Abstract
The purpose of this prospective multicenter phase 2 trial was to investigate the long-term outcome of reduced-intensity conditioning allogeneic stem cell transplantation (alloSCT) in patients with poor-risk chronic lymphocytic leukemia. Conditioning was fludarabine/ cyclophosphamide-based. Longitudinal quantitative monitoring of minimal residual disease (MRD) was performed centrally by MRD-flow or real-time quantitative polymerase chain reaction. One hundred eligible patients were enrolled, and 90 patients proceeded to alloSCT. With a median follow-up of 46 months (7-102 months), 4-year nonrelapse mortality, event-free survival (EFS) and overall survival (OS) were 23%, 42%, and 65%, respectively. Of 52 patients with MRD monitoring available, 27 (52%) were alive and MRD negative at 12 months after transplant. Four-year EFS of this subset was 89% with all event-free patients except for 2 being MRD negative at the most recent assessment. EFS was similar for all genetic subsets, including 17p deletion (17p−). In multivariate analyses, uncontrolled disease at alloSCT and in vivo T-cell depletion with alemtuzumab, but not 17p−, previous purine analogue refractoriness, or donor source (human leukocyte antigen-identical siblings or unrelated donors) had an adverse impact on EFS and OS. In conclusion, alloSCT for poor-risk chronic lymphocytic leukemia can result in long-term MRD-negative survival in up to one-half of the patients independent of the underlying genomic risk profile. This trial is registered at http://clinicaltrials.gov as NCT00281983.
Introduction
Although chronic lymphocytic leukemia (CLL) mostly demonstrates indolent behavior, some patients show an aggressive course and die within few years from diagnosis. Clinically, “poor-risk” CLL is characterized by resistance to chemotherapy, including modern purine analog-antibody combination regimens.1-4 Biologically, an unfavorable course can be predicted by a variety of parameters, such as the immunoglobulin variable heavy-chain (IGHV) mutational status,5,6 ZAP70 expression,7-9 serologic proliferation markers,2,10,11 and genomic aberrations identified by fluorescence in situ hybridization (FISH). The hierarchical model of genomic aberrations segregates distinct prognostic subgroups of patients with CLL with median survival estimates ranging from 3 years to more than 15 years.12 The hierarchical model has been validated prospectively, demonstrating its applicability also to modern upfront treatment regimens on the basis of purine analogue and/or rituximab combinations.13,14 The abnormality associated with the worst prognosis is 17p deletion (17p−), which reliably heralds a poor response to standard chemo(immuno)therapy and a median survival of less than 3 years.12-15
On the basis of its capacity to induce graft-versus-leukemia (GVL) activity,16-18 allogeneic stem cell transplantation (alloSCT) has been shown to provide long-term disease control in selected patients with poor-risk CLL, including those with 17p− and/or resistance to purine analogues.19-30 This has led some to advocate alloSCT as reasonable treatment option for eligible patients with these adverse features.31,32 However, it is unclear whether alloSCT can durably eradicate poor-risk CLL, thereby overcoming the prognostic relevance of genomic aberrations (ie, 17p−), and whether this might translate into a significant survival benefit. The aim of the present study was to prospectively investigate the feasibility and efficacy of reduced-intensity conditioning (RIC) alloSCT in patients with poor-risk CLL and to analyze clinical as well as biologic prognostic factors with particular focus on genomic aberrations.
By the use of central FISH karyotyping at study entry as well as central prospective monitoring of minimal residual disease (MRD) kinetics, our results show that RIC alloSCT from related or unrelated donors can completely abrogate the prognostic impact of the FISH hierarchical model in patients with poor-risk CLL, translating into long-term MRD-free survival in up to one-half of the patients independent of the underlying genomic risk profile, resistance to purine analogues, and donor source.
Methods
Patients and donors
Eligible were patients with poor-risk CLL as defined by one of the following: refractoriness or early relapse (within 12 months) after treatment with a purine analogue-containing regimen, relapse after autologous SCT, or progressive disease in the presence of an unfavorable genetic constellation (11q−, 17p−, and/or unmutated IGHV status and/or usage of the VH3-21 gene). Patients had to be between 18 and 65 years of age with normal organ function and an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or better. Patients with Richter transformation were excluded. Patients could be registered at any time before transplant once the eligibility criteria were met.
Donors had to be siblings or unrelated volunteers who were human leukocyte antigen (HLA) identical as defined by A/B identity at the serologic (antigen) level and DRB1 identity at the molecular (allele) level. During the course of the study, unrelated donor selection criteria were not adapted to improved HLA-typing methodology and matching standards, implying that donors who were only partially matched or even mismatched according to modern standards33 could be eligible.
Because the hematopoietic cell transplantation comorbidity index (HCT-CI)34 was not available when the trial was designed, it was extracted retrospectively from the case report forms by use of the items “concomitant diseases” and “detailed results of cardiac, pulmonary, hepatic and renal function tests.” Thirty patients studied here have been included in a previous preliminary report on MRD kinetics in the CLL3X trial35 ; in addition, 4 patients with 17p− deletion have been part—with limited follow-up—of a previously published registry analysis.30
Treatment
Conditioning consisted of the administration of daily fludarabine (30 mg/m2) and cyclophosphamide (500 mg/m2) from day −6 through day −2 (conditioning regimen FC). In case of unrelated donors, ATG (10 mg/kg/d; Fresenius) was added from day −4 through day −1. In 2 patients with documented fludarabine intolerance, this drug was replaced by cladribine 0.11 mg/kg from day −6 through day −2. Peripheral blood stem cell grafts from granulocyte colony-stimulating factor (G-CSF)–primed healthy donors were transfused without manipulation on day 0. From June 2002 through November 2005, an alternative conditioning regimen was active that could be selected at the discretion of the investigator: FC plus 2 Gy total body irradiation on day −9 and in vivo T-cell depletion with alemtuzumab (20 mg/d from day −8 through day −4; conditioning regimen TCD). TCD was used by 3 centers as standard conditioning for all consecutive patients until this regimen was abandoned because of engraftment problems. Instead, in November 2005 an intensified regimen was introduced for refractory patients only: fludarabine (30 mg/m2/d) from day −7 through day −3, busulfan (4 mg/kg/d by mouth) from day −7 through day −5, and cyclophosphamide (30 mg/kg/d) on days −3 and −2 (conditioning regimen FBC).
Graft-versus-host disease (GVHD) prophylaxis was performed with cyclosporin A. Short-course methotrexate or mycophenolat mofetil was added in arms A and C as described.35 Donor lymphocyte infusions (DLIs) were administered not earlier than 4 weeks after complete withdrawal of cyclosporin A in case of incomplete chimerism or MRD.
Trial objectives and assessments
The primary objective of this nonrandomized, multicenter phase 2 clinical study was to prospectively study the feasibility and safety of RIC alloSCT in patients with poor-risk CLL. Secondary objectives were the assessment of clinical response; event-free survival (EFS); overall survival (OS); incidence, kinetics, durability, and clinical significance of MRD response; and the prognostic impact of genetics on these parameters. Response evaluation was performed according to National Cancer Institute criteria.36 MRD was defined as being in clinical remission but having one or more CLL cells per 10 000 blood or marrow leukocytes.32 Clinical follow-up and sampling for MRD and chimerism assessment was performed as described previously.35 The protocol including the study-specific informed consent form was approved by all responsible institutional review boards in accordance with the Declaration of Helsinki.35
Genetic, chimerism, and MRD analyses
Assessments of genomic aberrations, IGHV sequence and mutational status, chimerism of unseparated blood leukocytes by short tandem repeat polymerase chain reaction, and MRD by MRD flow or allele-specific oligonucleotide primer IgH real-time quantitative polymerase chain reaction were performed in the central reference laboratories of the German CLL Study Group, as described.12,35,37,38 Both MRD assays allowed the quantitative assessment of MRD levels with a sensitivity of at least one CLL cell in 10 000 leukocytes. Only results obtained in the German CLL Study Group central laboratories were considered for the analyses performed in this study.
Statistical analyses
The Fisher exact test was used to compare categorical factors between 2 groups of patients. Survival time data were calculated by use of the Kaplan-Meier method for OS and EFS whereas cumulative incidence estimates were calculated in a competing risk framework for relapse, nonrelapse mortality (NRM), and acute and chronic GVHD. Events relevant for EFS were clinical progression, disease recurrence, nonengraftment, CLL-specific retreatment, secondary malignancy, or death from any cause. Preemptive DLI for treatment of MRD or incomplete chimerism was part of the transplant process and not considered as CLL-specific retreatment relevant for EFS. Accordingly, persistent or recurrent MRD without clinical relapse was not counted as event for EFS. Events relevant for relapse incidence were clinical progression or disease recurrence. Responses to therapeutic DLI were not considered for calculation of EFS and relapse, that is, all EFS curves represent real and not “current” EFS. Events determining NRM were all deaths before clinical progression or disease recurrence. Kaplan-Meier curves were compared with the log-rank test. Proportional hazards models (Cox regression) were fitted to investigate effects of prognostic factors for OS, EFS, relapse, and NRM. Univariate calculations were performed by the use of GraphPad Prism software (release 5.0), and Cox regression analyses were performed with SPSS (release 11.0). Significance levels were set at .05. Data were analyzed as of January 31, 2010.
Results
Patients and donors
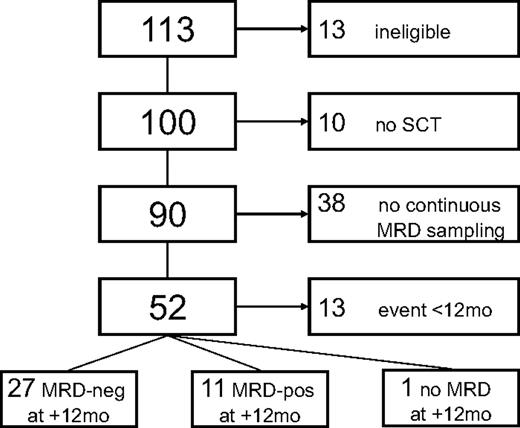
Between June 2001 and March 2007, 113 patients were accrued in 16 centers. Of these, 13 patients had to be excluded because of ineligibility (Figure 1). Reasons for ineligibility were no CLL (n = 2), previous Richter transformation (n = 1) comorbidity (n = 2), concomitant therapy-related myelodysplasia (n = 1), withdrawn consent (n = 3), and registration only after alloSCT (n = 4). Three of the 4 latter patients are alive 28, 38, and 62 months from study entry, and one has died from progressive CLL (supplemental Table 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Ten patients did not proceed to alloSCT because of death attributable to progressive disease (n = 1), development of Richter transformation (n = 3) refusal (n = 2), or because a suitable donor was not found (n = 4). Details of treatment and outcome of these 10 patients are summarized in supplemental Table 3.
Consort diagram. Of 113 patients registered, 13 had to be excluded because of ineligibility (no CLL, 2; Richter's transformation, n = 1; no informed consent, n = 3; comorbidity precluding eligibility as per protocol, n = 3; registration after alloSCT, n = 4), leaving 100 patients evaluable for analysis. Ten patients did not proceed to alloSCT because they lacked a donor (n = 4), died from progressive disease or developed Richter transformation before alloSCT (n = 4), or refused alloSCT (n = 2).
Consort diagram. Of 113 patients registered, 13 had to be excluded because of ineligibility (no CLL, 2; Richter's transformation, n = 1; no informed consent, n = 3; comorbidity precluding eligibility as per protocol, n = 3; registration after alloSCT, n = 4), leaving 100 patients evaluable for analysis. Ten patients did not proceed to alloSCT because they lacked a donor (n = 4), died from progressive disease or developed Richter transformation before alloSCT (n = 4), or refused alloSCT (n = 2).
Of the 90 remaining patients, 42 (47%) had a history of purine-analogue refractoriness according to the National Cancer Institute/International Workshop on Chronic Lymphocytic Leukemia definition.32 However, because of successful, mainly antibody-based salvage therapy, only 24 patients (21%) had uncontrolled, refractory disease at alloSCT. A total of 78 patients had informative samples for central genetic analyses available, revealing 17p− in 13 patients (18%), and 11q− without 17p− in 26 patients (36%). Further details on genomic aberrations and other patient characteristics are listed in Table 1. Because the inclusion criteria precluded enrolment of patients with significant comorbidity, only few patients had an advanced HCT-CI. Donors were HLA-identical siblings (40%) or well-matched (13%), partially matched (30%), or mismatched (2%) unrelated volunteers. In the remaining 15%, sufficient HLA typing information for assignment of score was not available. The conditioning regimen used was FC in 65 patients, TCD in 12 (per center consecutive) patients, and FBC in 12 patients of 89 patients with information available. Three patients were reconstituted with bone marrow grafts because peripheral blood stem cells were not available from their donors. Transplantations were performed between July 2001 and November 2007.
Patient characteristics
| . | All eligible patients (n = 100) . | Patients proceeding to alloSCT (n = 90) . |
|---|---|---|
| Sex, female/male | 33/67 | 30/60 |
| Age, y | 53 (27-65) | 53 (27-65) |
| IGHV unfavorable, n (%)* | 87 of 91 (96) | 78 of 82 (95) |
| FISH karyotype, n (%) | 78 of 100 (78) | 72 of 90 (80) |
| del 17p− ± others, n (%) | 16 of 78 (22) | 13 of 72 (18) |
| del 11q− ± others (except del 17p−), n (%) | 28 of 78 (36) | 26 of 72 (36) |
| +12 ± others (except del 17p−, del 11q−), n (%) | 4 of 78 (5%) | 4 of 72 (6) |
| del 13q− only, n (%) | 12 of 78 (15) | 12 of 72 (17) |
| Others, n (%) | 5 of 78 (6) | 5 of 72 (7) |
| Normal FISH karyotype, n (%) | 13 of 78 (17) | 12 of 72 (17) |
| Maximum Binet stage A/B/C/unknown | 10/42/45/3 | 9/40/40/1 (10%/44%/44%/1%) |
| Time from diagnosis, months (range) | 54 (2-270) | 57 (2-270) |
| Previous regimens, n (range) | 4 (1-11) | 4 (1-11) |
| Previous autoSCT, n (%) | 31 of 98 (32%) | 28 of 90 (31%) |
| Purine analogue resistant, n (%)† | 48 of 98 (49%) | 42 of 90 (47%) |
| ECOG score 0/1/unknown | 65/29/6 | 62/27/1 (69%/30%/1%) |
| EBMT indication 1/2/3/0‡ | 3/16/48/31 | 3/13/45/29 (3%/14%/50%/32%) |
| Alemtuzumab last regimen before transplant | NA | 22 of 90 (24%) |
| Refractory disease at SCT | NA | 21 of 89 (24%) |
| BM infiltration at SCT (%) | NA | 15 (0–100) |
| HCT-CI score at SCT 0/1/2/>2 | NA | 67/12/6/3 |
| Alternative donor§ | NA | 54 of 90 (60%) |
| Weisdorf score SIB/WMUD/PMUD/MM/unclassifiable¶ | NA | 36/12/27/2/13 (40%/13%/30%/2%/15%) |
| Conditioning FC/TCD/FBC/unknown | NA | 65/12/12/1 (72%/13%/13%/1%) |
| . | All eligible patients (n = 100) . | Patients proceeding to alloSCT (n = 90) . |
|---|---|---|
| Sex, female/male | 33/67 | 30/60 |
| Age, y | 53 (27-65) | 53 (27-65) |
| IGHV unfavorable, n (%)* | 87 of 91 (96) | 78 of 82 (95) |
| FISH karyotype, n (%) | 78 of 100 (78) | 72 of 90 (80) |
| del 17p− ± others, n (%) | 16 of 78 (22) | 13 of 72 (18) |
| del 11q− ± others (except del 17p−), n (%) | 28 of 78 (36) | 26 of 72 (36) |
| +12 ± others (except del 17p−, del 11q−), n (%) | 4 of 78 (5%) | 4 of 72 (6) |
| del 13q− only, n (%) | 12 of 78 (15) | 12 of 72 (17) |
| Others, n (%) | 5 of 78 (6) | 5 of 72 (7) |
| Normal FISH karyotype, n (%) | 13 of 78 (17) | 12 of 72 (17) |
| Maximum Binet stage A/B/C/unknown | 10/42/45/3 | 9/40/40/1 (10%/44%/44%/1%) |
| Time from diagnosis, months (range) | 54 (2-270) | 57 (2-270) |
| Previous regimens, n (range) | 4 (1-11) | 4 (1-11) |
| Previous autoSCT, n (%) | 31 of 98 (32%) | 28 of 90 (31%) |
| Purine analogue resistant, n (%)† | 48 of 98 (49%) | 42 of 90 (47%) |
| ECOG score 0/1/unknown | 65/29/6 | 62/27/1 (69%/30%/1%) |
| EBMT indication 1/2/3/0‡ | 3/16/48/31 | 3/13/45/29 (3%/14%/50%/32%) |
| Alemtuzumab last regimen before transplant | NA | 22 of 90 (24%) |
| Refractory disease at SCT | NA | 21 of 89 (24%) |
| BM infiltration at SCT (%) | NA | 15 (0–100) |
| HCT-CI score at SCT 0/1/2/>2 | NA | 67/12/6/3 |
| Alternative donor§ | NA | 54 of 90 (60%) |
| Weisdorf score SIB/WMUD/PMUD/MM/unclassifiable¶ | NA | 36/12/27/2/13 (40%/13%/30%/2%/15%) |
| Conditioning FC/TCD/FBC/unknown | NA | 65/12/12/1 (72%/13%/13%/1%) |
alloSCT indicates allogeneic stem cell transplantation; autoSCT, autologous stem cell transplantation; BM, bone marrow; EBMT, European Group for Blood and Marrow Transplantation; ECOG, Eastern Cooperative Oncology Group; FBC, FC + busulfan; FC, fludarabine-cyclophosphamide; FISH, fluorescence in situ hybridization; HCT-CI, hematopoietic cell transplantation comorbidity index; IGHV, immunoglobulin variable heavy-chain; MM, mismatched unrelated donor (Weisdorf and coworkers33 ); NA, not applicable; PMUD, partially matched unrelated donor; SIB, 10 of 10 allele-matched sibling; TCD, FC + total body irradiation 2Gy + in vivo alemtuzumab; and WMUD, well-matched unrelated donor.
Unmutated (> 98% homology to germline) or VH3–21.
Nonresponse or relapse within 6 months after fludarabine monotherapy or fludarabine-cyclophosphamide/fludarabine-rituximab combinations.
1, relapse within 24 months after intensive therapy; 2, del 17p−; 3, purine analogue refractory or relapse within 12 months after purine analogues; 0, other indication as defined in the protocol.
Unrelated donor or mismatched relative.
In 13 patients sufficient HLA typing information for assignment of score was not available (unclassifiable).
Engraftment and conditioning-related toxicity
Neutrophil and platelet recovery occurred in 85 of 86 patients with recovery data available. Median time to neutrophils greater than 0.5 × 109/L was 10 days (0-24 days) in 36 patients with G-CSF stimulation and 16 days (0-28 days) in 48 patients without G-CSF. Median time to platelets greater than 20 × 109/L was 8 days (0-30 days). A total of 36 patients (42%) never needed any platelet transfusions. Complete lymphohematopoietic chimerism was achieved in 66 of 80 patients (83%) who had chimerism results available after a median time of 80 days (25-600 days). Incomplete chimerism was attributable to persistent CLL in 6 patients and to nonengraftment in 7 patients. An additional patient showed graft rejection after transient complete chimerism, translating into a nonengraftment rate of 10%. Nonengraftment was observed only after conditioning with regimens FC or TCD. Six patients with nonengraftment had a donor with some degree of mismatch, and 2 had received marrow grafts. None of these 8 patients died as the result of persistent aplasia: 7 had autologous recovery, and one could be salvaged by a second transplant (supplemental Table 1).
Median time of hospitalization after alloSCT was 22 days (0-150 days). Nonhematopoietic toxicity was generally modest. Mucositis was observed in 36 of 81 patients (44%), but only 5 patients (6%) had grade 3/4 mucositis. Accordingly, parenteral nutrition was administered to only 29 patients (36%). Neutropenic fever occurred in 42 of 76 patients (55%) and lasted 4 days (1-30 days). No patient died during the conditioning-related hospitalization phase, and only 2 patients succumbed to NRM within the first 3 months, translating into a 100-day mortality of 2% (95% confidence interval [95% CI] 0%-5%).
GVHD and need for prolonged systemic immunosuppression
Clinically relevant acute GVHD (grade 2-4) developed in 38 of 85 patients (45%) after primary alloSCT or DLI but was severe (grade 3-4) in only 12 (14%). Chronic and extensive chronic GVHD occurred in 46 and 35 patients, respectively, of the 66 patients who survived longer than 100 days and had GVHD information available, translating into a cumulative incidence of chronic and extensive chronic GVHD at 2 years of 73% (95% CI 62%-85%), and 55% (95% CI 42%-65%), respectively. Among the 40 patients with chronic GVHD who were alive 1 year after transplant and had information available, 26 (65%) were still on systemic immunosuppression (all patients: 31 of 60; 52%).
Causes of death and survival
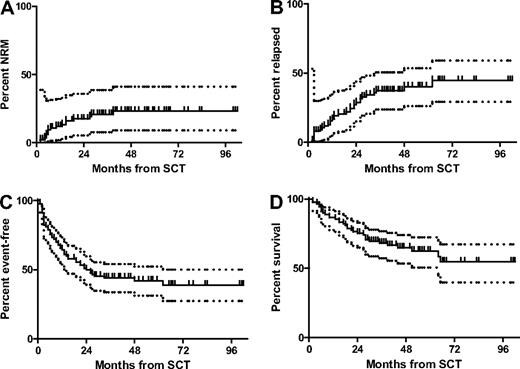
Thirty-three of all allografted patients died, 16 as the result of progressive CLL, and 17 as the result of nonrelapse causes as follows: acute GVHD, 4; chronic GVHD, 5; infection in the absence of GVHD, 5; transplantation-associated microangiopathy, 1; and unknown, 2. NRM and OS at 4 years was 23% (95% CI 9%-41%) and 65% (95% CI 53%-74%), respectively (Figure 2) with a median follow-up of 46 months (7-102 months). A total of 7 of the 10 patients not proceeding to transplant died; 5 as the result of CLL, and 2 as the result of complications of alloSCT off protocol, translating into a 4-year OS of all 100 patients by intent-to-treat of 61% (95% CI 51%-71%).
Outcome of the 90 allografted patients after SCT. NRM (A), relapse incidence (B), EFS (C), and OS (D) of the 90 allografted patients from time of SCT. Dotted lines indicate the 95% confidence intervals.
Outcome of the 90 allografted patients after SCT. NRM (A), relapse incidence (B), EFS (C), and OS (D) of the 90 allografted patients from time of SCT. Dotted lines indicate the 95% confidence intervals.
Disease control and MRD
A complete response after alloSCT was achieved in 61 of 84 patients (73%) with response information available. Seven of these were already in complete remission (CR) at the time of transplantation. Best response was partial response in 18 patients, giving an overall response rate of 94%. Including 4 nonresponders, disease progression or relapse was observed in 31 patients, resulting in a relapse incidence of 40% (95% CI 26%-54%) at 4 years. Taking into account relapse events together with the 17 NRM events, the single secondary malignancy, and the 8 nonengraftment events, 4-year EFS was 42% (95% CI 31%-52%; Figure 2).
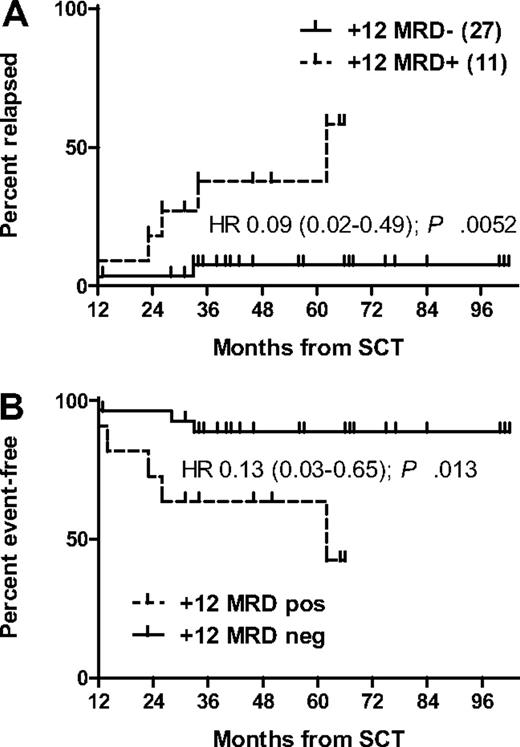
Continuous posttransplant MRD sampling was performed in only 4 centers representing 52 patients. Of the 39 of 52 patients (75%) from this cohort who were event-free 1 year after alloSCT, 27 of 52 (52%) were MRD negative, 11 of 52 (21%) were MRD positive, and 1 of 52 (2%) had no MRD sample available at this landmark. Only 3 events (2 relapses, 1 NRM) occurred in the subset being MRD negative at 12 months after transplant, translating into a strongly reduced risk for EFS compared with the 12-month MRD-positive subset (hazard ratio [HR] 0.13; 95% CI 0.03-0.65; P = .013; Figure 3). Apart from the 2 clinical relapses, only 2 patients of those who were MRD negative at the 12-month landmark became MRD positive again during follow-up, whereas in all others MRD remained undetectable. Despite the high incidence of chronic GVHD, only 12 of 26 MRD-negative patients with information available (46%) were still on systemic immunosuppression at 1 year after transplant.
Outcome according to MRD status 1 year after SCT. Relapse incidence (A) and EFS (B).
Outcome according to MRD status 1 year after SCT. Relapse incidence (A) and EFS (B).
MRD-based GVL response patterns were classified for the 52 patients with MRD monitoring according to the previously published criteria.35 A detailed summary of the results is presented in Table 2. Of note, the patterns reflecting a clear MRD response to immunomodulation (patterns 2 and 5) were both characterized by a very high risk of chronic GVHD (Table 2).
GVL response patterns as defined by MRD kinetics
| Pattern . | pattern description . | n (%) . | Patients with chronic GVHD (% of patients of pattern at risk for chronic GVHD) . | Clinical relapses (% of all patients of pattern) . |
|---|---|---|---|---|
| 0 | Unclassifiable* | 9 (17) | 3 of 7 (43) | 2 of 9 (22) |
| 1 | MRD− immediately after SCT | 11 (21) | 6 of 8 (75) | 1 of 11 (9) |
| 2 | MRD− after immunosuppression tapering | 21 (40)† | 18 of 20 (90)† | 2 of 21 (10)† |
| 3 | MRD− after DLI | 2 (4) | 1 of 2 (50) | 1 of 2 (50) |
| 4 | No MRD response | 4 (8) | 1 of 4 (25) | 3 of 4 (75) |
| 5 | Incomplete and transient MRD response upon immunosuppression tapering | 5 (10)† | 5 of 5 (100)† | 3 of 5 (60)† |
| Total | 52 (100) | 34 of 46 (74) | 12 of 52 (23) |
| Pattern . | pattern description . | n (%) . | Patients with chronic GVHD (% of patients of pattern at risk for chronic GVHD) . | Clinical relapses (% of all patients of pattern) . |
|---|---|---|---|---|
| 0 | Unclassifiable* | 9 (17) | 3 of 7 (43) | 2 of 9 (22) |
| 1 | MRD− immediately after SCT | 11 (21) | 6 of 8 (75) | 1 of 11 (9) |
| 2 | MRD− after immunosuppression tapering | 21 (40)† | 18 of 20 (90)† | 2 of 21 (10)† |
| 3 | MRD− after DLI | 2 (4) | 1 of 2 (50) | 1 of 2 (50) |
| 4 | No MRD response | 4 (8) | 1 of 4 (25) | 3 of 4 (75) |
| 5 | Incomplete and transient MRD response upon immunosuppression tapering | 5 (10)† | 5 of 5 (100)† | 3 of 5 (60)† |
| Total | 52 (100) | 34 of 46 (74) | 12 of 52 (23) |
alloSCT indicates allogeneic stem cell transplantation; DLI, donor lymphocyte infusions; GVHD, graft-versus-host disease; GVL, graft-versus-leukemia; MRD−, negative minimal residual disease; and SCT, stem cell transplantation.
Early death, nonengraftment, or MRD− before alloSCT.
Patterns with clear evidence of MRD response to immunomodulation.
Donor lymphocyte infusions
Altogether 32 fractions of DLI were given to 15 patients for persistent, increasing, or recurring MRD (n = 6; 40%), clinical relapse (n = 8; 53%), or incomplete chimerism (n = 1; 7%). Five patients (3 with MRD and 2 with clinical relapse) responded by achieving MRD-negative CR (supplemental Table 4).
Clinical and genomic prognostic factors
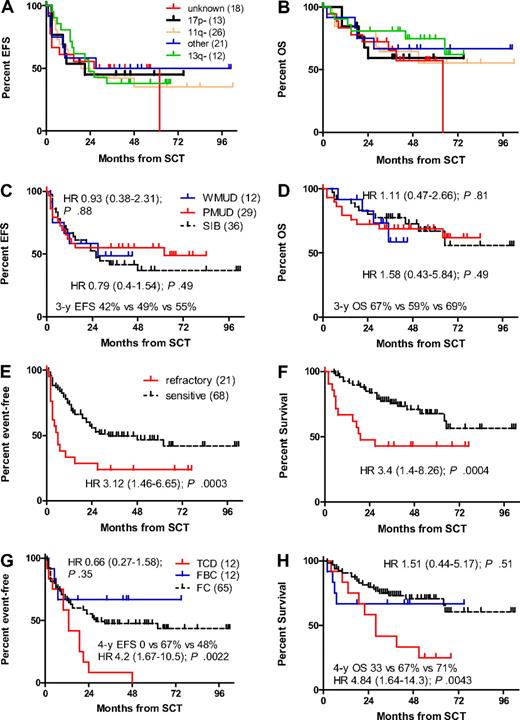
There were no significant differences in terms of EFS and OS among the individual genomic subsets as listed in Table 1 (Figure 4A-B). In particular, the 13 patients with 17p− had no significantly different risk for EFS and OS compared with the 59 patients with other genomic aberrations (HR for EFS 1.01; 95% CI 0.44-2.31; P = .98; HR for OS 1.33; 95% CI 0.45-3.91; P = .6). In contrast, uncontrolled disease at alloSCT and alemtuzumab TCD strongly affected EFS and OS, whereas the time interval from diagnosis to alloSCT (≥ 5 years vs < 5 years), the number of previous regimens (> 3 vs ≤ 3), previous autoSCT, use of alemtuzumab for pretransplant cytoreduction, ECOG score, HCT-CI, BM infiltration at alloSCT, conditioning regimen FBC versus FC, 55 years of age or older, and unrelated donor Weisdorf score did not show significant impact on EFS or OS in univariate comparisons (Table 3; Figure 4C-H).
Impact of pretransplant veriables on survival. FISH karyotype (A-B), donor match (SIB, sibling donor; WMUD, well-matched unrelated donor; PMUD, partially matched unrelated donors; panels C-D), disease status at SCT (E-F), and conditioning regimen (TCD, fludarabine/cyclophosphamide/total body irradiation/in vivo alemtuzumab T-cell depletion; FC, fludarabine/cyclophosphamide; and FBC, fludarabine/busulfan/cyclophosphamide, panels G-H).
Impact of pretransplant veriables on survival. FISH karyotype (A-B), donor match (SIB, sibling donor; WMUD, well-matched unrelated donor; PMUD, partially matched unrelated donors; panels C-D), disease status at SCT (E-F), and conditioning regimen (TCD, fludarabine/cyclophosphamide/total body irradiation/in vivo alemtuzumab T-cell depletion; FC, fludarabine/cyclophosphamide; and FBC, fludarabine/busulfan/cyclophosphamide, panels G-H).
Univariate prognostic factor analyses for allografted patients (log-rank)
| End point/variable . | Univariate analysis (Log-rank) . | |||
|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | |
| Overall survival | ||||
| 17p− vs other karyotypes | .60 | 1.33 | 0.45 | 3.91 |
| Refractory at SCT | < .001* | 3.4* | 1.4* | 8.26* |
| TCD vs FC | .004 | 4.84* | 1.64* | 14.3* |
| FBC vs FC | .51 | 1.51 | 0.44 | 5.17 |
| Age ≥ 55 vs < 55 years | .67 | 1.16 | 0.58 | 2.33 |
| Purine analogue refractory | .091 | 1.82 | 0.91 | 3.63 |
| WMUD vs sibling donor | .81 | 1.11 | 0.47 | 2.66 |
| PMUD vs sibling donor | .49 | 1.58 | 0.43 | 5.84 |
| Previous autoSCT | .54 | 0.8 | 0.39 | 1.64 |
| Previous regimens > 3 vs ≤ 3 | .085 | 1.85 | 0.92 | 3.7 |
| Years from diagnosis to SCT ≥ 5 vs <5 | .14 | 0.59 | 0.3 | 1.19 |
| Alemtuzumab for remission induction | .83 | 1.09 | 0.48 | 2.49 |
| ECOG 0 vs 1 | .78 | 0.92 | 0.50 | 1.69 |
| HCT-CI 0 vs > 0 | .99 | 0.99 | 0.50 | 1.97 |
| BM infiltration at SCT > 50% vs ≤50%* | .058 | 2.16 | 0.98 | 4.78 |
| Event-free survival | ||||
| 17p− vs other karyotypes | .98 | 1.01 | 0.44 | 2.31 |
| Refractory at SCT | .002 | 3.12* | 1.46* | 6.65* |
| TCD vs FC | <.001* | 4.2* | 1.67* | 10.5* |
| FBC vs FC | .35 | 0.66 | 0.27 | 1.58 |
| Age | .53 | 0.84 | 0.48 | 1.46 |
| Purine analogue refractory | .82 | 1.07 | 0.61 | 1.95 |
| WMUD vs sibling donor | .88 | 0.93 | 0.38 | 2.31 |
| PMUD vs sibling donor | .49 | 0.79 | 0.40 | 1.54 |
| Previous autoSCT | .37 | 0.77 | 0.43 | 1.38 |
| Previous regimens > 3 vs ≤ 3 | .36 | 1.31 | 0.73 | 2.33 |
| Years from diagnosis to SCT ≥ 5 vs < 5 | .33 | 0.76 | 0.44 | 1.33 |
| Alemtuzumab for remission induction | .60 | 0.84 | 0.44 | 1.61 |
| ECOG 0 vs 1 | .8 | 0.91 | 0.43 | 1.91 |
| HCT-CI 0 vs >0 | .73 | 0.86 | 0.37 | 1.99 |
| BM infiltration at SCT > 50% vs ≤ 50%* | .86 | 1.09 | 0.40 | 2.93 |
| End point/variable . | Univariate analysis (Log-rank) . | |||
|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | |
| Overall survival | ||||
| 17p− vs other karyotypes | .60 | 1.33 | 0.45 | 3.91 |
| Refractory at SCT | < .001* | 3.4* | 1.4* | 8.26* |
| TCD vs FC | .004 | 4.84* | 1.64* | 14.3* |
| FBC vs FC | .51 | 1.51 | 0.44 | 5.17 |
| Age ≥ 55 vs < 55 years | .67 | 1.16 | 0.58 | 2.33 |
| Purine analogue refractory | .091 | 1.82 | 0.91 | 3.63 |
| WMUD vs sibling donor | .81 | 1.11 | 0.47 | 2.66 |
| PMUD vs sibling donor | .49 | 1.58 | 0.43 | 5.84 |
| Previous autoSCT | .54 | 0.8 | 0.39 | 1.64 |
| Previous regimens > 3 vs ≤ 3 | .085 | 1.85 | 0.92 | 3.7 |
| Years from diagnosis to SCT ≥ 5 vs <5 | .14 | 0.59 | 0.3 | 1.19 |
| Alemtuzumab for remission induction | .83 | 1.09 | 0.48 | 2.49 |
| ECOG 0 vs 1 | .78 | 0.92 | 0.50 | 1.69 |
| HCT-CI 0 vs > 0 | .99 | 0.99 | 0.50 | 1.97 |
| BM infiltration at SCT > 50% vs ≤50%* | .058 | 2.16 | 0.98 | 4.78 |
| Event-free survival | ||||
| 17p− vs other karyotypes | .98 | 1.01 | 0.44 | 2.31 |
| Refractory at SCT | .002 | 3.12* | 1.46* | 6.65* |
| TCD vs FC | <.001* | 4.2* | 1.67* | 10.5* |
| FBC vs FC | .35 | 0.66 | 0.27 | 1.58 |
| Age | .53 | 0.84 | 0.48 | 1.46 |
| Purine analogue refractory | .82 | 1.07 | 0.61 | 1.95 |
| WMUD vs sibling donor | .88 | 0.93 | 0.38 | 2.31 |
| PMUD vs sibling donor | .49 | 0.79 | 0.40 | 1.54 |
| Previous autoSCT | .37 | 0.77 | 0.43 | 1.38 |
| Previous regimens > 3 vs ≤ 3 | .36 | 1.31 | 0.73 | 2.33 |
| Years from diagnosis to SCT ≥ 5 vs < 5 | .33 | 0.76 | 0.44 | 1.33 |
| Alemtuzumab for remission induction | .60 | 0.84 | 0.44 | 1.61 |
| ECOG 0 vs 1 | .8 | 0.91 | 0.43 | 1.91 |
| HCT-CI 0 vs >0 | .73 | 0.86 | 0.37 | 1.99 |
| BM infiltration at SCT > 50% vs ≤ 50%* | .86 | 1.09 | 0.40 | 2.93 |
autoSCT indicates autologous stem cell transplantation; BM, bone marrow; CL, confidence limit; ECOG, Eastern Cooperative Oncology Group; FBC, FC + busulfan; FC, fludarabine-cyclophosphamide; HCT-CI, hematopoietic cell transplantation comorbidity index; HR, hazard ratio; PMUD, partially matched unrelated donor; SCT, stem cell transplantation; SIB, 10 of 10 allele-matched sibling; TCD, FC + total body irradiation 2Gy + in vivo alemtuzumab; WMUD, well-matched unrelated donor.
Significant.
†A total of 60 patients with data available.
Cox modeling considering genomic aberrations (17p− vs other or unknown) together with the covariates purine analogue resistance, donor (sibling vs nonsibling), age (at 1-year increments), TCD with in vivo alemtuzumab, ECOG score, HCT-CI, and disease activity at alloSCT for the endpoints OS, EFS, NRM, and relapse confirmed that refractory disease at alloSCT and alemtuzumab TCD strongly affected EFS, OS, and NRM, whereas greater age was an adverse parameter for EFS and relapse. An ECOG score of 1 was associated with greater NRM. All other covariates including 17p− showed no significant effect on any outcome end point (Table 4). Four-year EFS and OS of those 59 patients who had sensitive disease and received T-replete alloSCT was 55% (95% CI 42%-67%) and 79% (95% CI 65%-89%).
Multivariate prognostic factor analyses for allografted patients (Cox backward; n = 88)
| End point/variable . | Multivariate analysis (Cox backward) . | |||
|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | |
| Overall survival | ||||
| Refractory at SCT | .023 | 2.31 | 1.13 | 4.73 |
| TCD vs no TCD | .024 | 2.44 | 1.13 | 5.27 |
| Event-free survival | ||||
| Refractory at SCT | < .001 | 2.77 | 1.50 | 5.09 |
| TCD vs no TCD | < .001 | 2.75 | 1.42 | 5.33 |
| Age (per year) | .038 | 0.96 | 0.924 | 0.998 |
| Relapse incidence | ||||
| TCD vs no TCD | .060 | 2.45 | 0.967 | 6.21 |
| Age (per year) | .008 | 0.943 | 0.902 | 0.985 |
| Nonrelapse mortality | ||||
| Refractory at SCT | < .001 | 5.06 | 1.96 | 13.12 |
| TCD vs no TCD | .057 | 2.75 | 0.969 | 7.80 |
| End point/variable . | Multivariate analysis (Cox backward) . | |||
|---|---|---|---|---|
| P . | HR . | Lower CL . | Upper CL . | |
| Overall survival | ||||
| Refractory at SCT | .023 | 2.31 | 1.13 | 4.73 |
| TCD vs no TCD | .024 | 2.44 | 1.13 | 5.27 |
| Event-free survival | ||||
| Refractory at SCT | < .001 | 2.77 | 1.50 | 5.09 |
| TCD vs no TCD | < .001 | 2.75 | 1.42 | 5.33 |
| Age (per year) | .038 | 0.96 | 0.924 | 0.998 |
| Relapse incidence | ||||
| TCD vs no TCD | .060 | 2.45 | 0.967 | 6.21 |
| Age (per year) | .008 | 0.943 | 0.902 | 0.985 |
| Nonrelapse mortality | ||||
| Refractory at SCT | < .001 | 5.06 | 1.96 | 13.12 |
| TCD vs no TCD | .057 | 2.75 | 0.969 | 7.80 |
Covariates considered for all end points were 17p− versus other or unknown karyotype; purine analogue-resistant versus purine analogue-sensitive versus untested; age (per year); alemtuzumab TCD; nonsibling donor versus sibling donor; refractory disease at SCT, ECOG 0 versus 1, and HCT-CI 0 versus > 0. Only covariates remaining in the final models are listed.
CL indicates confidence limit; SCT, stem cell transplantation; TCD, FC + total body irradiation 2Gy + in vivo alemtuzumab; WMUD, well-matched unrelated donor.
Long-term outcome of patients with 17p−
Altogether, 7 of 13 allografted patients with 17p− live currently in CR with a median follow-up of 43 months (18-75 months), 6 of these with documented MRD negativity and complete donor chimerism (Table 5), corresponding to a 4-year EFS and OS of 45% (95% CI 17%-73%) and 59% (95% CI 32%-87%), respectively. One event was attributable to a diffuse large cell lymphoma occurring 9 months after alloSCT, which was probably not related to the CLL clone (see Table 5 for details). Considering also the 3 patients who did not proceed to transplant, OS of all enrolled patients with 17p− was 48% (95% CI 23%-73%) at 4 years after study entry.
Outcome of all 16 patients with del 17p− at registration (intent-to-treat)
| UPN . | Age/sex . | FISH karyotype . | PA refractory . | Donor . | Status at SCT . | SCT (regimen) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 005004 | 63/M | del 17p− | Yes | SIB | SD | Yes (FC) | REL +3, died of PD +19 |
| 005008 | 56/M | del 17p− | Yes | NA (UD) | SD | Yes (FBC) | Alive in CR, 46+ mo (MRD− 46+) |
| 011002 | 39/M | del 17p−; del 13q− | No | PMUD | NA | Yes (FC) | REL +3, died of PD +10 |
| 012005 | 58/M | del 17p−; del 13q−; del 11q− | Yes | MMUD | PR2 | Yes (FBC) | Alive in CR, 43+ mo (MRD− 43+) |
| 012007 | 58/F | del 17p−; +12; t(14;?) | Yes | PMUD | PR3 | Yes (FC) | Alive in CR, 38+ mo (MRD− 38+) |
| 012008 | 56/M | del 17p− | No | WMUD | PR5 | Yes (FC) | Alive in CR, 39+ mo (MRD− 39+)* |
| 013001 | 54/F | del 17p− | Untested | PMUD | PR1 | Yes (FC) | Alive in CR, 59+ mo (MRD− 54+) |
| 019002 | 38/F | del 17p− | Untested | NA (UD) | PR3 | Yes (TCD) | REL +21, died of PD +22 |
| 026001 | 49/M | del 17p− | Yes | NA | NA | No | Died of PD (Richter transformation) +6 mo after registration without being transplanted |
| 033003 | 48/F | del 17p−; del 13q− | Yes | PMUD | PR3 | Yes (TCD) | Died of TRM +10 |
| 041008 | 62/M | del 17p− | Yes | NA | NA | No | Died of PD (Richter transformation) +9 mo after registration without being transplanted |
| 125012 | 62/F | del 17p− | Yes | SIB | SD | Yes | REL +3; alive 37+ mo |
| 125014 | 58/M | del 17p−; del 13q− | No | WMUD | PR5 | Yes (FBC) | Alive in CR, 18+ mo |
| 144005 | 51/F | del 17p−; del 11q− | Yes | PMUD | SD | Yes (FBC) | Alive in CR, 75+ mo (MRD− 59+) |
| 144011 | 44/F | del 17p− | Yes | Not found | NA | No | Died of PD +17 mo after registration (no donor) |
| 144010 | 43/M | del 17p−; del 6q− | Untested | SIB | PR2 | Yes (FC) | REL +11, died of PD +24 mo |
| UPN . | Age/sex . | FISH karyotype . | PA refractory . | Donor . | Status at SCT . | SCT (regimen) . | Outcome . |
|---|---|---|---|---|---|---|---|
| 005004 | 63/M | del 17p− | Yes | SIB | SD | Yes (FC) | REL +3, died of PD +19 |
| 005008 | 56/M | del 17p− | Yes | NA (UD) | SD | Yes (FBC) | Alive in CR, 46+ mo (MRD− 46+) |
| 011002 | 39/M | del 17p−; del 13q− | No | PMUD | NA | Yes (FC) | REL +3, died of PD +10 |
| 012005 | 58/M | del 17p−; del 13q−; del 11q− | Yes | MMUD | PR2 | Yes (FBC) | Alive in CR, 43+ mo (MRD− 43+) |
| 012007 | 58/F | del 17p−; +12; t(14;?) | Yes | PMUD | PR3 | Yes (FC) | Alive in CR, 38+ mo (MRD− 38+) |
| 012008 | 56/M | del 17p− | No | WMUD | PR5 | Yes (FC) | Alive in CR, 39+ mo (MRD− 39+)* |
| 013001 | 54/F | del 17p− | Untested | PMUD | PR1 | Yes (FC) | Alive in CR, 59+ mo (MRD− 54+) |
| 019002 | 38/F | del 17p− | Untested | NA (UD) | PR3 | Yes (TCD) | REL +21, died of PD +22 |
| 026001 | 49/M | del 17p− | Yes | NA | NA | No | Died of PD (Richter transformation) +6 mo after registration without being transplanted |
| 033003 | 48/F | del 17p−; del 13q− | Yes | PMUD | PR3 | Yes (TCD) | Died of TRM +10 |
| 041008 | 62/M | del 17p− | Yes | NA | NA | No | Died of PD (Richter transformation) +9 mo after registration without being transplanted |
| 125012 | 62/F | del 17p− | Yes | SIB | SD | Yes | REL +3; alive 37+ mo |
| 125014 | 58/M | del 17p−; del 13q− | No | WMUD | PR5 | Yes (FBC) | Alive in CR, 18+ mo |
| 144005 | 51/F | del 17p−; del 11q− | Yes | PMUD | SD | Yes (FBC) | Alive in CR, 75+ mo (MRD− 59+) |
| 144011 | 44/F | del 17p− | Yes | Not found | NA | No | Died of PD +17 mo after registration (no donor) |
| 144010 | 43/M | del 17p−; del 6q− | Untested | SIB | PR2 | Yes (FC) | REL +11, died of PD +24 mo |
CR indicates complete remission; F, female; FBC, FC + busulfan; FC, fludarabine-cyclophosphamide; FISH, fluorescence in situ hybridization; M, male; MMUD, mismatched unrelated donor; −, negative minimal residual disease; NA, not available; PA, purine analogue; PD, progressive disease; PMUD, partially matched unrelated donor; PR, partial remission; REL, relapse; SCT, stem cell transplantation; SIB, 10/ of 10 allele-matched sibling; TCD, FC + total body irradiation 2Gy + in vivo alemtuzumab; andWMUD, well-matched unrelated donor.
This patient was diagnosed with a diffuse large cell lymphoma (DLCL) 9 months after transplant, which was counted as event in the event-free survival analysis. Although a formal proof of clonal identity with the CLL by sequencing was not performed, the fact that paraffin slides of the DLCL were MRD negative with the CLL-specific primers and that the patient became a durably MRD-negative complete donor chimera at the time of DLCL occurrence suggest that DLCL and CLL were not clonally related.
Discussion
The CLL3X study is one of the largest disease-specific prospective trials on allogeneic transplantation in CLL to date and confirms that RIC alloSCT in a well-defined poor-risk population is feasible in a multicenter setting and has low acute-phase mortality and morbidity. NRM, which cumulates to 23% within the first 2 years, as well as the 4-year EFS and OS figures of 42% and 65%, respectively, is in line with previous studies.22,26-28,39
A specific drawback of the FC regimen used here seems to be the high nonengraftment rate, which affected EFS but did not translate into additional mortality because of autologous recovery of hematopoiesis. Because in CLL an increased risk of engraftment failure also has been observed with myeloablative conditioning by some investigators, it has been suggested that the nonengraftment risk in CLL might be correlated with the degree of marrow involvement.18 Indeed, in our study engraftment failure occurred 3 times more often in patients who had marrow infiltration of 50% or more in comparison with those whose marrow was less affected, translating into an adverse effect of BM infiltration on EFS in univariate analysis (Table 3). However, other variables, such as HLA mismatch and TCD, might also impact the nonengraftment risk, but the number of events was too small for an informative multivariate evaluation of this issue. However, all patients receiving the more intensive FBC regimen engrafted despite high marrow infiltration in 4 of them and use of partially mismatched donors in 5 instances. Because FBC has been associated with substantial toxicity, we are currently exploring escalation of the cyclophosphamide dose40 and the use of more immunosuppressive alkylators.41
When the protocol was designed, it was common policy in the absence of more mature data from RIC studies to exclude patients with significant comorbidity from trials on alloSCT. Therefore relatively few patients with advanced HCT-CI were enrolled in the CLL3X study, making analyses of the impact of this parameter on outcome difficult. However, given the missing or modest adverse effects of HCT-CI scores 1 to 2 on NRM and other outcome parameters observed in this and other trials,28 it seems to be justified to extend the indication for RIC alloSCT as performed here to patients with poor-risk CLL who have an intermediate comorbidity index.
A preplanned secondary objective of the CLL3X trial was to investigate whether the prognostic hierarchical model of recurrent genomic aberrations and especially the adverse impact of 17p− might be overcome by RIC alloSCT. Although few small case series have suggested some efficacy of RIC alloSCT in 17p− CLL,28,29 and a larger registry analysis documented long-term disease control in a proportion of selected patients with 17p− CLL,30 to date there has been no prospective information on the frequency of long-term responses after alloSCT for 17p− CLL. Moreover, comprehensive MRD data as surrogate parameters for profound CLL eradication have been missing for allografted patients with 17p− disease.
Although the CIs are still wide, this first prospective study on the basis of central FISH karyotyping of diagnostic samples obtained at study entry indicates that the hierarchical model loses its prognostic value after alloSCT. In particular, EFS of allografted patients with the otherwise very unfavorable 17p− was not different from patients with other or unknown karyotype. The 4-year EFS of 45% observed here for 17p− patients matches the results from the registry analysis.30 Moreover, the fact that all 6 patients holding the plateau on the relapse curve have been MRD negative at their last follow-up suggests that alloSCT may provide long-term disease control in 17p− CLL. Because of the design of the study, however, this conclusion is limited to patients who are younger than 65 years of age and have a low HCT-CI. However, among the 10 patients not receiving alloSCT, all 3 patients with 17p− could not proceed to transplant because of progression or Richter transformation while waiting for a donor, indicating that the window for successful alloSCT in this subset might be small.
Although all patients conditioned with in vivo TCD had an event, and our results by no means support the use of alemtuzumab TCD, the small number of TCD patients and the nonrandomized assignment to TCD conditioning precludes firm conclusions on this issue. Apart from TCD, the overriding adverse prognostic factor was active disease at the time of transplantation. This finding is in accordance with findings of previous studies,26,27,42 supporting the notion that RIC alloSCT might be best performed before CLL has advanced to a status of complete refractoriness. Because similar to previous reports27,28,42 in our trial the time interval from diagnosis and the number of previous treatment lines did not affect the outcome, “timeliness” of transplant is meant here in a biological rather than in a chronological sense, implying that alloSCT should be considered once the criteria for transplant indication (ie fludarabine resistance or 17p−) have been fulfilled.31 Bulky disease has been identified as prognostic factor in the Seattle series.28 Unfortunately, in our protocol reporting of this parameter was not mandated; nevertheless, it is very likely that bulky disease and CLL resistance at alloSCT are usually strongly correlated.
Because in the original version of the protocol HLA identity was defined as A/B match at the serologic level with DRB1 match at the molecular level and never amended, the study provided the opportunity to retrospectively identify different degrees of HLA-mismatched unrelated donor-recipient combinations and assign them to prognostic matching groups as defined by a study of Weisdorf and coworkers.33 This resulted in a rather large proportion (n = 29) of only partially or even mismatched pairs whose EFS and OS curves were nevertheless virtually identical to that of well-matched pairs or even siblings. Although the overall numbers are still small and some adverse effects of mismatching must be expected from large registry studies,33,43 our results suggest that the use of partially mismatched unrelated donors may be justified in patients with poor-risk CLL if no better match is available.
Another unique feature of the CLL3X trial was central longitudinal quantitative MRD monitoring after transplant. The aims of prospective MRD quantification were primarily to determine the likelihood of achieving MRD negativity, MRD durability, its prognostic impact on clinical outcome, and how MRD kinetics might help to understand patterns of GVL as the key therapeutic principle of alloSCT in CLL.16-18 This issue has been partially addressed in a previous analysis of a subset of the CLL3X study and some patients from a pilot phase.35 The results of the full analysis presented here confirm and extend the preliminary findings, namely that an MRD-negative status 1 year after alloSCT can be achieved in up to 50% of the patients, is durable, and—as also shown by others29,44,45 —predicts for long-term clinical remission. In keeping with our previous analysis,35 a measurable MRD response upon immunomodulatory interventions was associated with chronic GVHD in the majority of cases. However, only 46% of the 27 patients who were MRD-negative at 1 year after transplant were still in need for systemic immunosuppression at that landmark.
Because MRD monitoring was available in real-time, it allowed for preemptive immune intervention in response to the results of MRD assessment. Of note, the 52 patients from the 4 centers that performed MRD monitoring had a substantially and significantly lower relapse risk (HR 0.28; 95% CI 0.12-0.58) and a better EFS (HR 0.31; 95% CI 0.17-0.56) without increase in NRM (HR 0.62; 95% CI 0.23-1.69) in comparison with the 38 patients without MRD follow-up. This effect remained even after adjustment for TCD and disease status. MRD monitoring triggered preemptive DLI in 6 cases, resulting in MRD-negative CR in 3 of them. Moreover, the MRD-monitoring group tended to be less exposed to long-term systemic immunosuppression despite having more chronic GVHD, suggesting that this group was treated with more aggressive taper or reluctance to reinstitution of immunosuppression upon unfavorable MRD results. However, because the immunomodulating interventions upon MRD results were not defined precisely in the study protocol, it remains to be shown that MRD-guided preemptive immunomodulation indeed can improve the outcome of alloSCT for CLL.
Similar to other reports22,26,46 and in contrast to our preliminary data,35 the efficacy of DLI was only modest, with only 2 of 6 preemptive and 2 of 8 therapeutic applications resulting in sustained MRD-negative responses. Although the durable MRD responses to immunosuppression tapering observed in a large proportion of patients (GVL response pattern 2) basically indicate a good susceptibility of CLL to GVL, reduced DLI efficacy may be the result of the “secondary GVL resistance” phenomenon reflected by GVL response pattern 5 (incomplete transient MRD responses) or other GVL escape mechanisms that become effective over time. Therefore, strategies for augmenting DLI activity with additional immunomodulating interventions, such as rituximab,27 need to be explored.
In conclusion, RIC alloSCT from related and well-matched or partially matched unrelated donors can abrogate the prognostic impact of genomic aberrations in patients with poor risk CLL, translating into long-term MRD-negative survival in up to half of the patients independent of the underlying genomic risk profile, history of resistance to purine analogues, and donor source. Prospective trials comparing alloSCT with nontransplantation strategies in defined clinical/biological risk situations by intention-to-treat are warranted to prove whether alloSCT can indeed impact the natural history of aggressive CLL.
Presented in part in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The trial is registered at the US National Cancer Institute (protocol identity no. EU-20 554, NCT00281983).
This work was supported by grants from the Deutsche Jose-Carreras Leukämiestiftung e.V. Projects R02/18, R05/02 (to M.R., P.D., and M.K.).
Authorship
Contribution: P.D. designed and performed research, provided patients and samples, gave administrative support, analyzed data, and wrote the paper; H.D. designed research, gave administrative support, and helped write the paper; M.R. and S.B. contributed analytical tools and analyzed data; R.B. and S.D. analyzed data; D. Bunjes, S.C., J.S., U.H., D. Beelen, M.Z., M.S., J.H., L.U., and C.S. provided patients and samples; A.H., T.Z., and D.W. contributed analytical tools and analyzed data; M.H. provided patients and samples and gave administrative support; M.K. provided patients and samples and gave administrative support, contributed analytical tools, and analyzed data; N.S. designed research; and S.S. designed and performed research, provided patients and samples, gave administrative support, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: P.D. and M.K. were consultants for Roche; S.S. was a consultant for Amgen, Bayer, and Roche. S.B. received honoraria from Roche; S.C. received honoraria from Roche and Schering-Plough; P.D. received honoraria from Bayer, Roche, and Novartis; and S.S. received honoraria from Amgen, Bayer, Celgene, Glaxo Smith-Kline, and Roche. M.K. received research funding from Roche and Novartis; S.B. received research funding from Roche; P.D. received research funding from Bayer; and S.S. received research funding from Amgen, Bayer, Celgene, Glaxo Smith-Kline, and Roche.
A complete list of the German CLL Study Group CLL3X Investigators appears in the Appendix.
Correspondence: Dr. Peter Dreger, Department of Medicine V, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: peter.dreger@med.uni-heidelberg.de.
Appendix: CLL3X investigators
Lutz Uharek, Berlin, Charite Benjamin Franklin; Dietrich Beelen, Essen, Universitätsklinikum; Bertram Glass, Göttingen, Universitätsklinikum; Norbert Schmitz, Hamburg, AK St. Georg; Bernd Hertenstein, Michael Stadler, and Matthias Eder, Hannover, Medizinische Hochschule; Peter Dreger and Ute Hegenbart, Heidelberg, Innere Medizin V; Jörg Schubert, Homburg, Universitätsklinikum; Nadežda Basara, Idar-Oberstein, Klinik für KMT; Martin Gramatzki and Michael Kneba, Kiel, II. Medizinische Klinik; Michael Hallek and Christof Scheid, Köln, Universitätsklinikum; Andreas Burchert, Marburg, Universitätsklinikum;Sandra Cohen, Montreal, Hopital Maisonneuve Rosemont; Dietger Niederwieser, Leipzig, Universitätsklinikum; Ernst Holler, Regensburg, Universitätsklinikum; and Donald Bunjes, Stephan Stilgenbauer, and Hartmut Döhner, Ulm, III. Medizinische Klinik.