Abstract
Philadelphia chromosome–positive (Ph+) B-acute lymphoblastic leukemia (B-ALL) can initiate in committed B-cell progenitors. However, the stages of B-cell differentiation in which disease can initiate and the efficiency with which this occurs are unclear. We now demonstrate that B-cell progenitors, up to and including the pro-B cell, efficiently initiate Ph+ B-ALL. However, cells at the pre-B-cell stage of development did not initiate disease. We show that this difference in leukemia initiating potential is due to the level at which the Arf tumor suppressor gene is induced in specific stages of B lymphopoiesis. Whereas immature B-cell progenitors survive the relatively low levels of Arf that are induced after oncogene expression, pre-B cells express the tumor suppressor gene at high levels and undergo massive apoptosis. These data demonstrate that the molecular events that control Ph+ B-ALL initiation and tumor suppression in the B-cell lineage are developmentally regulated.
Introduction
In addition to being the predominant translocation observed in chronic myeloid leukemia (CML), the Philadelphia chromosome (Ph), which encodes the BCR-ABL oncogene,1-3 is associated with approximately one-third of adult-onset cases of B-acute lymphoblastic leukemia (B-ALL).4-6 Although recent reports indicate that imatinib in combination with conventional chemotherapy can significantly improve clinical outcomes,7 the general prognosis for Ph+ B-ALL patients has historically been poor and Ph+ B-ALL is highly resistant to therapy.8-10 Thus, more information about disease initiation is needed. In particular, the stages of B-cell differentiation in which disease can initiate and the factors that influence this process are not fully understood.
B lymphocytes develop from hematopoietic stem cells (HSCs). Common lymphoid progenitors (CLPs) are one of the earliest B-lineage specified precursors derived from HSCs. CLPs have initiated immunoglobulin (Ig) heavy chain gene rearrangements, and this process continues as they mature into pro-B cells. If Ig gene recombination is productive, pro-B cells mature into pre-B cells that express Ig μ heavy chain protein in their cytoplasm.11,12 Pre-B cells are the immediate precursors of surface Ig+ B lymphocytes. HSCs13,14 and CLPs15 can be identified based on distinctive phenotypes, and the stages of B-cell development between pro-B cells and newly produced surface Ig+ B cells have been resolved based on their differential expression of specific cell-surface determinants as described by Hardy and colleagues.16
Several groups have demonstrated that B-ALL can develop when unseparated bone marrow, or marrow enriched with HSCs and immature progenitors, is transduced with BCR-ABL in vitro and transferred in vivo to murine recipients.17-20 A stem cell origin of the disease is further supported by the observation that CML, which initiates in HSCs, can evolve to lymphoid blast crisis and B-ALL.21,22 In addition to HSCs, the analysis of blast cells from Ph+ B-ALL patients, including those with no prior history of myeloproliferative disease,21,23-25 suggests that B-cell progenitors can also be Ph+ B-ALL cells of origin. However, whether all stages of B-cell development have the potential to initiate B-ALL is unclear. Furthermore, the efficiency with which disease can initiate in committed B-cell progenitors has been called into question by recent studies.18,26
Defining the cellular stage of differentiation and specific genetic signature that together create a permissive or restrictive state for the initiation of cancer is dependent on the sensitivity of the experimental protocols used.27 We now demonstrate, using a recently described system,28 that B-cell progenitors including the pro-B cell efficiently initiate Ph+ B-ALL. However, in striking contrast, pre-B cells did not do so. We further show that this difference in leukemia initiating potential is due to the level at which progenitors at specific stages of B-cell development express the Arf tumor suppressor gene in response to oncogenic stress. Taken together, these data indicate that the response to oncogene-induced apoptosis is developmentally regulated in B-cell progenitors and that it controls their potential to initiate lymphoid leukemia.
Methods
Mice
C57BL/6J (wild-type) and B6.129S7-Rag1tm1Mom/J (Rag1−/−) 4- to 6-week-old mice were purchased from The Jackson Laboratory. Arf−/− and Ink4a−/− mice were provided by Dr Norman Sharpless (University of North Carolina at Chapel Hill). Eμ-Bcl2-22 transgenic mice were provided by Dr David Nemazee (The Scripps Research Institute). All mice had been backcrossed onto the C57BL/6 background. Animals were housed in the Division of Laboratory Animal Medicine and experiments were conducted according to UCLA Institutional Animal Care and Use Committee guidelines.
Identification of normal and malignant hematopoietic cells
HSCs were identified as lineage-negative (Lin−) CD117(c-kit)high Sca-1highCD150+ cells based on the protocols described by Spangrude et al13 and Kiel et al.14 CLPs were purified based on their Lin−CD117 (c-kit)lowSca-1lowCD127+ phenotype as described by Kondo et al.15 B-lineage cells were identified using the protocol and terminology described by Hardy and colleagues.16 Pro-B and pre-B cells were identified by their Lin−CD19+CD45R+CD43+CD93+IgM− and Lin−CD19+CD45R+CD43−CD93+IgM− phenotypes, respectively. Immunostaining was performed as described,28 and cells were sorted on a FACSAria (BD Biosciences). Representative FACS plots that show the gating strategies that were used to resolve HSCs, CLPs, pro-B and pre-B cells as well as their place in the hematopoietic hierarchy are presented in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These cell-surface antigens were combined with detection of green fluorescent protein (GFP) expression to define the phenotype of donor-derived leukemic cells by flow cytometry.28,29
Photographs were taken at room temperature on a Leitz Laborlux D or Nikon Diaphot-TMD microscope with an Olympus DP12 camera system. A 40× objective and 10× eyepiece were used. Magnifications are indicated in the figure legends.
Generation of retroviral stocks
pMSCV40 retroviral vectors containing either a 5′ LTR-driven p210 BCR-ABL internal ribosome entry site (IRES) enhanced GFP (EGFP) or a 5′ LTR-driven IRES EGFP were used to generate high-titer helper-free retrovirus after transient cotransfection of 293T cells. 293T cells were grown in 10-cm tissue culture–treated plates (Becton Dickinson; BD Biosciences) precoated with poly-l-lysine (Sigma-Aldrich) in Iscove modified Dulbecco minimum essential medium (IMDM; Mediatech) supplemented with 10% fetal calf serum (FCS; Hyclone), 1mM l-glutamine, 100 U/mL streptomycin, and 100 μg/mL penicillin (complete IMDM; Gibco). 293T transfections were performed by coprecipitating 15 μg of retroviral vector with 15 μg of the ecotropic packaging vector using the CalPhos Mammalian Transfection Kit (BD Biosciences). Medium was replaced every 12 hours for 3 days with complete IMDM. Viral stocks were prepared by pooling supernatants collected at 36 and 48 hours posttransfection. Viral titers were determined after infection of 3T3 cells with serial dilutions of the pooled virus supernatant and found to range between 2 × 106 and 7 × 106 virus particles/mL.
Cell culture and transductions
HSCs and lymphoid progenitors were transduced in vitro as described28 (supplemental Figure 2). Target cells were plated in 3 mL of medium (RPMI 1640, 10% fetal calf serum, 1mM l-glutamine,100 U/mL streptomycin, 100 μg/mL penicillin, 50μM β-mercapatoethanol, 50 μg/mL gentamicin, and 4 μg/mL polybrene) in 6-well plates. The medium in which CLPs, pro-B, and pre-B cells were cultured was supplemented with SCF (20 ng/mL), IL-3 (20 ng/mL), Flt-3L (10 ng/mL), and IL-7 (30 ng/mL) to potentiate their growth and survival. Conditions were tailored for HSCs by additionally supplementing the medium with IL-11 (10 ng/mL) and thrombopoietin (10 ng/mL). Regardless of the target cells, 0.4-μm transwell inserts preseeded with confluent layers of the S17 stromal cell line30 in 1.5 mL of medium were inserted into each well. Retrovirus supernatant was added to the bottom wells containing the sorted progenitor populations at t = 0 hours, 4 hours, 16 hours, and 20 hours. After 24 hours in culture at 37°C, 5% CO2 air–humidified incubator, the cells were harvested and viable cell counts were determined by eosin dye exclusion. The number of transduced viable cells injected into mice is indicated in the “Results” and figure legends.
Transplantation
Cells were injected intravenously into Rag1−/− mice31 that were preconditioned with 500 R from a 137Cs irradiator (120 R/min; Mark I-68A; JL Shepherd and Associates) 24 hours earlier to facilitate lymphoid cell engraftment.32 Lymphoid development in these recipients is blocked during early stages of development due to their deficiency in the Rag1 gene, which prevents them from rearranging their Ig or T-cell receptor genes.31 Although a few CD45R(B220)+ pro-B cells can be found in their bone marrow, Rag1−/− mice have no pre-B, mature B, or T cells.
Recipients of transduced cells were killed when they became moribund. The phenotype of the donor-derived leukemic cells was established by analysis of cells coexpressing GFP and the B-lineage cell-surface antigens.
qPCR
RNA extraction, cDNA preparation, and qPCR reactions were performed as previously described.28 RNA was extracted with the RNeasy Plus micro or mini kit (QIAGEN), and cDNA was synthesized with the RT2 First Strand kit (SA Biosciences). Reactions were run in 25-μL volumes with SYBR green qPCR master mix (Bio-Rad Laboratories) as recommended by the manufacturer. Presence of single PCR products was confirmed by melt curve analysis. Data were analyzed with Biorad IQ5 software using the Pfaffl method with β-Actin or Gapdh as reference genes. Amplification efficiencies were routinely found to be between 95% and 105%. All reactions were run twice. Arf primer sequences were 5′-GCTCTGGCTTTCGTGAACATG-3′ (forward) and 5′-TCGAATCTGCACCGTAGTTGAG-3′ (reverse). RT2 Primer sets for β-Actin, Gapdh, and Bcl-2 were purchased from SA Biosciences.
Statistical analysis
Data are expressed as mean plus or minus SEM. Differences between groups were tested by a 2-tailed unpaired Student t test.
Results
Ph+ B-ALL can initiate in immature B-cell progenitors
To determine the efficiency with which various B-cell progenitors initiate Ph+ B-ALL, HSCs, CLPs, pro-B, and pre-B cells from young adult C57BL/6 mice were isolated and transduced with the BCR-ABL/GFP oncogene using a protocol that limited the transduction period to 24 hours and maintained cells in the presence of salutary microenvironmental signals that included cytokines and stromal cells30 (supplemental Figure 2). The capacity of the transduced cells to initiate B-ALL was then tested by transplanting them into sublethally irradiated Rag1−/− mice.
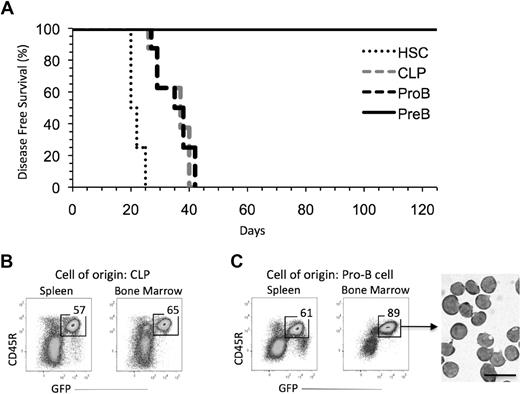
As shown in Figure 1A, all recipients of BCR-ABL/GFP transduced HSCs, CLPs, and pro-B cells had become moribund by 6 weeks after transplantation. The recipients of BCR-ABL/GFP transduced CLPs (Figure 1B) and pro-B cells (Figure 1C) had a significant fraction of GFP+ cells in their bone marrow and spleen, and many, if not most, of these cells were large lymphoblasts. In contrast, all recipients of BCR-ABL/GFP transduced pre-B cells remained healthy and free of disease; this was the case even though the number of pre-B cells (105) transplanted was on average 4-fold and approximately 2-fold greater, respectively, than the number of CLPs (2.5 × 104) and pro-B cells (6.5 × 104) injected.
Immature B-cell progenitors can generate B-ALL. (A) All recipients of BCR-ABL/GFP–transduced HSCs (n = 8; 11 000 cells/mouse), CLPs (n = 8; 25 000 cells/mouse), and pro-B cells (n = 8; 65 000 cells/mouse) developed leukemia by day 42 posttransplantation. Recipients of BCR-ABL/GFP–transduced pre-B cells (n = 12; 100 000 cells/mouse) showed no sign of disease. Results pooled from 3 independent experiments in which 2 to 4 recipients were reconstituted with the indicated cell population in each experiment. (B-C) FACS profiles of leukemic cells in the spleen and bone marrow of recipients of BCR-ABL/GFP–transduced CLPs (B) and pro-B cells (C). GFP+ cells purified from mice by FACS were large blasts (bar represents 20 μm).
Immature B-cell progenitors can generate B-ALL. (A) All recipients of BCR-ABL/GFP–transduced HSCs (n = 8; 11 000 cells/mouse), CLPs (n = 8; 25 000 cells/mouse), and pro-B cells (n = 8; 65 000 cells/mouse) developed leukemia by day 42 posttransplantation. Recipients of BCR-ABL/GFP–transduced pre-B cells (n = 12; 100 000 cells/mouse) showed no sign of disease. Results pooled from 3 independent experiments in which 2 to 4 recipients were reconstituted with the indicated cell population in each experiment. (B-C) FACS profiles of leukemic cells in the spleen and bone marrow of recipients of BCR-ABL/GFP–transduced CLPs (B) and pro-B cells (C). GFP+ cells purified from mice by FACS were large blasts (bar represents 20 μm).
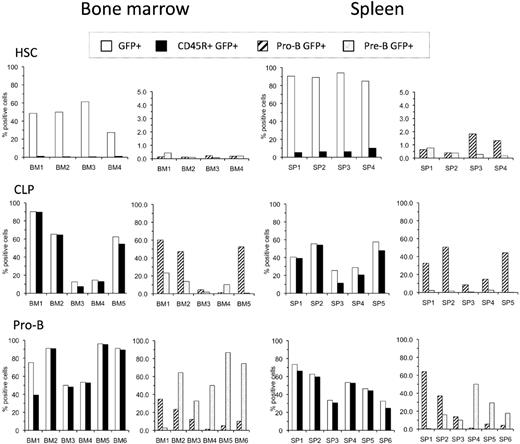
The bone marrow and spleen of all diseased recipients of BCR-ABL/GFP–transduced HSCs, CLPs, and pro-B cells included GFP+ cells, although there was mouse-to-mouse variation in tumor burden (Figure 2). As expected, tumors derived from transduced HSCs predominantly presented as a myeloproliferative disease characteristic of human CML,29 although CD45R(B220)+ cells were clearly detected in some mice. In contrast, most if not all of the leukemic GFP+ cells derived from BCR-ABL/GFP–transduced CLPs and pro-B cells expressed the CD45R(B220) B-lineage cell-surface determinant.
Phenotype of leukemias generated by HSCs, CLPs, and pro-B cells. Each cell population was transduced with BCR-ABL/GFP and transplanted into preconditioned Rag1−/− mice. Recipients were killed when they appeared moribund. Single-cell suspensions of bone marrow and spleen cells were prepared and examined for the expression of donor-derived GFP+ cells in combination with B-lineage cell-surface antigens. The left panel for each tissue shows the frequency of GFP+ cells in that tissue (□) and the proportion of these GFP+ cells that expressed CD45R(B220) (■). The right panel for each tissue shows the distribution of cells with a pro-B (▨) and pre-B-cell ( ) phenotype among the GFP+ CD45R(B220)+ cells. Phenotypic analysis was performed as shown in supplemental Figure 1. Total average number of BCR-ABL/GFP+ cells in the bone marrow of mice that received transduced HSCs, CLPs, and pro-B cells was 12.8 (± 4.8) × 106, 17.6 (± 5.7) × 106, and 17.3 (± 9.5) × 106, respectively. Total average number of BCR-ABL/GFP+ cells in the spleen of mice that received transduced HSCs, CLPs, and pro-B cells was 116.4 (± 51.1) × 106, 35.2 (± 22.8) × 106, and 44.0 (± 29.7) × 106, respectively.
) phenotype among the GFP+ CD45R(B220)+ cells. Phenotypic analysis was performed as shown in supplemental Figure 1. Total average number of BCR-ABL/GFP+ cells in the bone marrow of mice that received transduced HSCs, CLPs, and pro-B cells was 12.8 (± 4.8) × 106, 17.6 (± 5.7) × 106, and 17.3 (± 9.5) × 106, respectively. Total average number of BCR-ABL/GFP+ cells in the spleen of mice that received transduced HSCs, CLPs, and pro-B cells was 116.4 (± 51.1) × 106, 35.2 (± 22.8) × 106, and 44.0 (± 29.7) × 106, respectively.
Phenotype of leukemias generated by HSCs, CLPs, and pro-B cells. Each cell population was transduced with BCR-ABL/GFP and transplanted into preconditioned Rag1−/− mice. Recipients were killed when they appeared moribund. Single-cell suspensions of bone marrow and spleen cells were prepared and examined for the expression of donor-derived GFP+ cells in combination with B-lineage cell-surface antigens. The left panel for each tissue shows the frequency of GFP+ cells in that tissue (□) and the proportion of these GFP+ cells that expressed CD45R(B220) (■). The right panel for each tissue shows the distribution of cells with a pro-B (▨) and pre-B-cell ( ) phenotype among the GFP+ CD45R(B220)+ cells. Phenotypic analysis was performed as shown in supplemental Figure 1. Total average number of BCR-ABL/GFP+ cells in the bone marrow of mice that received transduced HSCs, CLPs, and pro-B cells was 12.8 (± 4.8) × 106, 17.6 (± 5.7) × 106, and 17.3 (± 9.5) × 106, respectively. Total average number of BCR-ABL/GFP+ cells in the spleen of mice that received transduced HSCs, CLPs, and pro-B cells was 116.4 (± 51.1) × 106, 35.2 (± 22.8) × 106, and 44.0 (± 29.7) × 106, respectively.
) phenotype among the GFP+ CD45R(B220)+ cells. Phenotypic analysis was performed as shown in supplemental Figure 1. Total average number of BCR-ABL/GFP+ cells in the bone marrow of mice that received transduced HSCs, CLPs, and pro-B cells was 12.8 (± 4.8) × 106, 17.6 (± 5.7) × 106, and 17.3 (± 9.5) × 106, respectively. Total average number of BCR-ABL/GFP+ cells in the spleen of mice that received transduced HSCs, CLPs, and pro-B cells was 116.4 (± 51.1) × 106, 35.2 (± 22.8) × 106, and 44.0 (± 29.7) × 106, respectively.
Ph+ B-ALL tumor cells exhibit phenotypic infidelity
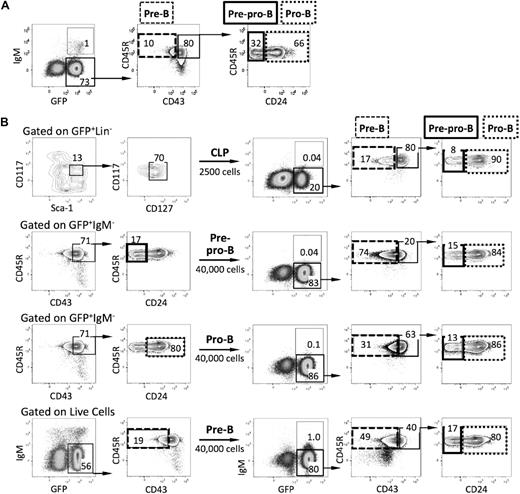
Additional phenotypic characterization of the leukemias derived from the various progenitors described above revealed that the tumor cells did not recapitulate the normal pattern of B-cell development. For example, recipients of BCR-ABL/GFP–transformed pro-B cells had GFP+ cells with a more immature CD45R+CD43+CD24− pre/pro-B-cell phenotype in their tissues (Figure 3A).
B-ALL blasts exhibit phenotypic infidelity. (A) FACS profiles of leukemic cells in the spleen of recipients of BCR-ABL/GFP–transduced pro-B cells. Leukemic GFP+ cells included pro-B cells, pre-B cells, sIgM+ B cells, and more immature pre/pro-B cells (CD45R+CD43+CD24−IgM−). (B) CLPs were isolated from the bone marrow of wild-type mice (n = 4), transduced with BCR-ABL/GFP, and transplanted into Rag1−/− recipients. Four weeks after the primary transplant, total splenocytes and leukemic GFP+ cells with a CLP, pre/pro-B-, pro-B-, and pre-B-cell phenotype were isolated from diseased primary recipients (n = 2) and each population was transplanted into 4 secondary Rag1−/− recipients, respectively. All secondary recipients developed B-ALL by day 19 after transplantation. Note that leukemic cells in recipients of GFP+ pro-B and pre-B cells had phenotypes characteristic of more immature stages of B-cell development.
B-ALL blasts exhibit phenotypic infidelity. (A) FACS profiles of leukemic cells in the spleen of recipients of BCR-ABL/GFP–transduced pro-B cells. Leukemic GFP+ cells included pro-B cells, pre-B cells, sIgM+ B cells, and more immature pre/pro-B cells (CD45R+CD43+CD24−IgM−). (B) CLPs were isolated from the bone marrow of wild-type mice (n = 4), transduced with BCR-ABL/GFP, and transplanted into Rag1−/− recipients. Four weeks after the primary transplant, total splenocytes and leukemic GFP+ cells with a CLP, pre/pro-B-, pro-B-, and pre-B-cell phenotype were isolated from diseased primary recipients (n = 2) and each population was transplanted into 4 secondary Rag1−/− recipients, respectively. All secondary recipients developed B-ALL by day 19 after transplantation. Note that leukemic cells in recipients of GFP+ pro-B and pre-B cells had phenotypes characteristic of more immature stages of B-cell development.
To further address this issue, spleen cells from 2 diseased primary recipients of BCR-ABL/GFP–transduced CLPs were pooled, and GFP+ populations were isolated according to their CLP, pre/pro-B-, pro-B-, and pre-B-cell phenotype and transplanted into sublethally irradiated Rag1−/− recipients. All secondary recipients of total spleen cells (supplemental Figure 3) and GFP+ cells with a CLP, pre/pro-B-, pro-B-cell, and pre-B-cell phenotype developed aggressive disease within 3 weeks of transplantation. Interestingly, while the GFP+ cells in mice transplanted with CLP and pre/pro-B leukemic cells included populations of all downstream B-cell progenitors, the tissues of recipients of GFP+ pro-B and pre-B cells included populations of leukemic cells with phenotypes characteristic of more immature stages of B-cell development (Figure 3B). Taken together, these observations suggest that transformation alters the normal expression of B- lineage cell-surface determinants and that caution must be exercised when staging tumors on the basis of phenotype alone.
Ph+ B-ALL does not initiate from pre-B cells
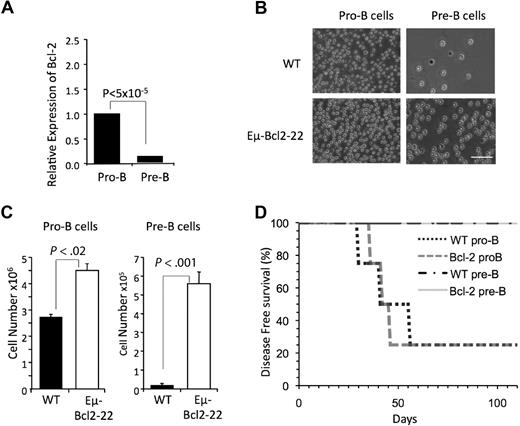
As noted in Figure 1A, we did not observe B-ALL in mice that received BCR-ABL/GFP–transformed pre-B cells (Figure 1A). Pre-B cells are generally thought to respond inefficiently to critical B lymphopoietic cytokines like IL-712 and express decreased levels of Bcl-233 (Figure 4A). Thus, we considered the possibility that their inability to initiate disease was an artifact related to their decreased survival in culture. To address this issue, pre-B cells were isolated from Eμ-Bcl-2-22 transgenic mice, that express a Bcl-2 transgene in all stages of B-cell development.34 Eμ-Bcl-2-22 pre-B cells exhibited enhanced in vitro survival compared with their wild-type counterparts (Figure 4B-C), indicating that the transgene is functional at that stage of development. However, BCR-ABL/GFP–transduced Eμ-Bcl-2-22 pre-B cells did not generate B-ALL after transplantation into Rag1−/− recipients (Figure 4D).
Bcl-2 expressing pre-B cells do not generate B-ALL. (A) Expression of Bcl-2 relative to β-Actin was assessed by qPCR in pro-B and pre-B cells isolated from the bone marrow of wild-type mice (n = 4). Reactions for each population were run 4 times. (B). Pro-B and pre-B cells were isolated from the bone marrow of wild-type or Eμ-Bcl2-22 transgenic mice and 25 000 cells were seeded in duplicate in wells of 6-well culture plates. Photomicrographs of cells after 10 days in culture are shown (bar represents 50 μm). (C) Total number of cells per well from cultures shown in panel B. (D) Pro-B cells, but not pre-B cells, from wild-type and Eμ-Bcl2-22 mice generated B-ALL. Cells were manipulated as described in Figure 1A. The indicated populations were sorted from pools of bone marrow from 4 wild-type and 4 Eμ-Bcl2-22 mice, respectively. Each cell population was transduced as described in supplemental Figure 2 and injected into 4 recipient mice.
Bcl-2 expressing pre-B cells do not generate B-ALL. (A) Expression of Bcl-2 relative to β-Actin was assessed by qPCR in pro-B and pre-B cells isolated from the bone marrow of wild-type mice (n = 4). Reactions for each population were run 4 times. (B). Pro-B and pre-B cells were isolated from the bone marrow of wild-type or Eμ-Bcl2-22 transgenic mice and 25 000 cells were seeded in duplicate in wells of 6-well culture plates. Photomicrographs of cells after 10 days in culture are shown (bar represents 50 μm). (C) Total number of cells per well from cultures shown in panel B. (D) Pro-B cells, but not pre-B cells, from wild-type and Eμ-Bcl2-22 mice generated B-ALL. Cells were manipulated as described in Figure 1A. The indicated populations were sorted from pools of bone marrow from 4 wild-type and 4 Eμ-Bcl2-22 mice, respectively. Each cell population was transduced as described in supplemental Figure 2 and injected into 4 recipient mice.
The pre-B-cell compartment has been reported to be minimally proliferative,35,36 making it necessary to consider the additional possibility that pre-B cells may not have been transduced with the retroviral vectors used. To examine this possibility, we determined whether pre-B cells could be transduced using a GFP-expressing retrovirus. As shown in Figure 5A, up to 11% of the pre-B cells were GFP+ at 40 hours posttransduction and this frequency had increased to 28% at 65 hours. In an independent experiment, cells were examined 120 hours after transduction, and GFP expression was similar to that in pro-B cells. The ability of pre-B cells to be transduced in this system is consistent with the observation that a significant proportion expressed Ki-67 after 18 hours of culture, although levels were lower than observed with pro-B cells (Figure 5B).
Pre-B cells can be retrovirally transduced. (A) Pro-B and pre-B cells were isolated from the bone marrow of wild-type mice, transduced with a GFP– containing retroviral vector, and placed in culture. GFP expression was assessed in cell aliquots harvested at 40, 65, and, in an independent experiment, 120 hours later. Cultures were initiated with 1.0 to 1.5 × 105 pro-B or pre-B cells. (B) Ki-67 labeling of pro-B and pre-B cells. Ki-67 expression was measured in these populations after 18 hours culture in vitro.
Pre-B cells can be retrovirally transduced. (A) Pro-B and pre-B cells were isolated from the bone marrow of wild-type mice, transduced with a GFP– containing retroviral vector, and placed in culture. GFP expression was assessed in cell aliquots harvested at 40, 65, and, in an independent experiment, 120 hours later. Cultures were initiated with 1.0 to 1.5 × 105 pro-B or pre-B cells. (B) Ki-67 labeling of pro-B and pre-B cells. Ki-67 expression was measured in these populations after 18 hours culture in vitro.
Deletion of Arf, but not Ink4a, allows pre-B cells to initiate B-ALL
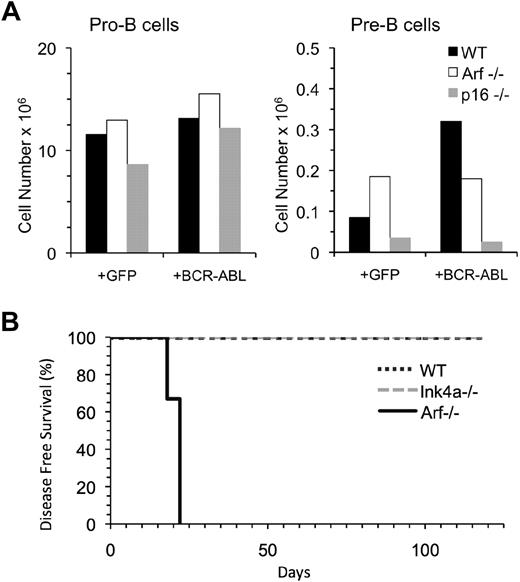
Lymphoid cells up-regulate tumor suppressor genes, including Arf and Ink4a, which are encoded by the Cdkn2a locus,37 in response to oncogenic stress, and it has been previously reported that B-cell progenitors that fail to express Arf efficiently generate B-ALL.18,26,38 To test how the lack of Arf expression in pre-B cells would affect their leukemogenic potential, we isolated pre-B cells from Arf−/− and wild-type mice and compared their ability to initiate B-ALL. Pre-B cells from Ink4a−/− mice were examined in parallel. Pre-B cells from each of these mice exhibited nearly comparable growth and survival in culture (Figure 6A), but only BCR-ABL/GFP–transduced Arf−/− pre-B cells developed B-ALL (Figure 6B).
Pre-B cells from Arf−/− but not Ink4a−/− mice can initiate B-ALL. (A) Pro-B and pre-B cells from wild-type (4), Arf−/− (n = 2), and Ink4a−/− (n = 2) mice were purified, transduced with retroviral vectors containing GFP only or BCR-ABL/GFP and 2.0 × 104 cells were seeded in a single well in culture. The number of cells harvested 7 days later is indicated. (B) Pre-B cells were isolated from the bone marrow of wild-type (n = 2), Ink4a−/− (n = 2), or Arf−/− (n = 2) mice, transduced with BCR-AB/GFPL, and 80 000 cells were transplanted into 3 Rag1−/− recipients per group. All recipients of the Arf−/− pre-B cells developed B-lineage leukemia by day 22 posttransplantation, whereas wild-type or Ink4a−/− pre-B cells showed no signs of disease.
Pre-B cells from Arf−/− but not Ink4a−/− mice can initiate B-ALL. (A) Pro-B and pre-B cells from wild-type (4), Arf−/− (n = 2), and Ink4a−/− (n = 2) mice were purified, transduced with retroviral vectors containing GFP only or BCR-ABL/GFP and 2.0 × 104 cells were seeded in a single well in culture. The number of cells harvested 7 days later is indicated. (B) Pre-B cells were isolated from the bone marrow of wild-type (n = 2), Ink4a−/− (n = 2), or Arf−/− (n = 2) mice, transduced with BCR-AB/GFPL, and 80 000 cells were transplanted into 3 Rag1−/− recipients per group. All recipients of the Arf−/− pre-B cells developed B-lineage leukemia by day 22 posttransplantation, whereas wild-type or Ink4a−/− pre-B cells showed no signs of disease.
Arf expression is differentially regulated in B-cell progenitors in response to oncogenic stress
The previously stated results suggest a distinct role of Arf in blocking the initiation of B-ALL in BCR-ABL transduced, wild-type pre-B cells. To investigate this possibility in more detail, we examined Arf expression, levels of apoptosis, and cell production in cultures initiated with GFP– or BCR-ABL/GFP–transduced pro-B or pre-B cells.
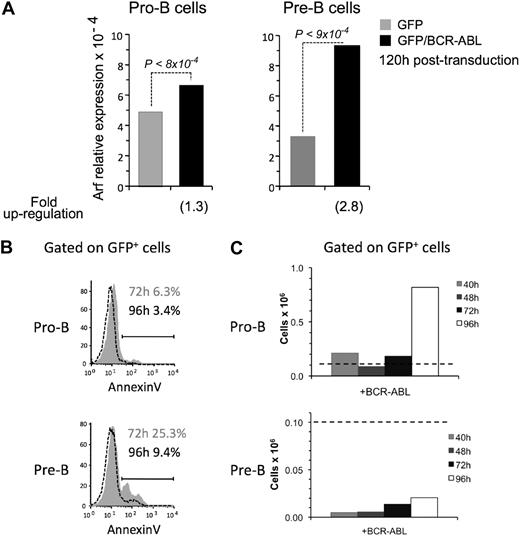
A time-course analysis at 40, 65, and 120 hours demonstrated that BCR-ABL/GFP–transduced pro-B cells up-regulated Arf to levels similar to that in cells transduced with GFP or YFP alone (Figure 7A and supplemental Figure 4). In contrast, the level of Arf expression in BCR-ABL/GFP–transduced pre-B cells was higher compared with cells transduced with GFP or YFP alone at these time points (Figure 7A and supplemental Figure 4). These data suggest that oncogene-induced stress results in higher levels of Arf induction in pre-B cells compared with pro-B cells.
Pre-B cells up-regulate Arf in response to BCR-ABL expression. (A) expression of Arf relative to β-Actin was assessed by qPCR at 120 hours after transduction with a retrovirus encoding either GFP or BCR-ABL/GFP. The results are pooled from 2 independent experiments, and reactions for each experiment were run 2 to 4 times. (B) Frequency of apoptotic BCR-ABL/GFP–transduced pre-B cells and pro-B cells at 72 hours (gray shaded area) and 96 hours (area under dashed line) after transduction. Apoptosis was measured by annexin V staining. (C) Number of BCR-ABL/GFP–expressing pro-B and pre-B cells harvested from cultures at 40, 48, 72, and (in an independent experiment) 96 hours after culture initiation. Dashed horizontal line indicates the number of cells (105) used to initiate cultures.
Pre-B cells up-regulate Arf in response to BCR-ABL expression. (A) expression of Arf relative to β-Actin was assessed by qPCR at 120 hours after transduction with a retrovirus encoding either GFP or BCR-ABL/GFP. The results are pooled from 2 independent experiments, and reactions for each experiment were run 2 to 4 times. (B) Frequency of apoptotic BCR-ABL/GFP–transduced pre-B cells and pro-B cells at 72 hours (gray shaded area) and 96 hours (area under dashed line) after transduction. Apoptosis was measured by annexin V staining. (C) Number of BCR-ABL/GFP–expressing pro-B and pre-B cells harvested from cultures at 40, 48, 72, and (in an independent experiment) 96 hours after culture initiation. Dashed horizontal line indicates the number of cells (105) used to initiate cultures.
Consistent with this hypothesis, we observed that the frequency of apoptotic cells in cultures initiated with BCR-ABL/GFP–transduced pre-B cells at 72 and 96 hours after transduction was higher compared with pro-B cells (Figure 7B). As a result of this high rate of apoptosis, the number of cells recovered from cultures initiated with BCR-ABL/GFP–transduced pre-B cells was lower than the 105 cells initially seeded. In contrast, expansion of BCR-ABL/GFP–expressing pro-B cells had occurred by 96 hours (Figure 7C).
Discussion
Despite being a focus of continuing investigation over the past decade,39 the stage(s) of B-cell development in which Ph+ B-ALL can initiate and the efficiency with which this occurs are unclear. The aim of the present study was to address these issues. One clear result is that Ph+ B-ALL can initiate with high efficiency in immature B-cell progenitors like CLPs and pro-B cells but not in more mature pre-B cells. A second finding is that, in contrast to recently published observations,26 pre-existing deletions of Arf are not required for efficient initiation of B-ALL from these stages of development. Finally, we demonstrate that the level at which the Arf tumor suppressor gene is up-regulated in distinct stages of B-cell development determines whether or not disease will initiate in a particular progenitor. These results provide new insights into the cellular origins of Ph+ B-ALL and demonstrate that the activation of tumor suppressor mechanisms in B-cell progenitors is affected by developmental status.
B-lineage leukemia was observed in a majority of mice that received BCR-ABL/GFP–transduced CLPs or pro-B cells. The kinetics with which animals became moribund was similar for both populations, although direct comparisons are difficult because we did not inject comparable cell numbers into recipients. Although the leukemic GFP+ cells in the mice expressed B-lineage cell-surface determinants, our data suggest that the tumor cells did not maintain the regulated expression of B-lineage cell-surface determinants characteristic of normal cells. The latter finding is consistent with another report showing that phenotypic infidelity is a characteristic of non-Ph+ B-ALL as well.40 In view of this promiscuous expression of B-lineage antigens, caution should be exercised in using cell-surface phenotype to determine the developmental status of tumor cells.
That point aside, our data raise the question of why we were able to generate B-ALL from wild-type B-cell progenitors with high efficiency while others have reported inefficient initiation of disease in the absence of predisposing genetic mutations.18,26 Our experience is that the conditions under which B-cell progenitors are manipulated directly affect their potential to initiate leukemia. In this regard, a distinguishing feature of our transduction protocol is that the total ex vivo culture period after isolation of progenitors was shortened from several days to 24 hours and the cells were grown in the presence of salutary microenvironmental signals that included B lymphopoietic cytokines and stromal cells known to support B lymphopoiesis.30 These modifications were introduced after our observation that lengthening the ex vivo transduction period resulted in a marked reduction in the potential of pro-B cells to initiate leukemia.29 Limiting the transduction period to 24 hours is particularly important to avoid the maturation of precursors into pre-B cells, a population which we demonstrate cannot initiate disease. In addition, extended times in culture may result in cell stress that activates Arf expression,41 which is also obviated by using a short transduction period.
In striking contrast to the efficient generation of lymphoid leukemia from immature B-cell progenitors, we did not observe disease initiation from pre-B cells. It was important to document that this result was not an artifact of the culture conditions. In this regard, the number of cells recovered from cultures initiated with wild-type pre-B cells was lower than from their upstream precursors. This in turn raised the possibility that decreased pre-B-cell survival in vitro was the reason for the inability to initiate disease; however, this was not the case. This conclusion is supported by data showing that pre-B cells that expressed a Bcl-2 transgene exhibited enhanced survival in culture but still failed to initiate leukemia. It is interesting that expression of a Bcl-2 transgene in myeloid progenitors42 is sufficient for induction of leukemia after transduction with BCR-ABL. This dichotomy probably results from the fact that, in contrast to pre-B cells, myeloid progenitors do not express Arf,28 and because the gene is not deleted in myeloid leukemias,43 they probably do not express it at all after transformation. Therefore, it seems that the prolonged survival conferred on myeloid progenitors by Bcl-2 would facilitate their acquisition of secondary mutations, such as activation of β-catenin,44 and allow them to initiate and propagate CML.
Because the pre-B-cell fraction (Hardy Fraction D) we tested has been reported to be postmitotic,35,36 we also considered the possibility that they were not efficiently transduced with retroviral vectors. However, our analysis showed that this was not the case. Consistent with this finding was the demonstration that a significant proportion of pre-B cells retained the potential to proliferate. This contrasts with earlier studies that reported small pre-B cells to be noncycling cells.35,36 However, it is important to appreciate that those reports used microscopy to enumerate a limited number of in vivo tritiated thymidine labeled cells that were subsequently stained with IgM antibodies. Our current ability to identify proliferating cells by Ki-67 expression and to count several hundred thousand events using flow cytometry revealed that a proportion of small pre-B cells are in cycle.
As reviewed by Williams and Sherr,10 B-cell progenitors and their progeny are poised to respond to oncogenic stress by activating the Ink4a-Arf locus. Our data are in agreement with this conclusion, but our results further demonstrate that the level at which Arf is induced and the response of progenitors to this event differs according to developmental status. Thus, although Arf is induced in pro-B cells after oncogenic stress, at least some cells survive this relatively small increase, undergo significant expansion over time, and initiate leukemia. It is important to emphasize that secondary events may subsequently occur in these cells and exacerbate disease. This would seem to be a relatively frequent event in view of data showing that between 50% and 80% of human Ph+ B-ALLs exhibit deletions or mutations in Arf and/or Ikaros,43,45 respectively. In contrast to what is observed in pro-B cells, Arf levels in BCR-ABL/GFP–transduced pre-B cells are significantly elevated compared with the cells transduced with GFP only. This in turn has deleterious effects, as evidenced by the high levels of apoptosis and a reduction in the number of cells harvested from the short-term cultures. Taken together, these data suggest that Arf expression in pre-B cells results in massive apoptosis, which, in turn, explains why we did not observe B-ALL in recipients of these cells.
In normal tissues, p19Arf has been shown to have cell stage–specific functions in stem and progenitor cells.46 However, it has not been reported that induction of Arf in response to oncogenic stress is a developmentally regulated event that, in turn, determines the potential of progenitors to initiate Ph+ B-ALL. It will be of interest to analyze levels of Arf expression in specific T-cell progenitor populations in the bone marrow and thymus in view of recent findings which suggest that Arf expression may also be differentially regulated in transformed T-cell progenitors.47
How cells within the same developmental pathway differentially control levels of oncogene-induced Arf expression is unknown. In particular, it will be of interest to determine whether there is any link between pre-B-cell receptor (BCR) signaling and Arf induction in BCR-ABL–transformed pre-B cells in view of a recent report that the pre-BCR mediates cell-cycle arrest in BCR-ABL–transformed pre-B cells through activation of Ikaros.48 Insights may emerge as a greater understanding of Cdkn2a gene regulation is attained.49
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs David Nemazee, Charles Sherr, Norman Sharpless, and Francisco Zaera for reagents, mice, and/or advice.
This work was supported by grants from the National Institutes of Health (AG021450 and AI21256). O.N.W. is an investigator of the Howard Hughes Medical Institute. R.A.J.S. was supported by a fellowship from the California Institute for Regenerative Medicine (TI-00005). The UCLA Flow Cytometry Core Facility is supported by National Institutes of Health grants CA-16042 and AI-28697.
National Institutes of Health
Authorship
Contribution: R.A.J.S. and E.M.-R. designed and conducted experiments and contributed equally to this work; O.N.W. provided reagents, contributed to experimental design, and helped write the paper; and K.D. supervised work, helped write the paper, and had overall responsibility for this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for R.A.J.S. is Center for Stem Cell Biology, University of Michigan, Ann Arbor, MI.
Correspondence: Kenneth Dorshkind, Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at UCLA, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail: kdorshki@mednet.ucla.edu.
References
Author notes
R.A.J.S. and E.M.-R. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal