Abstract
Evidence suggests that tumor microenvironment is critically involved in supporting survival of chronic lymphocytic leukemia (CLL) cells. However, the molecular mechanisms of this effect and the clinical significance are not fully understood. We applied a microenvironment model to explore the interaction between CLL cells and stromal cells and to elucidate the role of phosphatidylinositol 3 kinase (PI3-K)/Akt/phosphatase and tensin homolog detected on chromosome 10 (PTEN) cascade in this process and its in vivo relevance. Primary human stromal cells from bone marrow, lymph nodes, and spleen significantly inhibited spontaneous apoptosis of CLL cells. Pan–PI3-K inhibitors (LY294002, wortmannin, PI-103), isotype-specific inhibitors of p110α, p110β, p110γ, and small interfering RNA against PI3-K and Akt1 counteracted the antiapoptotic effect of the stromal cells. Induction of apoptosis was associated with a decrease in phosphatidylinositol-3,4,5-triphosphate, PI3-K–p85, and dephosphorylation of phosphatidylinositol-dependent kinase-1 (PDK-1), Akt1, and PTEN. Freshly isolated peripheral blood mononuclear cells from patients with CLL (n = 44) showed significantly higher levels of phosphorylated Akt1, PDK-1, PTEN, and CK2 than healthy persons (n = 8). CK2 inhibitors (4,5,6,7-tetrabromo-1H-benzotriazole, apigenin, and 5,6-dichloro-1-β-D-ribofuranosylbenzimidazol) decreased phosphorylation of PTEN and Akt, induced apoptosis in CLL cells, and enhanced the response to fludarabine. In conclusion, bone marrow microenvironment modulates the PI3-K/Akt/PTEN cascade and prevents apoptosis of CLL cells. Combined inhibition of PI3-K/Akt and recovery of PTEN activity may represent a novel therapeutic concept for CLL.
Introduction
Major progress has been achieved in the past decade in understanding the biology of B-cell chronic lymphocytic leukemia (CLL), leading to new therapeutic concepts and a trend toward improved survival. However, the disease remains incurable, and many patients develop drug resistance.1-3 Despite the long lifespan of CLL cells in vivo, these cells undergo rapid and spontaneous apoptosis in vitro but can be rescued by marrow stromal cells,4-6 nurse-like cells,7 and follicular dendritic cells.8 There are also indications that the stromal cells mediate resistance to chemotherapy in CLL cells.9 This point to the dependence of CLL cells on antiapoptotic stimuli that could be provided in vivo by marrow microenvironment10,11 and may have a major effect on disease progression and response or resistance to therapy.
Bone marrow microenvironment is a complex structure that comprises accessory cells (stromal cells, adipocytes, reticulum cells, endothelial cells, follicular dendritic cells, T cells, and macrophages), matrix proteins, and soluble factors, including growth factors and cytokines.11,12 Bone marrow stromal cells (BMSCs) represent a major component of the marrow microenvironment. They originate from the mesenchymal stem cells and have hematopoietic supportive properties. They also produce matrix proteins, express integrin ligands, and release several cytokines and growth factors that are involved in the generation and maturation of normal and leukemic B cells.11-13 Therefore, BMSCs could provide a suitable milieu for the development and survival of CLL cells. However, the antiapoptotic cascades and the molecular events that are activated upon the interaction between CLL cells and BMSCs are not fully identified.
Several stimuli that are endogenously produced in the microenvironment were shown to activate the antiapoptotic phosphatidylinositol 3-kinase (PI3-K)/Akt pathway. These include cytokines and growth factors,14 adhesion molecules and matrix proteins,15 and involve receptors that are expressed on the surface of CLL cells, including the B-cell receptor, CD19, and CD5.16-18 This strongly suggests that the PI3-K/Akt pathway might play a central role in the interaction between CLL cells and the bone marrow microenvironment. The PI3-K/Akt cascade contributes to the regulation of many cellular processes, including motility, proliferation, apoptosis, and tumorigenesis.14,19 Class I of PI3-K family is the best characterized and comprises p110α, p110β, p110γ, and p110δ isotypes.14,19 The first generation of pan–PI3-K inhibitors (LY294002 and wortmannin) provided substantial information on the molecular mechanism of action and proof of concept of targeting the PI3-K for cancer therapy.20 Subsequently, major progress has been achieved in drug development and more specific pan–PI3-K inhibitors (NVPBEZ235, GDC-0941, and ZSTK474), and isotype-specific inhibitors (CAL-101, TGX-221, IC87114, and AS-605240) are already in clinical trials.21
Activation of PI3-K in response to extracellular stimuli leads to the generation of phosphatidylinositol-3,4,5-triphosphate (PIP3) and activation of 3-phosphoinositide-dependent kinase 1 (PDK1) that in turn mediates the phosphorylation/activation of serine-threonine protein kinase Akt/protein kinase B. The downstream targets of Akt, including Bcl-2, Bad, BclxL, caspases, nuclear factor-κ B, and the tumor suppressor phosphatase and tensin homolog detected on chromosome 10 (PTEN), are involved in cell survival and apoptosis. The end effect of PI3-K/Akt cascade activation is inhibition of apoptosis and regulation of cell proliferation.14,19 However, the primary target of PTEN is PIP; therefore, it functions as the natural negative regulator of PI3-K signaling.22
The PI3-K cascade also plays a key role in B-cell development and differentiation23 and may therefore have a substantial function in CLL cells. It has also been reported that the PI3-K pathway is activated in CLL cells and is related to cell survival.24-29 However, it is not precisely known how PI3-K/Akt cascade is activated and how its downstream targets PDK1 and Akt and its natural inhibitor PTEN are regulated in CLL cells within the lymphoid microenvironment. Therefore, we applied a coculture model that we have previously shown to be suitable for investigating the crosstalk between the leukemic cells and their microenvironment.30 This model is based on using primary human nontransformed BMSCs in coculture with CLL cells under serum-free conditions. We provide evidence that the interaction between CLL cells and BMSCs leads to the activation of PI3-K/Akt cascade, inactivation of PTEN, and inhibition of apoptosis in CLL cells. We also demonstrate that the inhibition of PI3-K/Akt cascade and recovery of PTEN activity by casein kinase 2 (CK2) inhibitors apigenin (4′,5,7,-trihydroxyflavone), TBB (4,5,6,7-tetrabromo-1H-benzotriazole), and DRB (5,6-dichloro-1-β-D-ribofuranosylbenzimidazol) overcome the antiapoptotic effect of BMSCs and facilitate the induction of apoptosis in CLL cells.
Methods
Patients and sample collection
Forty-four patients with CLL were enrolled in the study after obtaining informed consent, following the Declaration of Helsinki according to the approval of the ethical committee of the Medical University of Vienna (EK No. 036/2007). Sixteen patients were in clinical stage Binet A, 14 in B, and 14 in C. Twenty-three patients had mutated immunoglobulin variable region heavy chain (IgVH) genes, and 21 patients had unmutated IgVH genes. Twenty-nine patients had del(13q), 10 had del(11q), 7 had del(17p), and 7 had trisomy 12. Peripheral blood mononuclear cells were isolated with Ficoll-Hypaque (GE Healthcare, BioScience AB) and immunophenotyping was performed by flow cytometric analysis. The mean percentage of the CD19+/CD5+/CD23+ cells was 82.5% (range, 35%-98%). Magnetic cell sorting was applied to purify CD19+ cells with the use of B-cell isolation kit as described by the manufacturer (magnetically activated cell sorting; Miltenyi Biotec GmbH).
Stromal cell isolation and cocultures
BMCSs were isolated from patients with CLL or other hematologic disorders and healthy persons from bone marrow mononuclear cells as previously described.30 Cells between the third and fifth passage were used for coculture experiments. Isolation of lymph node stromal cells (LNSCs) and spleen stromal cells (SSCs) were performed on postoperative materials. Briefly, tissue was minced, and 1-mm3 fragments were explanted into tissue culture plates with α-minimum essential medium that contained 20% fetal calf serum. To initiate the coculture, medium from stromal cell cultures was removed, and cells were washed twice with serum-free α-minimum essential medium. PBMCs or purified CLL cells were suspended in serum-free medium at a cell density of 2 × 106/mL and added to the culture plates. Cocultures were continued for different incubation periods at 37°C in 5% CO2 either untreated or treated with the inhibitors. The PI3-K inhibitor LY294002 was obtained from Cell Signaling Technologies. Wortmannin and CK2 inhibitor apigenin were obtained from Sigma Chemical Co. TBB, DRB, PI-103, PI3-K isotype inhibitors against p110α (inhibitor VIII), p110β (TGX-221), and p110γ (inhibitor II) were purchased from Merck/Calbiochem.
Detection of cell viability and apoptosis
Cell viability was evaluated by a nonisotopic MTT (3-(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium bromide) assay (Ez4U) in 96-well culture plates as described by the manufacturer (Biomedica). For detection of apoptosis, annexin V/propidium iodide (PI) staining kits were used according to the instructions of the manufacturer (Bender MedSystems). Fluorescence-activated cell sorting (FACS) analysis was performed on a FACSCalibur with the use of CellQuest-Pro software Version 5.2.1 (BD Biosciences). In some experiments, acridine orange/ethidium bromide (AO/EtBr) staining was performed to visualize cell viability.
Immunofluorescence studies
Immunofluorescence studies were performed on adhesion slides (Marienfeld) or chamber slides (LabTech; Nalge Nunc International). Cells from suspension or cocultures were fixed with paraformaldehyde for 10 minutes, washed with phosphate-buffered saline (PBS) and permeabilized with 0.05% NP40 in PBS for 10 minutes. Nonspecific binding was suppressed by incubation with 10% AB serum (PAA) for 20 minutes. After washing with PBS, cells were incubated with the primary antibodies overnight at 4°C and washed 3 times with PBS. Cells were then incubated with Alexa fluorochrome-488– or -595–conjugated secondary antibodies for 45 to 60 minutes and washed extensively in PBS. Negative controls were included by omitting the first antibodies from the staining and by applying the isotype- and species-matched negative controls. Monoclonal mouse-anti–human anti-PtdIns (3,4,5)P3 antibody (RC6F8) was used at 1:100 dilution and was obtained from Molecular Probes. Polyclonal rabbit-anti–human Akt-pS473 antibody (Santa Cruz Biotechnology Inc) was used at 1:100 dilution. Images were visualized using an Olympus IX 70 microscope equipped with appropriate filter and objectives; LCPlanFI 20×/0.40 for immunofluorescence studies and LCPlanFI 20×/0.40Ph1 for phase contrast microscopy combined with eyepiece WH10×-H/22 (total magnification 200×). Photographs were taken with an Olympus DP71 digital camera and processed using Olympus Cell∧F software Version 3.1 and displayed using Microsoft Office PowerPoint.
Transfection of CLL cells with PI3-K p110 and Akt-1 small interfering RNA
To simultaneously evaluate the transfection efficacy and the effect of small interfering RNA (siRNA), we used fluorescein isothiocyanate–labeled synthetic siRNA (VBC; Vienna Biocenter). Oligonucleotides were resuspended in RNase-free TE buffer (final concentration of 100μM), and aliquots of equal volume were combined (final concentration of 50μM). For formation of duplexes the siRNAs were incubated at 50°C for 2 minutes in annealing buffer (25mM NaCl, 5mM MgCl2) and were cooled down to room temperature before use. The siRNAs have the following sequences: p110β, 5′-aaa uuc cag ugg uuc auu cca-TT; p110, 3′-TT-uuu aag guc acc aag uaa ggu; for Akt-1, 5′-cga ggg gag uac auc aag a-TT; Akt-1, 3′-TT-g cuc ccc uca ugu agu ucu. Nucleofector kit (Lonza/Amaxa Biosystems) and the optimized protocol for CLL cells (program U-13, VCA-1003 kit) were applied according to the manufacturer's instruction. Briefly, 1 × 107 freshly isolated PBMCs of patients with CLL were suspended in 100 μL of Nucleofector solution and mixed with siRNA (final concentration of 1-5nM). Cells were nucleofected and resuspended in prewarmed culture media and incubated in coculture for 24 hours. Negative controls comprised cells transfected with 100nM silencer negative control no. 1 siRNA (Applied Biosystems/Ambion Inc) or pmaxGFP (Lonza/Amaxa Biosystems).
Western blotting
Cells were lysed with RIPA buffer (50mM Tris [tris(hydroxymethyl) aminomethane-HCL]; pH7.4), 150mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% Triton X-100, 1% deoxycholic acid sodium salt, 0,1% sodium dodecylsulfate, 1mM phenylmethylsulfonyl fluoride, 1mM Na2VO4, and Complete Protease Inhibitor Cocktail (Roche) for 30 minutes at 4°C. Cell lysates (20 μg of proteins) were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis, then electrotransferred on Immobilon-P membranes (Millipore) or nitrocellulose membranes (Amersham Pharmacia). The membranes were saturated for 1 hour in blocking buffer: 0.2% I-Block (Tropix Inc)/0.1% Tween 20/PBS and further incubated with the first antibody in blocking buffer for 1 hour to 24 hours. After 3 washes, the immunoblotted proteins were incubated with an alkaline phosphatase–conjugated secondary antibody for 30 minutes. The membranes were washed and developed with a chemiluminescence system (CDP-Star or CSPD-Star; Tropix Inc). The membranes were hybridized with anti-actin monoclonal antibody (clone A-2066; Sigma) for monitoring of protein content. The following antibodies were used: rabbit-anti–human β-actin (Sigma Chemical Co), mouse-anti–human PTEN (A2B1), rabbit-anti–human Akt-pS473, and mouse-anti–human p85 (B-9; Santa Cruz Biotechnology Inc); rabbit-anti–human PTEN-pS380, rabbit-anti–human Akt1, rabbit-anti–human PDK1, and rabbit-anti–human PDK1-pS241 (Cell Signaling Technology Inc); rabbit-anti–human PTEN-pS370, rabbit-anti–human PTEN-pS380/T382/S385, rabbit-anti–human Akt-pT308, and rabbit-anti–human CK2β-pS209 (Biosource, Life Technologies Corporation). Densitometric scanning was performed with the software program E.A.S.Y Win32 (Herolab).
Statistical analysis
Grouped data are expressed as mean plus or minus SEM. The results were compared for statistical significance with the use of analysis of variance, and P values less than .05 were considered statistically significant.
Results
Stromal cells of different lymphoid organs support survival of CLL cells
Monocyte-depleted PBMCs of 3 patients with CLL were incubated in coculture with BMSCs, SSCs, or LNSCs. The overall viability of CLL cells was assessed by CD19/PI staining after 4 weeks. As shown in Figure 1A, BMSCs, SSCs, and LNSCs were effective in supporting survival of CLL cells, and the percentages of viable CD19+ cells were 89% (± 4%), 88% (± 4%), and 81% (± 4%), respectively, whereas no viable cells could be detected in the absence of stromal cells. There was a moderate increase (20%-30%) in the cell number compared (Figure 1B). All subsequent experiments were performed with BMSCs (CD90+, CD73+, and CD105+). As shown in Figure 1Ci to ii, CLL cells formed 2 compartments, most of the CLL cells were in suspension or loosely adherent, and a smaller proportion (5%-20%) formed small islands of cells that were in close contact with or migrated beneath the BMSCs. The efficiency of BMSCs in supporting survival of CLL cells in short-term cocultures (4 days) was also assessed by AO/EtBr and with annexin V/propidium iodide stainings. Cell viability was higher in cocultures compared with suspension cultures as shown by AO/EtBr staining (Figure 1Di-ii) and flow cytometry (Figure 1Diii-iv), and this was confirmed in samples from 44 patients with CLL (Figure 1E). These results are in accordance with the previously published data.4-6 In addition, they showed that the stromal cells from bone marrow as well as spleen and lymph nodes have comparable endogenous survival supportive effect. Furthermore, they point to the need for direct communication between CLL cells and viable BMSCs for survival (see also supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article; Figure 1)
Stromal cells support survival of CLL cells in long- and short-term cocultures. PBMCs from 3 patients with CLL (> 94% CD19+/CD5+/CD23+) were monocyte depleted by plastic adhesion and then incubated either with BMSCs, SSCs, and LNSCs in a long-term coculture experiment (4 weeks). (A) Cell viability was consistently greater than 80% in parallel to a moderate increase in cell number (20%-30%) compared with the input cell number (B). (C) Phase contrast microscopy shows the nonadherent (i) and adherent cellular compartment including islands of closely adherent cells and cells which migrate beneath the stromal cells (ii). The supportive effect of BMSCs was validated in short-term cultures (4 days) with the use of CLL cells from 44 patients. A representative experiment is showing cell viability with AO/EtBr staining (Di-ii) where green fluorescence of the nuclei indicates viable cells, whereas nuclear red fluorescence indicates cell death and by FACS analysis (Diii-iv). The overall supportive effect of BMSCs in cocultures compared with suspension cultures as measured by FACS analysis in samples (n = 44) is shown in panel E (P < .01). FITC indicates fluorescein isothiocyanate.
Stromal cells support survival of CLL cells in long- and short-term cocultures. PBMCs from 3 patients with CLL (> 94% CD19+/CD5+/CD23+) were monocyte depleted by plastic adhesion and then incubated either with BMSCs, SSCs, and LNSCs in a long-term coculture experiment (4 weeks). (A) Cell viability was consistently greater than 80% in parallel to a moderate increase in cell number (20%-30%) compared with the input cell number (B). (C) Phase contrast microscopy shows the nonadherent (i) and adherent cellular compartment including islands of closely adherent cells and cells which migrate beneath the stromal cells (ii). The supportive effect of BMSCs was validated in short-term cultures (4 days) with the use of CLL cells from 44 patients. A representative experiment is showing cell viability with AO/EtBr staining (Di-ii) where green fluorescence of the nuclei indicates viable cells, whereas nuclear red fluorescence indicates cell death and by FACS analysis (Diii-iv). The overall supportive effect of BMSCs in cocultures compared with suspension cultures as measured by FACS analysis in samples (n = 44) is shown in panel E (P < .01). FITC indicates fluorescein isothiocyanate.
PI3-K inhibitors induce apoptosis in CLL cells in coculture
To investigate the involvement of the PI3-K/Akt pathway, we first tested the effect of pan–PI3-K inhibitors LY294002 and wortmannin on the supportive capacity of BMSCs and induction of apoptosis in CLL cells in cocultures. After 3 days of incubation in cocultures, nonadherent and loosely adherent CLL cells from 10 individual experiments were harvested and plated onto 96-well plates, and MTT assays were performed. As shown in Figure 2A, both substances led to a dose-dependent decrease in cell viability (concentration that inhibits 50%, 0.5μM and 1μM for wortmannin and LY294002, respectively). For annexin V/PI labeling and FACS analysis, CLL cells were harvested by gentle pipetting and followed by adding PBS to collect the remaining cells. Gating was performed on the small lymphocyte population to exclude the large granular BMSCs. As shown in Figure 2, wortmannin (0.5μM) and LY294002 (1μM) significantly induce apoptosis in a time-dependent manner. This effect was observed after 16 hours of incubation (P < .05) and became more significant after 3 and 7 days (P < .01 and P < .001, respectively). The antiproliferative effect of LY294002 and wortmannin was comparable in MTT assays, whereas LY294002 was more effective in the execution of apoptosis as evaluated by FACS analysis. This might be due to the presence of additional downstream targets for LY294002 in CLL cells or due to chemical instability of wortmannin in culture.31 LY294002 and wortmannin also accelerated the spontaneous apoptosis of CLL cells in suspension cultures (Figure 2Ci-iii). These data are in agreement with the previously published reports,25,28,29 and, in addition, they show that both PI3-K inhibitors could overcome the supportive effect of BMSCs and induce cell death in the nonadherent (Figure 2Cv-vi) and adherent (Figure 2Cviii-ix) compartment of CLL cells.
Effect of PI3-K inhibitors on the viability of CLL cells in cocultures. CLL cells from 10 patients with CLL were exposed to LY294002 (1μM) and wortmannin (0.5μM) in cocultures. Cell viability was assessed by MTT assays in triplicates, by annexin V/PI staining, and by acridine orange staining. A dose-dependent decrease in cell viability by both inhibitors is shown by MTT assays as reflected by the decrease in optical density (OD) and presented as a percentage of the control sample (A). A significant time-dependent proapoptotic effect is shown by annexin V/PI staining and FACS analysis (B); *P < .5, **P < .01, ***P < .001 after 1, 3, and 7 days, respectively. AO/EtBr staining was performed to visualize cell viability in suspension cultures (Ci-iii) and cocultures in nonadherent cells (Civ-vi) and adherent cell compartments (Cvii-ix) after exposure to LY294002 or wortmannin for 3 days. (A representative example of 10 experiments is shown in panel C).
Effect of PI3-K inhibitors on the viability of CLL cells in cocultures. CLL cells from 10 patients with CLL were exposed to LY294002 (1μM) and wortmannin (0.5μM) in cocultures. Cell viability was assessed by MTT assays in triplicates, by annexin V/PI staining, and by acridine orange staining. A dose-dependent decrease in cell viability by both inhibitors is shown by MTT assays as reflected by the decrease in optical density (OD) and presented as a percentage of the control sample (A). A significant time-dependent proapoptotic effect is shown by annexin V/PI staining and FACS analysis (B); *P < .5, **P < .01, ***P < .001 after 1, 3, and 7 days, respectively. AO/EtBr staining was performed to visualize cell viability in suspension cultures (Ci-iii) and cocultures in nonadherent cells (Civ-vi) and adherent cell compartments (Cvii-ix) after exposure to LY294002 or wortmannin for 3 days. (A representative example of 10 experiments is shown in panel C).
The proapoptotic effect of PI3-K inhibitors is selective for CLL cells
As shown in the supplementary data (supplemental Figure 2), the effect of the PI3-K inhibitors was selective for the leukemic B cells (supplemental Figure 2A), whereas the T cells, monocytes (supplemental Figure 2B), and BMSCs (supplemental Figure 2C) appeared to be much less sensitive to the inhibitors. We could also confirm that purified CD19+ cells of healthy persons are far less sensitive to the PI3-K inhibitors in comparison to CLL cells (supplemental Figure 2D).
To test whether the proapoptotic function of the PI3-K inhibitors on CLL cells could be due to an indirect effect on BMSCs, we preincubated the BMSCs with inhibitors for 6 hours, then inhibitors were removed, and cocultures with CLL cells were initiated and continued for 3 days. As shown in the supplemental data and supplemental Figure 3, preincubation of BMSCs with the inhibitors did not influence their supportive capacity for CLL cells. We also compared the effect of pan–PI3-K inhibitors (LY294002, wortmannin, and PI-103) and isotype-specific inhibitors of PI3-K–p110α, p110β, and p110γ. As shown in supplemental Figure 4, the inhibitors had a minimal effect on BMSCs (supplemental Figure 4A), whereas CLL cells were sensitive to the PI3-K inhibitors in suspension (supplemental Figure 4B) and in coculture (supplemental Figure 4C).
Inhibition of PI3-K and Akt1 by siRNA prevents the supportive effect of BMSCs
To confirm the specific effect of the PI3-K/Akt pathway inhibitors we applied siRNA against Akt1 and PI3-K–p110β catalytic subunit. CLL cells were transfected with siRNA and immediately incubated with BMSCs in cocultures. Transfection efficiency and cell viability was assessed after 24 hours. A representative example of 6 independent experiments is shown in Figure 3A. The transfection efficiency of the fluorescein isothiocyanate–labeled siRNA was high (64% ± 15% for Akt1 and 68% ± 13% for PI3-K). There was also an effective knockdown of Akt1 (Figure 3B) and p110β that was followed by a decrease in the phosphorylation of its downstream target Akt-pS473 (Figure 3B-C). A significant induction of cell death was obtained by both siRNAs (P < .01). However, siRNA against PI3-K–p110β was more effective in the induction of cell death compared with siRNA against Akt1. Figure 3D and E summarizes the data obtained from 6 different CLL cases. These results confirm that specific inhibition of PI3-K catalytic subunit and Akt1 leads to apoptosis in CLL cells and that an intact PI3-K/Akt pathway is essential for CLL cells to acquire the survival advantage in coculture with BMSCs. Interestingly, p110β exerts its oncogenic properties independent of gain of function mutations32 and is essential for oncogenic transformation in cancer-associated PTEN deficiency.33 This might be relevant for CLL, particularly because of the loss of PTEN activity as shown in “Inactivation of PTEN by phosphorylation.”
siRNA against p110 catalytic subunit of PI3-K and Akt1 prevents the supportive effect of BMSCs. CLL cells were transfected by Amaxa Nucleofector with the use of fluorescein isothiocyanate (FITC)–labeled siRNA against Akt1 and PI3-K p110 subunit (1 or 5nM) or with silencer negative control siRNA in electroporation buffer (control siRNA) or with pmaxGFP as a second control. Cells were incubated for 24 hours in cocultures. Transfection efficiency was estimated by FITC positivity (Aii-iii), and the effect on the viability is shown by PI/FITC positivity (a representative case, Aiv-vii). The percentages in the dotplots represent the transfection efficiency (Aii-iii), dead cells (Aiv), dead-transfected (top right; Avi-vii) and living transfected (bottom right; Av-vii). The efficiency of siRNA to knockdown Akt1, PI3-K p110, and its downstream target phospho-Akt is shown by Western blotting in (B-C). The mean ± SEM of the transfection efficiency and effect on cell viability obtained from 6 independent experiments is shown in panels D and E (control: untreated). A significant effect of the transfection on cell viability is shown in comparison to the controls (E). (**P < .01).
siRNA against p110 catalytic subunit of PI3-K and Akt1 prevents the supportive effect of BMSCs. CLL cells were transfected by Amaxa Nucleofector with the use of fluorescein isothiocyanate (FITC)–labeled siRNA against Akt1 and PI3-K p110 subunit (1 or 5nM) or with silencer negative control siRNA in electroporation buffer (control siRNA) or with pmaxGFP as a second control. Cells were incubated for 24 hours in cocultures. Transfection efficiency was estimated by FITC positivity (Aii-iii), and the effect on the viability is shown by PI/FITC positivity (a representative case, Aiv-vii). The percentages in the dotplots represent the transfection efficiency (Aii-iii), dead cells (Aiv), dead-transfected (top right; Avi-vii) and living transfected (bottom right; Av-vii). The efficiency of siRNA to knockdown Akt1, PI3-K p110, and its downstream target phospho-Akt is shown by Western blotting in (B-C). The mean ± SEM of the transfection efficiency and effect on cell viability obtained from 6 independent experiments is shown in panels D and E (control: untreated). A significant effect of the transfection on cell viability is shown in comparison to the controls (E). (**P < .01).
Activation of PI3-K pathway in coculture
As shown in Figure 4A, in suspension culture, a weak staining for Akt1-pS473 was observed in purified CLL cells in the control cultures (Figure 4Ai) that was further decreased after 2 days of exposure to LY294002 (Figure 4Avi) and wortmannin (Figure 4Axi). In the cocultures, a bright staining for Akt1-pS473 was seen in the nonadherent (Figure 4Aii) and adherent (Figure 4Aiii) compartment of CLL cells. Fibronectin staining (Figure 4Aiv) showed that CLL cells form clusters or islands of adherent cells within and in close contact with BMSCs (Figure 4Av). The addition of LY294002 or wortmannin to the coculture considerably diminished the intensity of staining for Akt1-pS473 in the nonadherent (Figure 4Avii-x) and adherent (Figure 4Aix-xv) CLL cells. This effect was confirmed by Western blotting (Figure 4B).
Stromal cells sustain the activation of PI3-K/Akt pathway. Sorted CD19+ CLL cells were incubated in suspension and in coculture for 2 days either untreated or treated with LY294002 or wortmannin. As shown in panel A, a weak staining for Akt-pS473 is observed in suspension cultures, and a further decrease occurred after exposure to LY294002 and wortmannin (Ai,vi,xi). An intense staining for Akt pSer-473 is shown in the nonadherent and adherent compartments in the coculture (ii-iii), which was diminished by exposure to the inhibitors LY294002 (vii-viii) and wortmannin (xii-xiii). Red fluorescence shows the fibronectin matrix that is produced by bone marrow fibroblasts (iv,ix,xiv). Islands of adherent CLL cells could be seen above or in close contact with BMSCs and fibronectin matrix (v). Cell adhesion was decreased after exposure to the inhibitors (x,xv). Western blotting confirmed the effect of coculture and PI3-K inhibitors on Akt phosphorylation (B; S indicates suspension; NAd, nonadherent cells; Ad, adherent cells). Panel C shows weak staining for PIP3 in CLL cells in suspension cultures (i) that was absent after LY294002 and wortmannin treatment (ii-iii). A bright fluorescence for PIP3 was seen in CLL cells from cocultures (iv) that was reduced after LY294002 and wortmannin treatment (v-vi). The pattern of expression of the main components of the PI3-K pathway (PI3-K–p85, PDK1, Akt, and PTEN) is shown in sorted (CD19+) CLL cells from 2 patients before and after exposure to LY294002 (1μM) under both culture conditions (D). As shown by Western blotting, the amounts of PI3-K p85, PDK1, pPDK1, pAkt were relatively lower in suspension cultures compared with cocultures and were further decreased after exposure to LY294002 under both culture conditions.
Stromal cells sustain the activation of PI3-K/Akt pathway. Sorted CD19+ CLL cells were incubated in suspension and in coculture for 2 days either untreated or treated with LY294002 or wortmannin. As shown in panel A, a weak staining for Akt-pS473 is observed in suspension cultures, and a further decrease occurred after exposure to LY294002 and wortmannin (Ai,vi,xi). An intense staining for Akt pSer-473 is shown in the nonadherent and adherent compartments in the coculture (ii-iii), which was diminished by exposure to the inhibitors LY294002 (vii-viii) and wortmannin (xii-xiii). Red fluorescence shows the fibronectin matrix that is produced by bone marrow fibroblasts (iv,ix,xiv). Islands of adherent CLL cells could be seen above or in close contact with BMSCs and fibronectin matrix (v). Cell adhesion was decreased after exposure to the inhibitors (x,xv). Western blotting confirmed the effect of coculture and PI3-K inhibitors on Akt phosphorylation (B; S indicates suspension; NAd, nonadherent cells; Ad, adherent cells). Panel C shows weak staining for PIP3 in CLL cells in suspension cultures (i) that was absent after LY294002 and wortmannin treatment (ii-iii). A bright fluorescence for PIP3 was seen in CLL cells from cocultures (iv) that was reduced after LY294002 and wortmannin treatment (v-vi). The pattern of expression of the main components of the PI3-K pathway (PI3-K–p85, PDK1, Akt, and PTEN) is shown in sorted (CD19+) CLL cells from 2 patients before and after exposure to LY294002 (1μM) under both culture conditions (D). As shown by Western blotting, the amounts of PI3-K p85, PDK1, pPDK1, pAkt were relatively lower in suspension cultures compared with cocultures and were further decreased after exposure to LY294002 under both culture conditions.
Because the activation of the PI3-K leads to the generation of PIP3, we performed immunofluorescence staining with the use of an antibody against PIP3. As shown in Figure 4C, a weak staining for PIP3 was observed in purified CLL cells obtained from suspension cultures (Figure 4Ci) and was absent after treatment with LY294002 and wortmannin (Figure 4Cii-iii). An intense fluorescence was observed in CLL cells harvested from cocultures (Figure 4Civ), which was also decreased after exposure to the inhibitors (Figure 4Cv-vi). The decrease in PIP3 was associated with induction of apoptosis as shown by nuclear morphology and visualized by Hoechst staining.
Western blotting analysis was used to evaluate the protein expression and the phosphorylation status of the main PI3-K pathway components (PI3-K, PDK1, Akt1, and PTEN) in suspension and in cocultures. As depicted in Figure 4D, the amounts of PI3-K–p85 adapter domain were relatively lower in suspension cultures than in cocultures. Lower amounts of PDK-pS241 and Akt-pS473 were noticed in the suspension cultures in comparison to coculture. A decrease in the phosphorylation of PDK1-pS241, Akt1-pS473, and PTEN-pS380 was observed upon exposure to LY294002.
PI3-K/Akt pathway is activated in freshly isolated CLL cells
To get insight into the clinical relevance of the in vitro data to the in vivo situation in patients with CLL, we analyzed the expression of the main components of the PI3-K/Akt pathway (PDK1, Akt1, and PTEN) and CK2, which is involved in the regulation of PTEN activity, in freshly isolated PBMCs from 44 patients with CLL and 8 healthy persons. Western blotting was performed with phosphospecific antibodies, and densitometric scanning of the obtained signals and the relative amount of the target protein in each sample were corrected to β-actin levels (Table 1).
Comparison of the total amount and phosphorylation state of the major components of the PI3-K/Akt/PTEN and CK2 by densitometric scanning
| . | CLL (n = 44) . | HD (n = 8) . | P . |
|---|---|---|---|
| PDK1-total | 1.72 ± 0.6 | 0.25 ± 0.2 | < .001 |
| PDK1-pS241 | 1.43 ± 0.3 | 0.13 ± 0.1 | < .001 |
| Akt1-total | 1.82 ± 0.6 | 1.45 ± 0.2 | NS |
| Akt1-pT308 | 1.35 ± 0.7 | 0.12 ± 0.09 | < .01 |
| Akt1-pS473 | 1.53 ± 0.45 | 1.1 ± 0.56 | < .05 |
| PTEN-total | 1.72 ± 0.73 | 1.69 ± 0.63 | NS |
| PTEN-pS380 | 1.65 ± 0.69 | 0.65 ± 0.67 | < .01 |
| PTEN-pS370 | 1.63 ± 0.55 | 0.43 ± 0.15 | < .01 |
| PTEN-pSTS | 1.43 ± 0.6 | 0.27 ± 0.13 | < .01 |
| CK2-pS209 | 1.53 ± 0.54 | 0.3 ± 0.12 | < .001 |
| . | CLL (n = 44) . | HD (n = 8) . | P . |
|---|---|---|---|
| PDK1-total | 1.72 ± 0.6 | 0.25 ± 0.2 | < .001 |
| PDK1-pS241 | 1.43 ± 0.3 | 0.13 ± 0.1 | < .001 |
| Akt1-total | 1.82 ± 0.6 | 1.45 ± 0.2 | NS |
| Akt1-pT308 | 1.35 ± 0.7 | 0.12 ± 0.09 | < .01 |
| Akt1-pS473 | 1.53 ± 0.45 | 1.1 ± 0.56 | < .05 |
| PTEN-total | 1.72 ± 0.73 | 1.69 ± 0.63 | NS |
| PTEN-pS380 | 1.65 ± 0.69 | 0.65 ± 0.67 | < .01 |
| PTEN-pS370 | 1.63 ± 0.55 | 0.43 ± 0.15 | < .01 |
| PTEN-pSTS | 1.43 ± 0.6 | 0.27 ± 0.13 | < .01 |
| CK2-pS209 | 1.53 ± 0.54 | 0.3 ± 0.12 | < .001 |
The expression of the total amount and phosphorylation state of PDK1, Akt1, PTEN and CK2 was measured by Western blotting, and signals were quantified by densitometric scanning and corrected to β-actin levels. Values are mean ± SD. The table corresponds to the Western blotting data shown in Figure 5
CLL indicates chronic lymphocytic leukemia; HD, healthy donor; and NS, not significant.
Activated PDK1.
As shown in Figure 5A and Table 1, remarkably higher amounts of PDK1 and its phosphorylated form at Ser-241 were detected in most CLL samples but not in samples from healthy persons. Note that the phosphorylation of PDK1 at Ser-241 is essential for its enzymatic activity and for the phosphorylation of its downstream target Akt1 essentially at Thr-308.34
Activation of the PI3-K/Akt cascade and inactivation of PTEN in freshly isolated CLL cells. Basal levels of main components of PI3-K/Akt cascade in freshly isolated PBMCs of patients with CLL (n = 44) and healthy donors (HDs) were evaluated by Western blotting (the corresponding densitometric scanning is shown in Table 1). A CLL sample (*) was included in Western blotting 2 times together with the HD sets as an internal control. As shown (A), the total amount of PDK1 protein and its phosphorylated form is significantly higher in CLL samples compared with HDs (P < .001 for PKD1 and PDK1-pSer241). As shown (B), the total amounts of Akt and Akt pSer-473 varied between patients with CLL but were higher than in HD samples (P < .05 for Akt pSer-473). The amounts of Akt pThr-308 were significantly higher in most of the CLL samples compared with HD samples (P < .01). As shown (C), comparable amounts of total PTEN were detected in CLL and HD samples. Higher amounts of phosphorylated PTEN at Ser-380, pSer-370, pSTS (pSer-380/pThr-382/pSer-385; P < .01 for all) and CK2 pSer-209 (P < .001) were detected in CLL samples. As demonstrated in panel D, the expression of total PTEN (t) and PTEN pSer-380 in the cytoplasmic (c) and nuclear (n) compartments of purified CLL cells from 3 patients. SOD and ORC2 represent markers for cytoplasmic and nuclear subfraction and show the purity of cellular fractions. Cytoplasmic and nuclear distribution of PTEN-p380 is visualized by immunofluorescence staining in purified CLL cells (E).
Activation of the PI3-K/Akt cascade and inactivation of PTEN in freshly isolated CLL cells. Basal levels of main components of PI3-K/Akt cascade in freshly isolated PBMCs of patients with CLL (n = 44) and healthy donors (HDs) were evaluated by Western blotting (the corresponding densitometric scanning is shown in Table 1). A CLL sample (*) was included in Western blotting 2 times together with the HD sets as an internal control. As shown (A), the total amount of PDK1 protein and its phosphorylated form is significantly higher in CLL samples compared with HDs (P < .001 for PKD1 and PDK1-pSer241). As shown (B), the total amounts of Akt and Akt pSer-473 varied between patients with CLL but were higher than in HD samples (P < .05 for Akt pSer-473). The amounts of Akt pThr-308 were significantly higher in most of the CLL samples compared with HD samples (P < .01). As shown (C), comparable amounts of total PTEN were detected in CLL and HD samples. Higher amounts of phosphorylated PTEN at Ser-380, pSer-370, pSTS (pSer-380/pThr-382/pSer-385; P < .01 for all) and CK2 pSer-209 (P < .001) were detected in CLL samples. As demonstrated in panel D, the expression of total PTEN (t) and PTEN pSer-380 in the cytoplasmic (c) and nuclear (n) compartments of purified CLL cells from 3 patients. SOD and ORC2 represent markers for cytoplasmic and nuclear subfraction and show the purity of cellular fractions. Cytoplasmic and nuclear distribution of PTEN-p380 is visualized by immunofluorescence staining in purified CLL cells (E).
Activated Akt1.
We then investigated the expression of total Akt1 and its phosphorylated forms at Ser-473 and Thr-308, which are both required for the enzymatic activity of Akt. As shown in Figure 5B and Table 1, high amounts of total Akt1, Akt1–pSer-473, and Akt–pThr 308 were detected in most CLL samples compared with samples from healthy persons. Interestingly, the amounts of Akt1 pThr-308 were significantly higher in CLL samples and very weak to undetectable in healthy donor samples.
Inactivation of PTEN by phosphorylation.
The function of PTEN as a phosphatase and inhibitor of PI3-K pathway depends on its phosphorylation at the tail domain. Therefore, we evaluated the total amount of PTEN and its phosphorylated forms with the use of phosphospecific antibodies against PTEN pSer-380, pSer-370, and an antibody that recognizes PTEN phosphorylated at 3 sites in this domain (Ser-380, Thr-382, and Ser-385), which decrease its activity as a tumor suppressor.35 As shown in Figure 5C and Table 1, the total amounts of PTEN in samples of patients with CLL were comparable to those of healthy donors. However, PTEN was highly phosphorylated at Ser-370, Ser-380, Thr-382, and Ser-385 in CLL samples, whereas PTEN phosphorylation at these sites was minimal or undetectable in healthy donor samples. In addition, the expression of CK2 was significantly higher in the CLL samples. Interestingly, PTEN and PTEN pSer-380 were also detectable in the cytosolic and nuclear compartments of purified CLL cells (Figure 5D-E). These data suggest that inactivation of PTEN by phosphorylation at several sites at the tail domain may lead to loss of its function as a phosphatase and contribute to the persistent activation of PI3-K/Akt cascade in CLL cells in vivo.
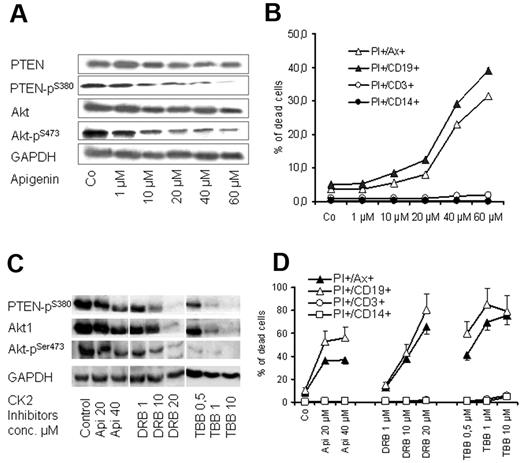
CK2 inhibition leads to dephosphorylation of PTEN and induction of apoptosis of CLL cells
CK2 is an ubiquitous enzyme and has been reported to phosphorylate PTEN primarily at Ser-370 and Ser-385 and further primes its phosphorylation at Ser-380, Thr-382, and Thr-383, leading to the inhibition of its tumor suppressor functions.36,37 As shown in Figure 5, CK2β pSer-209 subunit, which is involved in the regulation of CK2 activity and target binding,37 is highly expressed in CLL samples. Therefore, we tested the effect of CK2 inhibitor apigenin (plant flavonoid) that has been reported to diminish the phosphorylation of PTEN and Akt.38 Exposure of CLL cells to apigenin in cocultures led to a dose-dependent decrease in the phosphorylation of PTEN at Ser-380 and Akt1 at Ser-473 (Figure 6A) and diminished the viability of CLL cell as shown by MTT assays (not shown) and by flow cytometry (Figure 6B). The proapoptotic effect of apigenin was remarkable in CD19+ cells of CLL samples (> 15-fold increase in apoptosis) compared with a moderate effect on T cells (2- to 3-fold), whereas no effect was observed on monocytes (Figure 6B). The effect of CK2 inhibition on cell viability and dephosphorylation of Akt1 and PTEN was confirmed with the use of another CK2 inhibitor (DRB) and a more selective CK2 inhibitor (TBB)39,40 (Figure 6C-D). Exposure to high doses of the CK2 inhibitors led to a decrease in total Akt and its phosphororylated form (Figure 6C). However, the lower concentrations of CK2 inhibitors show that dephosphorylation of Akt and PTEN preceded the incidence of apoptosis, whereas the total amount of both proteins were unchanged (Figure 6A-B). This is in agreement with the recently published data that CK2 inhibitors decrease Akt phosphorylation and induce apoptosis.41 The data suggest that CK2 inhibitors may recover the activity of PTEN and consequently inhibit PI3-K/Akt signaling, resulting in induction of apoptosis in CLL cells.
Effect of CK2 inhibitors on PTEN and Akt1 phosphorylation. PBMCs from 6 patients with CLL were treated with apigenin for 2 days in cocultures. Apigenin inhibited phosphorylation of PTEN at Ser-380 residue and its downstream target Akt1 pSer-473 as shown by Western blotting in a representative case (A), and this was associated with a significant decrease in cell viability as shown by annexin V/PI staining (B). The effect of CK2 inhibitor is more significant on CD19+ cells compared with T cells and monocytes. The effect of 3 CK2 inhibitors (apigenin, TBB, DRB) on PTEN and Akt1 dephosphorylation and on cell viability in coculture was confirmed in samples of 3 patients with CLL as shown (C and D, respectively). GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase
Effect of CK2 inhibitors on PTEN and Akt1 phosphorylation. PBMCs from 6 patients with CLL were treated with apigenin for 2 days in cocultures. Apigenin inhibited phosphorylation of PTEN at Ser-380 residue and its downstream target Akt1 pSer-473 as shown by Western blotting in a representative case (A), and this was associated with a significant decrease in cell viability as shown by annexin V/PI staining (B). The effect of CK2 inhibitor is more significant on CD19+ cells compared with T cells and monocytes. The effect of 3 CK2 inhibitors (apigenin, TBB, DRB) on PTEN and Akt1 dephosphorylation and on cell viability in coculture was confirmed in samples of 3 patients with CLL as shown (C and D, respectively). GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase
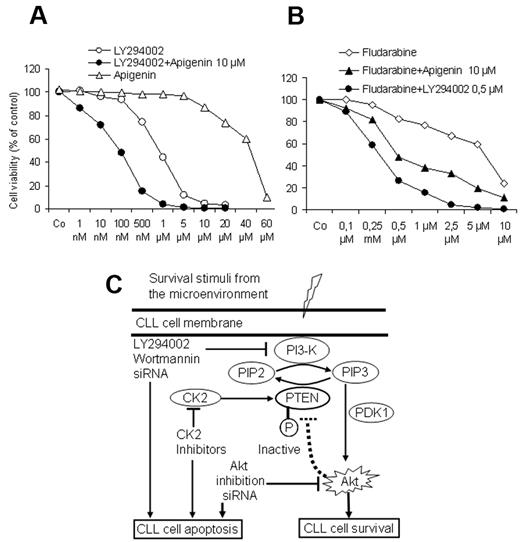
Combined targeting of PI3-kinase and recovery of PTEN enhances the response to fludarabine
To explore whether combined targeting of the PI3-K/Akt/PTEN cascade by PI3-K inhibitors together with the recovery of the PTEN activity by CK2 inhibitors may exert a proapoptotic advantage at lower doses, CLL cells from 5 patients were exposed to increasing concentrations of PI3-K inhibitor LY294002 for 3 days in the presence of a suboptimal dose of apigenin (10μM). As shown in Figure 7A, apigenin significantly enhanced the response to LY294002. Furthermore, LY294002 and apigenin at suboptimal concentrations (0.5μM and 10μM, respectively) were able to enhance the effect of fludarabine on the viability of CLL cells and counteract the supportive effect of the stromal cells. Drug combination index was calculated as previously published29 and showed that combination of apiginin with LY294002 or with fludarabine results in a synergistic effect. These results suggest that the combined use of PI3-K inhibitors or CK2 inhibitors or both may add an advantage to the current therapeutic regimens in CLL. A model depicting the regulation of PI3-K/Akt/PTEN cascade in CLL and the possible option for targeting this pathway is shown in Figure 7C.
Effect of drug combination on cell viability. As shown by MTT assays (A), a suboptimal concentration of CK2 inhibitor apigenin (10μM) augmented the effect of the PI3-K inhibitor LY294002 on cell viability in cocultures in a synergistic manner. Similarly both compounds synergistically enhanced the effect of fludarabine and could overcome the supportive effect of BMSCs (B). Incubation time was 3 days in coculture, and the data represent the means ± SD of 5 independent experiments. The drug combination index was calculated as reported in Vazquez35 according to the formula: a/A + b/B = I, where (a) is the concentration that inhibits 50% (IC50) of fludarabine in combination with apigenin or LY294002 at concentration (b), A is the IC50 of fludarabine alone; and B is the IC50 apigenin or LY294002 in the absence of fludarabine. (I < 1 means synergistic interaction, I = 1 means additive, and I > 1 means antagonistic interaction.) (C) This model depicts the interaction between CLL cells and the lymphoid microenvironment represented by BMSCs. In this model, CLL cells receive several stimuli from the microenvironment that converge to activate the PI3-K, leading to generation of PIP3, phosphorylation of PDK1 and its downstream target Akt1, resulting in inhibition of apoptosis and prolongation of the lifespan of CLL cells. Because of the phosphorylation of PTEN, which diminishes its ability to convert PIP3 into PIP2, the activation of antiapoptotic PI3-K/Akt cascade persists. This antiapoptotic arm could be effectively counteracted at different sites by the inhibitors of the PI3-K and Akt and the recovery of PTEN activity by the CK2 inhibitors, consequently permitting the induction of spontaneous apoptosis and enhancing the response to cytotoxic compounds in CLL cells.
Effect of drug combination on cell viability. As shown by MTT assays (A), a suboptimal concentration of CK2 inhibitor apigenin (10μM) augmented the effect of the PI3-K inhibitor LY294002 on cell viability in cocultures in a synergistic manner. Similarly both compounds synergistically enhanced the effect of fludarabine and could overcome the supportive effect of BMSCs (B). Incubation time was 3 days in coculture, and the data represent the means ± SD of 5 independent experiments. The drug combination index was calculated as reported in Vazquez35 according to the formula: a/A + b/B = I, where (a) is the concentration that inhibits 50% (IC50) of fludarabine in combination with apigenin or LY294002 at concentration (b), A is the IC50 of fludarabine alone; and B is the IC50 apigenin or LY294002 in the absence of fludarabine. (I < 1 means synergistic interaction, I = 1 means additive, and I > 1 means antagonistic interaction.) (C) This model depicts the interaction between CLL cells and the lymphoid microenvironment represented by BMSCs. In this model, CLL cells receive several stimuli from the microenvironment that converge to activate the PI3-K, leading to generation of PIP3, phosphorylation of PDK1 and its downstream target Akt1, resulting in inhibition of apoptosis and prolongation of the lifespan of CLL cells. Because of the phosphorylation of PTEN, which diminishes its ability to convert PIP3 into PIP2, the activation of antiapoptotic PI3-K/Akt cascade persists. This antiapoptotic arm could be effectively counteracted at different sites by the inhibitors of the PI3-K and Akt and the recovery of PTEN activity by the CK2 inhibitors, consequently permitting the induction of spontaneous apoptosis and enhancing the response to cytotoxic compounds in CLL cells.
Discussion
The expansion of the malignant clones in CLL appears to be due to reduced apoptosis rate and the presence of a proliferation pool in the lymphoid organs.1 However, the complex role of the human lymphoid microenvironment in this process remains to be dissected. Here, we show that primary human nontransformed stromal cells have an endogenous capacity to support the survival of CLL cells through the activation of the antiapoptotic PI3-K/Akt pathway and inhibition of the tumor suppressor PTEN in CLL cells. We also demonstrate that the inhibition of this pathway and recovery of PTEN activity in CLL cells by CK2 inhibitors overcome the protective effect of the stromal cells and propose a rationale for drug combinations for therapy of CLL.
In a comparative approach we could present evidence that stromal cells from different lymphoid organs (bone marrow, lymph nodes, and spleen) possess a comparable capacity to support survival and proliferation in short- and long-term in vitro cultures and under serum-free conditions. This is in agreement with the published data that BMSCs play a main role in B-cell lymphopoiesis and leukemogenesis.4,5,9,13 However, the data presented here were shown in primary CLL cells and are based on using primary human and in several cases autologous bone marrow stromal cells under serum-free conditions. This model may reflect the situation in vivo and may give insight into the biologic responses and molecular events that take place in the bone marrow of patients with CLL.
Because several factors might be involved in inhibiting apoptosis of CLL cells on their interaction with stromal cells, we explored the PI3-K/Akt/PTEN pathway that is a central antiapoptotic cascade and plays a major role in human neoplasia. It is also well documented that many of the extracellular stimuli, which also exist in the lymphoid microenvironment, are converging in this pathway.15-18 We first showed that the inhibition of PI3-K and Akt by pharmacologic compounds and siRNA deprive the leukemic cells of the survival advantage provided by the stromal cells. We also demonstrated that interaction between CLL cells and stromal cells leads to Akt phosphorylation, maintains the activation state of PI3-K/Akt pathway, and causes the inhibition of the tumor suppressor PTEN through phosphorylation at its tail domain.
PTEN is the natural inhibitor of the PI3-K signaling and was found to be mutated in several types of cancer but not in CLL.42,43 However, the functions of PTEN are complex and regulated at transcriptional and posttranscriptional levels by phosphorylation, oxidation, ubiquitinylation, acetylation, and nuclear and cytoplasmic localization.44,45 The cytoplasmic localization of PTEN is required for apoptosis, whereas its nuclear localization regulates cell cycle progression.45 The data presented here show that PTEN is phosphorylated in freshly isolated cells from the most patients with CLL and is detectable in the cytosolic and nuclear compartments of CLL cells. This strongly suggests that a deregulated tumor suppressor function of PTEN together with the activation of PI3-K/Akt cascade are of clinical relevance and contribute to prolonging the lifespan and proliferation of CLL cells in vivo. Similarly, we could demonstrate in the coculture model that the phosphorylation state of PTEN in CLL cells was maintained upon the interaction between CLL cells and stromal cells and is associated with the inhibition of spontaneous apoptosis. The molecular mechanisms leading to phosphorylation/inactivation of PTEN in the microenvironment remain to be clarified. However, it could be due to an excessive or persistent activation of the PI3-K/Akt pathway through multiple extracellular stimuli in the lymphoid microenvironment that lead to the activation of Akt and subsequently cause the phosphorylation of PTEN.46 Alternatively, the increased CK2 activity in CLL cells, as shown in this report, might be responsible for the phosphorylation of PTEN and the activation of Akt leading to inhibition of apoptosis as has been shown in T-cell leukemia47 and other types of cancer.37,41 In accordance with this possibility, the exposure of CLL cells to the CK2 inhibitors apigenin, TBB, and DRB led to a decrease in the phosphorylation of PTEN and Akt and was associated with the induction of apoptosis selectively in CLL cells.
Taken together, this report gives a closer insight into the role of the microenvironment in regulating the PI3-K/Akt/PTEN cascade in CLL cells and rendering them resistant to apoptosis. The clinical relevance of these data are substantiated by showing the activated state of the PI3-K/Akt cascade together with inactivation of PTEN in freshly isolated blood samples in a reasonable cohort of patients with CLL. The prognostic value of the PI3-K pathway components and the significance of the variation in protein levels and phosphorylation status between CLL cases remains to be evaluated in a larger cohort of patients. The data, however, support the concept of targeting the microenvironment as a novel approach for therapy of CLL and other B-cell malignancies.48 A major advantage could be taken from the rapid progress in drug development and the discovery of several specific pharmacologic inhibitors of PI3-K, Akt, and CK2, which became available for use in humans.49,50 Finally, targeting the PTEN/PI3-K/Akt cascade has high therapeutic potential and warrants further validation in clinical settings in patients with CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Austrian National Bank (M.S.), “Jubilaeumsfonds” (grants 11051 and 13012; M.S.), the “Fellinger Krebsforschungsverein” (AP001840FF, AP00136OFF; M.S.), the “Initiative Krebsforschung” (UE71104005, UE7114017; M.S.), and “Medizinisch-Wissenschaftlicher Fonds des Buergermeisters der Bundeshauptstadt Wien”/Medical Scientific Fund of the Mayor and City of Vienna (BGM-2522; M.S.).
Authorship
Contribution: M.S. designed and performed experiments and wrote the manuscript; S.S., D.D., M.H., R.H., E.P., S.B., C.L., A.H., and M.D. performed experiments and reviewed the manuscript; and A.G., C.Z., J.D.S, and U.J. provided materials and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Medhat Shehata, Department of Internal Medicine I, Division of Hematology and Hemostaseology, Waehringer Guertel 18-20, A-1090, Vienna, Austria; e-mail: medhat.shehata@meduniwien.ac.at.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal