Abstract
Cardiac biomarkers provide prognostic information in light-chain amyloidosis (AL). Thus, a novel high-sensitivity cardiac troponin T (hs-TnT) assay may improve risk stratification. hs-TnT was assessed in 163 patients. Blood levels were higher with cardiac than renal or other organ involvement and were related to the severity of cardiac involvement. Increased sensitivity was not associated with survival benefit. Forty-seven patients died during follow-up (22.3 ± 1.0 months). Nonsurvivors had higher hs-TnT than survivors. Outcome was worse if hs-TnT more than or equal to 50 ng/L and best less than 3 ng/L. Survival of patients with hs-TnT 3 to 14 ng/L did not differ from patients with moderately increased hs-TnT (14-50 ng/L), but was worse if interventricular septum was more than or equal to 15 mm. Discrimination according to the Mayo staging system was only achieved by the use of the hs-TnT assay, but not by the fourth-generation troponin T assay. Multivariate analysis revealed hs-TnT, NT-proBNP, and left ventricular impairment as independent risk factors for survival. hs-TnT and NT-proBNP predicted survival, even after exclusion of patients with impaired renal function. Plasma levels of the hs-TnT assay are associated with the clinical, morphologic, and functional severity of cardiac AL amyloidosis and could provide useful information for clinicians on cardiac involvement and outcome.
Introduction
Light-chain amyloidosis (AL) is a plasma cell dyscrasia characterized by extracellular deposition of pathologic insoluble β-fibrillar immunoglobulin light chains in diverse organs. Cardiac and renal involvement occurred in more than half of the patients diagnosed with AL. The extent of cardiac amyloidosis (CA) is the most important determinant of clinical outcome, resulting in a median survival of approximately 6 months without therapy.1,2 Approximately two-thirds of the patients with CA die of sudden death or congestive heart failure within the first year after cardiac symptoms have occurred.3,4 Moreover, CA may be considered one of the most therapeutically refractory forms of heart failure because medical treatment strategies for heart failure are neither effective nor well tolerated and patients are ineligible for treatment approaches that are capable to rapidly stop the production of the amyloidogenic light chains (eg, high-dose melphalan chemotherapy and autologous stem cell support).
Because of the potential impact of cardiac involvement for the prognosis of systemic AL, several parameters describing the cardiac morphology and function have been described for risk assessment.4-8 In addition, cardiac biomarkers (eg, plasma levels of cardiac troponin T and N-terminal pro-brain natriuretic peptide [NT-proBNP]) provided potent prognostic information in patients with AL.9-11 Cardiac troponin T is a highly specific and sensitive marker of myocardial injury,12-14 whereas NT-proBNP may be considered a sensitive indicator of cardiac overload.
Hitherto commercially available cardiac troponin assays (fourth generation) had a limit of detection of 0.01 μg/L with a recommended diagnostic threshold of 0.03 μg/L. Recently, improvements in the technology of cardiac troponin assays have allowed to provide fully automated assays with a limit of detection that is below the 99th percentile of the fourth-generation troponin assays (3 ng/L) in a normal reference population15 with a recommended diagnostic threshold of 50 ng/L. Even minor elevations in patients with acute coronary syndrome are associated with an increased risk of an adverse outcome,16 and the use of the novel high-sensitivity troponin assays in patients with chest pain substantially improved the early diagnosis and risk stratification of acute myocardial infarction by a single troponin measurement.17,18
Thus, it is possible that even minor cardiac troponin elevations below the conventional limit of previous assays improve risk stratification in AL. Therefore, we retrospectively assessed a single measurement of plasma troponin T levels by the high-sensitivity assay (hs-TnT) in 163 patients newly diagnosed with AL to test the significance of the very low cardiac troponin T levels for risk assessment in patients with AL.
Methods
Between December 2005 and November 2008, 163 patients (92 male, 71 female) were assessed at the Heidelberg Amyloidosis Center within 3 months after diagnosis of systemic AL. All patients included in the present analysis had biopsy-proven AL confirmed by Congo red staining and immunohistochemistry of any tissue specimen. In all patients, plasma cell dyscrasia was documented by serum/urine immunofixation electrophoresis and serum free light-chain test (Binding Site GmbH).
Cardiac involvement was defined as an endomyocardial biopsy specimen containing amyloid (n = 41) or by a history of congestive heart failure with myocardial wall thickening on cardiac ultrasound in the absence of arterial hypertension or valvular or coronary disease in a patients with extracardiac-proven AL (n = 61). Patients with extracardiac-proven AL, but no definite exclusion as well as definite diagnosis of cardiac involvement by noninvasive methods, were grouped as suspected cardiac involvement (n = 24). Because of potential risk of pericardial effusion and lack of clinical consequence, endomyocardial biopsies were not performed in this subgroup of patients to obtain definite diagnosis. Further organ involvement was defined according to the guidelines of AL.19
Patients were evaluated by a detailed history, physical examination, standard blood tests (including estimated glomerular filtration rate according to the modified diet in renal disease formula20 ), 12-lead electrocardiography, and echocardiography. Electrocardiography was analyzed for low voltage pattern, which was considered present if no QRS complex deflection was less than 0.5 mV in any limb lead or the sum of the S-wave deflection in lead V1 and R-wave deflection in lead V5-6 was less than 1.5 mV.21 All transthoracic echocardiograms were analyzed for surrogate markers of CA (eg, left atrial diameter, diastolic interventricular septum [IVS] thickness, diastolic posterior wall thickness, end-diastolic left ventricular [LV] cavity diameter, LV end-systolic cavity diameter, LV systolic function, and pericardial effusion).19 LV function was assessed by echocardiography according to standard definitions22 and was considered markedly impaired at less than 45% by the Simpson method (2D/4D). Myocardial mass was computed by the Devereux formula and indexed to body surface area.23 An additional plasma sample of each patient was frozen at −80°C until it was thawed and immediately used for the hs-TnT assay.
Approval for the study was obtained from the Medical University of Heidelberg Institutional Review Board, conforming to the Declaration of Helsinki.24 Informed consent forms were obtained from all patients before the investigation.
Quantification of cardiac biomarkers
Cardiac troponin T was measured using the fourth-generation assay and a novel high-sensitivity assay (Roche Diagnostics) on an ELECSYS 2010 automated analyzer that uses chemiluminescence technology. The interassay coefficient of variation of the high-sensitivity assay is 8% at 10 ng/L and 2.5% at 100 ng/L; the intra-assay coefficient of variation is 5% at 10 ng/L and 1% at 100 ng/L. The diagnostic range of this assay is 3 to 10 000 ng/L.25,26 The lower limit of detection for the fourth-generation assay is 0.01 μg/L. The interassay coefficients of variation were 20% at 0.015 μg/L, 10% at 0.03 μg/L, and 5% at 0.08 μg/L. NT-proBNP was measured using an electrochemiluminescence immunoassay (Elecsys proBNP; Roche Diagnostics). The measurement range extends from 5 to 35 000 pg/mL. The minimal detectable concentration is 5 pg/mL, and the coefficient of variation is 5.7% at 64 pg/mL.
Statistical analysis
Continuous data were expressed as mean plus or minus SEM. Categorical variables were expressed as absolute numbers and percentages. To test for significant differences between means, nonparametric Mann-Whitney U test or analysis of variance with Newman-Keuls post test as appropriate after testing for normal distribution by the Kolmogorov-Smirnov test were used. Linear regression models were used to assess the influence of variables on hs-TnT. All tests were 2-tailed, and a P value of less than 5% was regarded as statistically significant.
Receiver operating characteristic (ROC) curves for diagnosis of CA and all-cause mortality were used to assess the predictive accuracy of hs-TnT on the presence of CA and overall survival. Differences in overall survival, defined as the time between diagnosis and death from any cause, were assessed using log-rank analysis with right censoring. Differences in overall survival were analyzed using univariable and multivariable Cox proportional hazard models and displayed by the Kaplan-Meier product-limit method. In multivariate analyses, the main models were adjusted for variables that were associated with the clinical course of AL patients. A separate multivariate analysis, excluding patients with markedly impaired renal function, has been performed. Statistical analyses were performed using StatView (Version 5.0; SAS Institute).
Results
Patient characteristics
The main characteristics of the 163 AL patients included in the study are shown in detail in Table 1. In more than half of the patients, 3 or more organs were involved at diagnosis of AL. Cardiac involvement was established in 102 (62.6%) and was suspected in a further 24 (14.7%) of the patients. Renal involvement was present in 107 (65.6%) patients. Eighty-two patients (50.3%) had an intraventricular septum (IVS) thickness of more than or equal to 15 mm. Mean troponin T plasma level was 69.7 plus or minus 7.2 ng/L by the hs-TnT assay and 0.07 plus or minus 0.01 μg/L by the fourth-generation troponin assay. A strong correlation between hs-TnT and TnT was observed (r = 0.884; P < .001). Among the 72 patients with negative TnT (< 0.01 μg/L), 33 had hsTnT plasma values above the 99th percentile of the reference population (14 ng/L). Remarkably, only in 10 (6.1%) patients was hs-TnT plasma level lower than the detection limit of the hs-TnT (3 ng/L).
Clinical characteristics of the study patients
| Characteristic . | AL patients (n = 163) . |
|---|---|
| Age, y | 61.0 ± 0.7 |
| Male/female | 92 (56.4%)/71 (43.6%) |
| Body mass index, kg/m2 | 24.7 ± 0.3 |
| Lambda/kappa | 128 (56.4%)/35 (43.6%) |
| Amyloid organ involvement | 2.7 ± 0.1 |
| 1 organ involved | 23 (14.1%) |
| 2 organs involved | 55 (33.7%) |
| 3 or more organs involved | 85 (52.2%) |
| Dominant organ involvement | |
| Kidney | 79 (48.5%) |
| Heart | 64 (39.3%) |
| Liver | 15 (9.2%) |
| Peripheral nervous system | 5 (3.1%) |
| Soft tissues | 14 (8.6%) |
| Gastrointestinal | 7 (4.3%) |
| Clinical evidence of kidney involvement | |
| EGFR, mL × s−1 × 1.73 m−2 | 61.8 ± 2.7 |
| EGFR ≤ 60 mL × s−1 × 1.73 m−2 | 81 (49.7%) |
| EGFR ≤ 30 mL × s−1 × 1.73 m−2 | 39 (23.97%) |
| Dialysis at study inclusion | 27 (16.6%) |
| Clinical evidence of heart involvement | |
| Heart failure NYHA class | 1.7 ± 0.1 |
| NYHA I | 31 (19.0%) |
| NYHA II | 48 (29.5%) |
| NYHA III | 33 (20.2%) |
| NYHA IV | 1 (0.6%) |
| LA diameter, mm | 40.6 ± 0.5 |
| IVS thickness, mm | 15.5 ± 0.3 |
| Myocardial mass index, g × m−2 | 61.7 ± 1.5 |
| Fractional shortening, percentage | 33.2 ± 0.8 |
| Ejection fraction, < 45% | 30 (18.4%) |
| Granular sparkling | 72 (44.2%) |
| NT-proBNP, pg/mL | 7796 ± 1274 |
| Characteristic . | AL patients (n = 163) . |
|---|---|
| Age, y | 61.0 ± 0.7 |
| Male/female | 92 (56.4%)/71 (43.6%) |
| Body mass index, kg/m2 | 24.7 ± 0.3 |
| Lambda/kappa | 128 (56.4%)/35 (43.6%) |
| Amyloid organ involvement | 2.7 ± 0.1 |
| 1 organ involved | 23 (14.1%) |
| 2 organs involved | 55 (33.7%) |
| 3 or more organs involved | 85 (52.2%) |
| Dominant organ involvement | |
| Kidney | 79 (48.5%) |
| Heart | 64 (39.3%) |
| Liver | 15 (9.2%) |
| Peripheral nervous system | 5 (3.1%) |
| Soft tissues | 14 (8.6%) |
| Gastrointestinal | 7 (4.3%) |
| Clinical evidence of kidney involvement | |
| EGFR, mL × s−1 × 1.73 m−2 | 61.8 ± 2.7 |
| EGFR ≤ 60 mL × s−1 × 1.73 m−2 | 81 (49.7%) |
| EGFR ≤ 30 mL × s−1 × 1.73 m−2 | 39 (23.97%) |
| Dialysis at study inclusion | 27 (16.6%) |
| Clinical evidence of heart involvement | |
| Heart failure NYHA class | 1.7 ± 0.1 |
| NYHA I | 31 (19.0%) |
| NYHA II | 48 (29.5%) |
| NYHA III | 33 (20.2%) |
| NYHA IV | 1 (0.6%) |
| LA diameter, mm | 40.6 ± 0.5 |
| IVS thickness, mm | 15.5 ± 0.3 |
| Myocardial mass index, g × m−2 | 61.7 ± 1.5 |
| Fractional shortening, percentage | 33.2 ± 0.8 |
| Ejection fraction, < 45% | 30 (18.4%) |
| Granular sparkling | 72 (44.2%) |
| NT-proBNP, pg/mL | 7796 ± 1274 |
EGFR indicates estimated glomerular filtration rate; NYHA, New York Heart Association; and LA, left atrial.
High-sensitivity cardiac troponin T and cardiac involvement
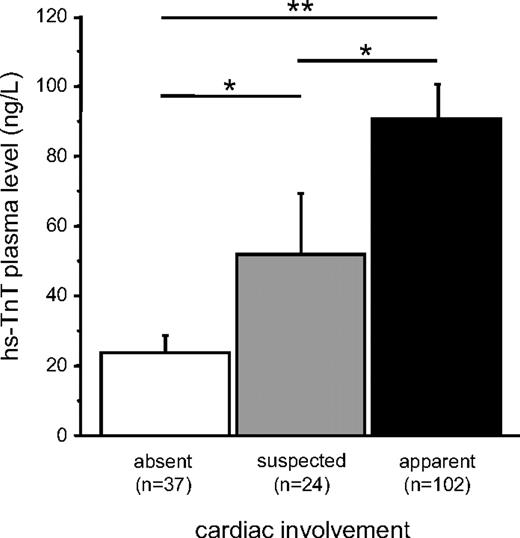
Troponin levels in blood show a consistent increase with AL. Even in the patients without evidence of CA by clinical indices, the median hs-TnT levels were above the 99th percentile of a normal reference population. In patients with suspected CA independent of further organ involvement, mean hs-TnT plasma levels were significantly higher compared with patients without CA, but lower than in patients with CA (Figure 1). Highest levels were observed in patients with cardiac (and additional renal) involvement (Figure 1). Mean plasma hs-TnT levels of patients with isolated renal involvement did not differ from patients with noncardiac organ involvement but were significantly lower than in patients with predominant CA (Figure 2) and increased with deterioration of glomerular filtration rate (Figure 3).
Comparison of hs-TnT plasma levels of patients with light-chain AL: cardiac involvement. ■ indicates apparent cardiac involvement; □, without cardiac involvement; and  , suspected cardiac involvement. Data are mean ± SEM. *P < .05. **P < .01.
, suspected cardiac involvement. Data are mean ± SEM. *P < .05. **P < .01.
Comparison of hs-TnT plasma levels of patients with light-chain AL: cardiac involvement. ■ indicates apparent cardiac involvement; □, without cardiac involvement; and  , suspected cardiac involvement. Data are mean ± SEM. *P < .05. **P < .01.
, suspected cardiac involvement. Data are mean ± SEM. *P < .05. **P < .01.
Comparison of hs-TnT plasma levels of patients with light-chain AL: organ involvement. Sole cardiac (■), sole renal ( ), as well as both cardiac and renal (
), as well as both cardiac and renal ( ) involvement compared with patients with involvement other than heart and kidneys (□). Data are mean ± SEM. *P < .05. **P < .01.
) involvement compared with patients with involvement other than heart and kidneys (□). Data are mean ± SEM. *P < .05. **P < .01.
Comparison of hs-TnT plasma levels of patients with light-chain AL: organ involvement. Sole cardiac (■), sole renal ( ), as well as both cardiac and renal (
), as well as both cardiac and renal ( ) involvement compared with patients with involvement other than heart and kidneys (□). Data are mean ± SEM. *P < .05. **P < .01.
) involvement compared with patients with involvement other than heart and kidneys (□). Data are mean ± SEM. *P < .05. **P < .01.
Comparison of hs-TnT plasma levels of patients with light-chain AL according to the severity of renal impairment assessed by estimated glomerular filtration rate. Data are mean ± SEM. *P < .05. **P < .01. ***P < .001.
Comparison of hs-TnT plasma levels of patients with light-chain AL according to the severity of renal impairment assessed by estimated glomerular filtration rate. Data are mean ± SEM. *P < .05. **P < .01. ***P < .001.
hs-TnT plasma levels were associated with a number of different clinical indices reflecting cardiac AL mass and heart failure severity, such as New York Heart Association functional class (Figure 4A), LV systolic dysfunction (Figure 4B), and IVS thickness (Figure 4C). hs-TnT plasma levels were also higher in patients with more advanced CA as indicated by low voltage pattern (LVP; no LVP, 53.4 ± 7.0 ng/L vs LVP, n = 37; 125.0 ± 20.8 ng/L; P < .001), granular sparkling (n = 72; 90.2 ± 12.8 ng/L vs 56.3 ± 7.9 ng/L; P < .05), or pericardial effusion (n = 54; 99.2 ± 10.5 ng/L vs 58.4 ± 4.7 ng/L; P < .05). The association of plasma hs-TnT levels with the clinical variables is shown in Table 2. These correlations remained significant after adjustment for renal function (data not shown).
Comparison of hs-TnT plasma levels of patients with light-chain AL according to the severity of heart failure. (A) New York Heart Association functional class. (B) Impairment of LV ejection fraction. (C) IVS thickness. Data are mean ± SEM. *P < .05. **P < .01. ***P < .001.
Comparison of hs-TnT plasma levels of patients with light-chain AL according to the severity of heart failure. (A) New York Heart Association functional class. (B) Impairment of LV ejection fraction. (C) IVS thickness. Data are mean ± SEM. *P < .05. **P < .01. ***P < .001.
Correlation of hs-TnT with cardiac parameters in patients with cardiac amyloidosis
| . | Correlation coefficient . | P . |
|---|---|---|
| LA | 0.321 | < .001 |
| IVS | 0.239 | .027 |
| LV-EDD | 0.038 | .641 |
| LV-ESD | 0.073 | .364 |
| LV mass index | 0.371 | < .001 |
| LV dysfunction | 0.300 | < .001 |
| FS | 0.123 | .183 |
| NT-proBNP | 0.706 | < .001 |
| . | Correlation coefficient . | P . |
|---|---|---|
| LA | 0.321 | < .001 |
| IVS | 0.239 | .027 |
| LV-EDD | 0.038 | .641 |
| LV-ESD | 0.073 | .364 |
| LV mass index | 0.371 | < .001 |
| LV dysfunction | 0.300 | < .001 |
| FS | 0.123 | .183 |
| NT-proBNP | 0.706 | < .001 |
LA indicates left atrial diameter; EDD, end-diastolic cavity diameter; ESD, end-systolic cavity diameter; and FS, fractional shortening.
The best cutoff value of hs-TnT for the diagnosis of cardiac involvement in AL was 20.1 pg/L (ROC-area under the curve = 0.80; 95% confidence interval, 0.72-0.88). With this cutoff, sensitivity was 70.3% and specificity was 81.4%. Based on the prevalence of cardiac involvement observed in the present cohort (62.6%), the positive and negative predictive values were 86.4% and 62.1%, respectively.
Ninety-four (57.7%) patients were treated with potentially curative high-dose melphalan chemotherapy and autologous stem cell transplantation within 4.9 plus or minus 0.4 months after diagnosis of AL. These patients had lower hs-TnT plasma levels at diagnosis compared with patients who were deemed ineligible for this treatment (104.6 ± 112.6 ng/L vs 34.6 ± 43.2 ng/L; P < .001).
Survival analyses
Forty-seven patients (28.8%) died during mean overall follow-up of 22.3 plus or minus 1.0 months. The mean follow-up for the survivors was 27.2 plus or minus 1.1 months and 10.7 plus or minus 1.5 months for the deceased patients. Deceased patients had a significantly higher plasma level of hs-TnT at diagnosis than survivors (52.5 ± 79.3 ng/L vs 112.8 ± 109.4 ng/L; P < .001). The hs-TnT cutoff value discriminating survivors and nonsurvivors with highest sensitivity (81.25%) and specificity (59.5%) was 70.9 ng/L. The area under the ROC curve for the death was 0.71 (95% confidence interval, 0.61-0.81). Outcome was worse in patients with plasma levels more than or equal to 50 ng/L and best with plasma levels below the detection limit (< 3 ng/L). Interestingly, survival of the patients was similar survival with plasma hs-TnT from 3 to 14 ng/L compared with patients with hs-TnT plasma levels between 14 and 50 ng/L, and in trend, but not significantly, different from the survival curve of patients with hs-TnT less than 3 ng/L (Figure 5A). Additional information for risk assessment of this subgroup is given by the IVS thickness (Figure 5B). A second analysis excluding patients on dialysis revealed similar survival data (data not shown). Among the patients with troponin T below the 99th percentile of the fourth-generation assay (< 0.01μg/L), an additional 34 patients had a troponin plasma level above the 99th percentile of the hs-TnT assay (> 14 ng/L). Outcome of these patients identified by the hs-TnT assay did not differ from survival of patients with plasma levels below the 99th percentile of the hs-TnT assay. A discrimination of a patient cohort at intermediate risk for survival according to the Mayo staging system10 was only achieved by the use of the hs-TnT assay, but not by the use of the fourth-generation troponin T assay in this independent patient cohort (Figure 6).
Risk stratification of patients with AL amyloidosis by plasma troponin T levels. (A) Association of hs-TnT plasma levels of patients with light-chain AL with survival stratified by 4 subgroups of patients. n.s. indicates not significant. (B) The subgroup of patients with hs-TnT plasma level between 3 and 50 ng/L were stratified by the IVS thickness more than versus less than 15 mm.
Risk stratification of patients with AL amyloidosis by plasma troponin T levels. (A) Association of hs-TnT plasma levels of patients with light-chain AL with survival stratified by 4 subgroups of patients. n.s. indicates not significant. (B) The subgroup of patients with hs-TnT plasma level between 3 and 50 ng/L were stratified by the IVS thickness more than versus less than 15 mm.
Risk stratification of patients with AL amyloidosis by a modified Mayo staging system. Association of troponin plasma levels of patients with light-chain AL with survival according to the Mayo staging system using the fourth-generation (A) or the novel high-sensitivity (B) troponin assay. n.s. indicates not significant.
Risk stratification of patients with AL amyloidosis by a modified Mayo staging system. Association of troponin plasma levels of patients with light-chain AL with survival according to the Mayo staging system using the fourth-generation (A) or the novel high-sensitivity (B) troponin assay. n.s. indicates not significant.
In univariate analysis, age, autologous stem cell transplantation, glomerular filtration rate, LV ejection fraction, New York Heart Association class, NT-proBNP, LVP, myocardial mass index, and pericardial effusion significantly affected overall survival. The Cox univariate model and the multivariate model with the highest prognostic power, according to model validation statistics, are reported in Table 3. The multivariate model with highest statistical power revealed NT-proBNP, hs-TnT, and LV impairment as independent parameters of survival (Table 3, model 1). Both biomarkers remained independent predictors of survival in a separate multivariate analysis, excluding patients with markedly impaired renal function (Table 3, model 2). When troponin plasma levels assessed by the fourth-generation assay were included in the multivariate analysis instead of hs-TnT values, NT-proBNP, troponin T, LV myocardial mass, and glomerular filtration rate were independent predictors of survival at diagnosis of AL (Table 3, model 3).
Univariate and multivariate Cox proportional hazard models for overall survival in cardiac amyloidosis
| . | Univariate . | Multivariate model 1 . | Multivariate model 2 . | Multivariate model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 . | P . | χ2 . | P . | χ2 . | P . | χ2 . | P . | |
| Male sex | 0.502 | .479 | 0.284 | .594 | 0.800 | .371 | 0.370 | .543 |
| Age, y | 4.103 | .043 | 1.982 | .159 | 0.399 | .528 | 1.453 | .228 |
| No. of organs involved | 0.256 | .613 | 0.010 | .919 | 0.005 | .945 | 0.036 | .849 |
| Lambda type of light chain | 1.061 | .303 | 2.171 | .141 | 1.673 | .196 | 2.110 | .146 |
| LV ejection fraction (< 45%) | 18.541 | < .001 | 5.725 | .017 | 0.001 | .992 | 3.010 | .083 |
| NYHA (< 3) | 50.617 | < .001 | 2.267 | .132 | 0.711 | .399 | 2.531 | .112 |
| EGFR | 5.696 | .017 | 3.644 | .054 | 1.210 | .271 | 3.989 | .046 |
| hs-TnT plasma level | 21.402 | < .001 | 6.349 | .012 | 3.930 | .047 | — | — |
| Troponin T (fourth generation) | — | — | — | — | — | — | 6.442 | .011 |
| NT-proBNP plasma level | 17.814 | < .001 | 19.397 | < .001 | 11.487 | < .001 | 15.883 | < .001 |
| Myocardial mass | 7.065 | .008 | 1.800 | .180 | 0.04 | .950 | 3.952 | .047 |
| . | Univariate . | Multivariate model 1 . | Multivariate model 2 . | Multivariate model 3 . | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 . | P . | χ2 . | P . | χ2 . | P . | χ2 . | P . | |
| Male sex | 0.502 | .479 | 0.284 | .594 | 0.800 | .371 | 0.370 | .543 |
| Age, y | 4.103 | .043 | 1.982 | .159 | 0.399 | .528 | 1.453 | .228 |
| No. of organs involved | 0.256 | .613 | 0.010 | .919 | 0.005 | .945 | 0.036 | .849 |
| Lambda type of light chain | 1.061 | .303 | 2.171 | .141 | 1.673 | .196 | 2.110 | .146 |
| LV ejection fraction (< 45%) | 18.541 | < .001 | 5.725 | .017 | 0.001 | .992 | 3.010 | .083 |
| NYHA (< 3) | 50.617 | < .001 | 2.267 | .132 | 0.711 | .399 | 2.531 | .112 |
| EGFR | 5.696 | .017 | 3.644 | .054 | 1.210 | .271 | 3.989 | .046 |
| hs-TnT plasma level | 21.402 | < .001 | 6.349 | .012 | 3.930 | .047 | — | — |
| Troponin T (fourth generation) | — | — | — | — | — | — | 6.442 | .011 |
| NT-proBNP plasma level | 17.814 | < .001 | 19.397 | < .001 | 11.487 | < .001 | 15.883 | < .001 |
| Myocardial mass | 7.065 | .008 | 1.800 | .180 | 0.04 | .950 | 3.952 | .047 |
Multivariate analysis model 1 indicates all patients, hs-TnT; Multivariate analysis model 2, EGFR > 30 mL/min × 1.73, hs-TnT; Multivariate analysis model 3, all patients, fourth-generation assay TnT; —, not applicable; NYHA, New York Heart Association; and EGFR, estimated glomerular filtration rate.
Discussion
Risk stratification of patients with AL (and cardiac involvement) is crucial. In the past, multiple clinical indices have been reported to be useful as indicators of an adverse outcome, such as electrocardiographic, echocardiographic, and laboratory parameters. In the present study, the diagnostic and prognostic power of a novel troponin T assay with higher sensitivity and lower limit of detection was evaluated in 163 consecutive patients with AL. We observed that a single hs-TnT determination at the time of diagnosis provides important information on morphology and function, as well as even prognosis of patients with AL. These findings also point to the clinical significance of very low detectable levels of troponin release (beneath the detection limit of the currently available fourth troponin T assay).
Cardiac troponin T has become the preferred marker for the diagnosis of acute myocardial infarction27 as it correlates with acute myocardial infarction risk and with infarct size.28 Furthermore, even small elevations in patients with non–ST-deviation acute coronary syndrome, as measured by the fourth-generation troponin T assay, are associated with an increased risk of an adverse outcome.16 The use of a novel high-sensitivity cardiac troponin (I and T) assay increases the diagnostic accuracy and discrimination for the early diagnosis of myocardial infarction by a single measurement compared with a conventional troponin T assay and other markers of myocardial necrosis.17,18 In patients with clinically stable coronary artery disease, cardiac troponin T levels measured by the hs-TnT assay were elevated in 21% and significantly associated with the incidence of cardiovascular death and heart failure.29
These data were in line with reports from the ValHeft trial, indicating a dose relationship of a single hs-TnT measurement with adverse outcome in patients with heart failure.30 Here we show another clinical cause of nonischemic troponin elevation, namely, AL. Cardiac involvement was detected in more than half of the patients with AL. The extent of CA is the most important determinant of clinical outcome.1,2 Plasma troponin levels were associated with poor outcome of patients with AL.9,10 Therefore, it seems plausible that cardiac troponin levels below the conventional limit of detection may indicate early cardiac involvement and may further discriminate between subjects at high risk and those at low risk for survival.
High-sensitivity troponin T and severity of cardiac AL
As some of the tools for diagnosis of CA require very much experience for early detection of cardiac involvement, assessment of the severity of CA by a single blood test offers a valuable benefit for clinicians. In the present study, plasma troponin T levels determined by a novel assay with improved sensitivity and lower detection limit were strongly associated with the presence and severity of cardiac involvement. Because of the increased sensitivity, 34 patients with troponin T lower than the detection limit had troponin T above the reference value of healthy reference population by the hs-TnT assay, most probably indicating minor cardiac amyloid deposition.
Interestingly, even in patients with AL but no apparent cardiac involvement by clinical criteria, mean troponin T levels were above the 99th percentile of normal reference populations. This might be explained by the low sensitivity of current clinical indices to detect cardiac abnormalities in AL.1,31 Patients presenting with renal, but no clinical, evidence for cardiac involvement did not reveal significantly higher hs-TnT plasma level than patients with other noncardiac organ involvement. Therefore, even slightly increased hs-TnT might indicate cardiac involvement escaping detection by echocardiography or electrocardiography. Endomyocardial biopsy, which is still regarded as gold standard for diagnosis of CA, was performed in only 25% of the study patients. We recently demonstrated that a large number of patients with normal septum thickness had amyloid depositions in their endomyocardial biopsy specimens.32 Unfortunately, in this study, we could not relate histologic analyses to microelevations of troponin T. This, however, needs further evaluation as improvement in the early diagnosis of cardiac involvement in such patients is of critical importance to initiate causative treatment early (eg, high-dose melphalan chemotherapy and autologous stem cell transplantation).33
As demonstrated in the present study, hs-TnT is associated in concentration-dependent fashion with clinical, morphologic, and functional state of the disease severity in a single parameter. Because of its wide measurement, the excellent reproducibility, and low costs, hs-TnT quantitation appears to be a valuable marker in the clinical routine for the evaluation of patients with AL. Because the extent of cardiac involvement is an adverse factor for autologous stem cell transplantation,34-36 hs-TnT may aid in patient selection to avoid high treatment-related mortality.37
High-sensitivity troponin T and prognosis of patients with AL
Determining levels of circulating cardiac biomarkers has been demonstrated to be a powerful tool for the clinical management of patients with AL.38 The data of the present study demonstrated that even minimally raised plasma cardiac troponins are associated with poorer prognosis of patients with AL that surpassed well-established predictors of survival in patients with AL.4-6,8,39 Interestingly, outcome of patients with any detectable hs-TnT above the detection limit of 3 ng/L but even below the 99th percentile of normal reference populations was associated with a similar outcome as observed in patients with hs-TnT plasma levels between 14 and 50 ng/L. IVS thickness is necessary for risk stratification of these patients with intermediate hs-TnT (3-50 ng/L). Finally, there is a slight, but not significant, difference from the survival curve of patients with hs-TnT less than 3 ng/L, most probably because of the limited patient number in this subgroup. Furthermore, by the use of the hs-TnT, but not fourth-generation troponin T assay in the present analysis, a group of patients with intermediate risk for survival was identified according to the Mayo staging system.10 These differences might be explained because Dispenzieri et al excluded patients evaluated several years ago who did not undergo peripheral blood stem cell transplantation.10
This emphasizes the impact of the use of the hs-TnT assay in patients with AL and indicates that, in such highly selected and well-characterized patients, the hs-TnT method may even be analyzed below the 99th percentile because even the levels in the upper range of a normal distribution seem to carry prognostic information.
Limitations
Many of the AL patients were first referred to our Amyloidosis Center several months after their initial diagnoses. Thus, there might be a bias in the present patient cohort as indicated by a high rate of patients with cardiac involvement in previous studies31 as well as the present study most probably because of the heart transplantation program40 or because of poor health with inability to visit our Amyloidosis Center. To avoid upward bias in the survival curves, only patients with diagnosis within the last 3 months were included in the present study.
The best sensitivity and specificity according to the ROC-area under the curve analysis are low; however, with a decrease in the diagnostic cutoff by implementation of more sensitive and precise assay, sensitivity is further increased, but specificity is decreased because of detection of more acute, subacute, and chronic cardiac diseases.29,30 However, a multitude of differential diagnoses of elevated troponin T levels are excluded in the present study by the preselection of patients with AL; thus, there is no need for a high specificity in contrast to the diagnosis of myocardial infarction when the troponin assay is used as a diagnostic criterion. Serial measurement of cardiac biomarkers for the response to chemotherapeutic treatment is of paramount interest for risk stratification as it has been demonstrated for NT-proBNP11 ; however, the present study evaluated the prognostic value of a single measurement of hs-TnT as it has been demonstrated for patients with stable coronary artery disease29 and stable heart failure.30 The impact of serial measurement of hs-TnT for risk prediction needs to be addressed in future studies.
In conclusion, this is the first report on the use of a novel hs-TnT assay in a large cohort of patients with newly diagnosed AL. Our data show that plasma hs-TnT values in AL patients are invariably associated with the severity of cardiac involvement. In addition, this high-sensitivity assay could provide useful information for clinicians, particularly for the improvement of risk stratification of AL patients according to the Mayo staging system as well as patients with insignificant troponin release, and probably may improve the early diagnosis of CA. Further studies are needed to evaluate hs-TnT plasma levels for monitoring the effect of treatment on cardiac involvement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The excellent technical assistance of Heidi Deigentasch, Erika Müller, Melanie Wusk, and Christa Dewald is gratefully acknowledged.
Authorship
Contribution: A.V.K. analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; E.G. contributed vital new reagents or analytical tools and wrote the manuscript; S.L. analyzed and interpreted data and performed statistical analysis; U.H. performed research and collected data; M.K., D.L., and C.M. collected data; S.H., P.A.S., and C.R. analyzed and interpreted data; S.O.S. and A.D.H. designed research; T.J.D. designed research and wrote the manuscript; and H.A.K. designed research and contributed vital new reagents or analytical tools.
Conflict-of-interest disclosure: E.G. has received financial support for clinical trials from Roche Diagnostics Germany, is a consultant to Roche Diagnostics, and receives honoraria for lectures from Roche Diagnostics. H.A.K. has developed the cTnT assay, holds a patent jointly with Roche Diagnostics, has received grants and research support from several companies, and has received honoraria for lectures from Roche Diagnostics. The remaining authors declare no competing financial interests.
Correspondence: Arnt V. Kristen, Department of Cardiology, Angiology, and Respiratory Medicine, University Hospital Heidelberg, Im Neuenheimer, Feld 410, D-69120 Heidelberg, Germany; e-mail: Arnt_Kristen@med.uni-heidelberg.de.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal