3–transduced T cells had lymphoma regression (Figure 7A) and long-term survival (Figure 7B). Mice resisted a subsequent intraperitoneal lymphoma challenge after subcutaneous masses were eradicated by 1D3-28Z.1-3–transduced T cells (Figure 7C).
1D3-28Z.1-3–transduced T cells are superior to the 1D3 monoclonal antibody at treating lymphoma. Mice received 5 Gy of TBI, and later the same day they were injected subcutaneously with 38c13 lymphoma cells. Four days later when the mice had established lymphoma masses, they were separated into 4 treatment groups. One group received 1D3-28Z.1-3–transduced T cells. A second group received an injection of the 1D3 monoclonal antibody. A third group received an isotype-matched control antibody, and the fourth group was left untreated. Tumor sizes (A) and survival (B) of each group are shown. The P value of .004 refers to the comparison of the 1D3-28Z.1-3–transduced T-cell group and the 1D3 monoclonal antibody group. The tumor size curves of antibody-treated and untreated mice end when the first mouse of a group was killed. This is 1 of 2 representative experiments with 5 mice per group, except the No treatment group that contained 4 mice. (C) Mice that were successfully treated for lymphoma with 1D3-28Z.1-3–transduced T cells were resistant to lymphoma rechallenge. Three groups of mice received an intraperitoneal injection of 100 000 38c13 cells. Untreated mice had not received any prior treatment. Twenty weeks before rechallenge, mice in Treatment group 1 had lymphoma masses eradicated after treatment with 1D3-28Z.1-3-transduced T cells and IL-2 as detailed in Figure 6A. Sixteen weeks before rechallenge, mice in Treatment group 2 had lymphoma masses eradicated after treatment with 1D3-28Z.1-3 transduced T cells without IL-2 as described in panel A. The survival of the 3 groups is shown. The P value refers to the comparison of the untreated mice and the Treatment group 2.
1D3-28Z.1-3–transduced T cells are superior to the 1D3 monoclonal antibody at treating lymphoma. Mice received 5 Gy of TBI, and later the same day they were injected subcutaneously with 38c13 lymphoma cells. Four days later when the mice had established lymphoma masses, they were separated into 4 treatment groups. One group received 1D3-28Z.1-3–transduced T cells. A second group received an injection of the 1D3 monoclonal antibody. A third group received an isotype-matched control antibody, and the fourth group was left untreated. Tumor sizes (A) and survival (B) of each group are shown. The P value of .004 refers to the comparison of the 1D3-28Z.1-3–transduced T-cell group and the 1D3 monoclonal antibody group. The tumor size curves of antibody-treated and untreated mice end when the first mouse of a group was killed. This is 1 of 2 representative experiments with 5 mice per group, except the No treatment group that contained 4 mice. (C) Mice that were successfully treated for lymphoma with 1D3-28Z.1-3–transduced T cells were resistant to lymphoma rechallenge. Three groups of mice received an intraperitoneal injection of 100 000 38c13 cells. Untreated mice had not received any prior treatment. Twenty weeks before rechallenge, mice in Treatment group 1 had lymphoma masses eradicated after treatment with 1D3-28Z.1-3-transduced T cells and IL-2 as detailed in Figure 6A. Sixteen weeks before rechallenge, mice in Treatment group 2 had lymphoma masses eradicated after treatment with 1D3-28Z.1-3 transduced T cells without IL-2 as described in panel A. The survival of the 3 groups is shown. The P value refers to the comparison of the untreated mice and the Treatment group 2.
Discussion
Several clinical trials of anti-CD19–CAR-expressing T cells have been initiated.1 CD19 is expressed by B-cell malignancies and normal B cells.12 We studied adoptive T-cell therapies aimed at CD19 using a mouse model in which murine CD19 is targeted by anti-CD19–CAR-transduced T cells in syngeneic mice. Several investigators have conducted murine studies to evaluate CAR-expressing T cells in vivo. In many of these studies, immunodeficient mice with severe combined immunodeficiency were engrafted with primary human leukemia cells or human tumor cell lines and treated with CAR-expressing human T cells.1,6,34,35 These studies have demonstrated the ability of human T cells to eradicate human malignancies from immunodeficient mice; however, studies of human T cells in immunodeficient mice have several drawbacks that might limit the ability of these studies to guide development of clinical T-cell transfer protocols. When human T cells are transferred to immunodeficient mice, the human cells recognize murine xenoantigens and sometimes cause graft-versus-host disease.36,37 Immunodeficient mice have low levels of endogenous lymphocytes,38 and multiple studies have demonstrated that host lymphocytes can reduce the antitumor efficacy of transferred T cells.29-31 In particular, T-regulatory cells exert an important inhibitory effect on T cells.23,39 In another type of experimental model, murine T cells expressing CARs that target human tumor-associated antigens have been used to treat mice bearing murine tumor cells that were genetically engineered to express the targeted human tumor-associated antigens.14,32,40 There are several drawbacks to murine tumor immunology studies that use murine tumor cells expressing foreign antigens, such as human tumor-associated antigens. Mice might be able to generate an endogenous immune response against the human antigens,41 and autoimmunity cannot be assessed in such models because the targeted human antigens are not expressed by normal murine cells. Self-antigen-specific T cells can be tolerized by mechanisms of peripheral tolerance that depend on expression of the targeted self-antigen on normal tissues.42-45 These mechanisms of peripheral tolerance might not be operational when tumor cells expressing a foreign antigen are targeted.
To evaluate the ability of anti-CD19–CAR-transduced T cells to treat lymphoma in syngeneic mice, we constructed 2 different CARs. One of the CARs, designated 1D3-28Z, had all 3 CD3-ζ ITAMs intact. The second CAR, designated 1D3-28Z.1-3, had the first and third CD3-ζ ITAMs inactivated. Compared with 1D3-28Z–transduced murine T cells, 1D3-28Z.1-3–transduced T cells exhibited increased viability, decreased apoptosis (Figure 1D), and decreased nonspecific IFN-γ production (Table 1).
Although CAR-transduced human T cells exhibit antigen-specific recognition of target cells in vitro, the cells sometimes produce much smaller, but possibly important, amounts of cytokines, such as IFN-γ, when the targeted antigen is absent.16,24 One possible approach to control nonspecific cytokine production by CAR-expressing T cells is to design CARs with the first and third CD3-ζ ITAMs inactivated. We demonstrated a decrease in nonspecific IFN-γ production by anti-CD19–CAR-transduced T cells with inactivation of the first and third CD3-ζ ITAMs (Table 1). Although our results did not demonstrate an enhancement of in vivo antilymphoma efficacy with 1D3-28Z.1-3–transduced T cells compared with 1D3-28Z–transduced T cells, inactivation of the first and third ITAMs of CD3-ζ moieties in CARs is a reasonable strategy to test with other CARs to improve survival and decrease nonspecific cytokine production of human CAR-transduced T cells. Apoptosis that occurs in human T cells expressing an anti-ErbB2 CAR after stimulation with ErbB2-expressing target cells can be decreased by inactivating the first and third CD3-ζ ITAMs of the CAR.16 Pinthus et al have previously shown that human T cells transduced with a CAR containing the FcRIγ signaling domain exhibited antitumor activity in a prostate carcinoma xenograft model.46 Like 1D3-28Z.1-3, the FcRIγ signaling domain contains only 1 ITAM.47 The results of Pinthus et al demonstrate that human T cells expressing a CAR with only 1 functional ITAM can cause an antitumor response.46
We tried to accurately model clinical adoptive T-cell transfer by transferring 6 × 106 total T cells to each mouse in our experiments. This dose is approximately equal to 3 × 108 total T cells/kg, which is a dose that has been achieved in human clinical trials.48 A total of 40% to 70% of these cells expressed detectable levels of CARs at the time of infusion. This level of expression is nearly identical to the level achieved with our anti–human-CD19 CAR.24
Our experiments demonstrated a complete and prolonged absence of normal B cells after anti-CD19 CAR therapy (Figure 4; supplemental Figures 2,4). CAR-transduced T cells were not detected by flow cytometry 63 days after infusion in mice with absent B cells (supplemental Figure 2). We hypothesize that elimination of early CD19+ B-cell precursors soon after anti-CD19–CAR-transduced T-cell infusion permanently or temporarily blocks recovery of B cells; however, we have not ruled out suppression of B-cell recovery by a very small population of persisting anti-CD19–CAR-transduced T cells. The B-cell depletion demonstrated the effectiveness of anti-CD19–CAR-transduced T cells at destroying cells expressing the targeted antigen. The prolonged B-cell depletion is also a warning that patients enrolled on clinical trials of anti-CD19 CARs might experience profound B-cell depletion. Fortunately, the effects of B-cell depletion can be partially alleviated in patients by intravenous immunoglobulin replacement.49 We are currently conducting a clinical trial of anti-CD19–CAR-expressing T cells. Early results from this trial have shown a complete and prolonged depletion of B-lineage cells in the first patient treated on this trial. This result was very similar to the profound depletion of normal B cells that occurred after anti-CD19–CAR-transduced T-cell infusions in mice.50
Cheadle et al have recently reported that T cells expressing an antimurine-CD19 CAR can cause an antilymphoma effect and temporarily deplete normal B cells when administered to syngeneic mice.11 Unlike the CAR used in our work, the CAR used by Cheadle et al11 did not include any costimulatory molecule, such as CD28. Interestingly, the B-cell depletion caused by anti-CD19–CAR-expressing T cells in the experiments of Cheadle et al11 was temporary. In contrast, our experiments demonstrated a complete absence of splenic B cells up to 209 days after anti-CD19–CAR-transduced T-cell infusion (Figure 4; supplemental Figures 2,4).
Mice that were successfully treated for lymphoma with 1D3-28Z.1-3–transduced T cells were resistant to a second lymphoma challenge (Figure 7C). This resistance to rechallenge could be the result of anti-CD19–CAR-transduced T cells persisting at levels below the limit of detection of the flow cytometry assay we used to detect CAR-transduced T cells. Alternatively, memory T-cell responses against antigens other than CD19 might have developed in the mice during the initial lymphoma challenge.
Our experiments demonstrated a critical role for nonmyeloablative TBI in enhancing anti-CD19–CAR-transduced T-cell therapy. We have conducted several clinical trials that use nonmyeloablative chemotherapy or chemotherapy plus TBI for lymphocyte depletion before adoptive T-cell transfer.48 When anti-CD19–CAR-transduced T cells were administered without irradiation, the cells caused only a minimal increase in survival compared with untreated mice (Figure 5B). In contrast, all mice that received anti-CD19–CAR-transduced T cells after irradiation survived long-term. This finding is consistent with previous studies that demonstrated an important role for lymphocyte depletion in enhancing the antitumor efficacy of T cells expressing major histocompatibility complex-restricted T-cell receptors.29-31 It is probable that other methods of lymphocyte depletion, such as chemotherapy or antibodies, would also enhance the antilymphoma efficacy of anti-CD19–CAR-transduced T cells. Indeed, B-cell depletion with antibodies has been shown to enhance the antileukemia efficacy of anti-CD20–CAR-transduced T cells.45
Lymphoma therapy with a monoclonal antibody has not previously been directly compared to therapy with T cells expressing a CAR incorporating the variable regions of the same monoclonal antibody. Our model allowed us to compare the antilymphoma efficacy of CAR-transduced T cells to therapy with the 1D3 monoclonal antibody from which the antigen-recognition moiety of the CAR was derived. This is an important comparison to make because monoclonal antibodies, such as rituximab, that target CD20 are clinically effective and convenient treatments for lymphoma, although most patients eventually become refractory to rituximab.51 To achieve widespread clinical use, CAR-expressing T cells need to be more effective than monoclonal antibodies at eradicating malignancy because adoptive cellular therapies require substantial technical and financial resources to produce gene-therapy vectors and to maintain a clinical cell production facility. In contrast, monoclonal antibodies can be manufactured and dispensed as typical drugs. Although the 1D3 monoclonal antibody has been shown by previous investigators to have in vivo antilymphoma activity,33 antilymphoma therapy with CAR-transduced T cells was superior to monoclonal antibody therapy in our model (Figure 7).
In conclusion, our findings should strongly encourage continued clinical development of CARs that target self-antigens in general and CARs that target CD19 in particular. Our results demonstrate a powerful antilymphoma effect of anti-CD19–CAR-transduced T cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Douglas Palmer and Robert Reger for assistance with animal experiments.
This work was supported by the Center for Cancer Research, National Cancer Institute, National Institutes of Health (intramural funding).
National Institutes of Health
Authorship
Contribution: J.N.K. designed and conducted experiments, analyzed data, and wrote the manuscript; Z.Y. and D.F. conducted experiments and revised the manuscript; N.P.R. revised the manuscript; and S.A.R. designed experiments, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James N. Kochenderfer, National Institutes of Health, 10 Center Dr, CRC Rm 3-3888, Bethesda, MD 20892; e-mail: kochendj@mail.nih.gov.