Abstract
Microvesicles (MVs) released by malignant cancer cells constitute an important part of the tumor microenvironment. They can transfer various messages to target cells and may be critical to disease progression. Here, we demonstrate that MVs circulating in plasma of B-cell chronic lymphocytic leukemia (CLL) patients exhibit a phenotypic shift from predominantly platelet derived in early stage to leukemic B-cell derived at advanced stage. Furthermore, the total MV level in CLL was significantly greater compared with healthy subjects. To understand the functional implication, we examined whether MVs can interact and modulate CLL bone marrow stromal cells (BMSCs) known to provide a “homing and nurturing” environment for CLL B cells. We found that CLL-MV can activate the AKT/mammalian target of rapamycin/p70S6K/hypoxia-inducible factor-1α axis in CLL-BMSCs with production of vascular endothelial growth factor, a survival factor for CLL B cells. Moreover, MV-mediated AKT activation led to modulation of the β-catenin pathway and increased expression of cyclin D1 and c-myc in BMSCs. We found MV delivered phospho-receptor tyrosine kinase Axl directly to the BMSCs in association with AKT activation. This study demonstrates the existence of separate MV phenotypes during leukemic disease progression and underscores the important role of MVs in activation of the tumor microenvironment.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) has been predominantly characterized as a clonal B-cell disorder1 in which the defective apoptosis of CLL B cells is ascribed not only to intrinsic defects of the neoplastic cells but also to extrinsic factors that influence their behavior in the tissue microenvironment. The issue of CLL heterogeneity and the exact reasons for the clinical variety of disease progression are unknown. One important factor associated with disease progression is unfavorable prognostic features that may influence apoptotic resistance in the CLL B-cell clone but could be related to the ability of the clone to manipulate the microenvironment to its advantage. A recent study2 demonstrated the importance of communication between tumor cells and their microenvironment through the shedding of membrane microvesicles (MVs), which can fuse to nearby cells within their circulatory pathways.
MVs are shed from the cell surface of normal healthy or malignant cells and can “hijack” membrane components and engulf cytoplasmic contents from either type of cell. The shedding of membrane-derived MVs is a physiologic phenomenon that accompanies cell activation and growth.3 MVs contain numerous proteins and lipids similar to those present in the membranes of the origination cells, and this likely facilitates their integration into cells they come in contact with during circulation.2 The content of MVs and their impact on biologic function are dependent upon the cell of origin.4 Thus, it is known that ovarian cancer MVs stimulate angiogenesis and that platelet-derived MVs promote tumor progression and metastasis of lung cancer cells.5,6 It is likely that a substantial percentage of the so-called soluble receptors identified in biologic fluids or molecules such as DNA or mRNA are in fact associated with circulating MVs.7-9 Given the attributes of the circulating MVs in terms of their ability to transfer their contents to resident tissue cells, we questioned (1) whether CLL plasma contained MV, (2) what their nature was, and (3) if they could influence the bone marrow stromal cells known to have close interactions that lead to both enhanced spontaneous and drug induced resistance of the CLL B cells.
Methods
Isolation of MVs from CLL plasma and cell culture
MVs were isolated as previously described,10 with minor modifications, from the plasma of untreated CLL patients (n = 60) or healthy human subjects (n = 5); each patient provided written informed consent, according to the Declaration of Helsinki, to the Mayo Clinic Institutional Review Board, which approved this study. The plasma samples were made free of platelets and cellular debris by centrifuging at 2500g for 20 minutes (repeated 2 more times). “Platelet-free plasma” was then centrifuged at 16 000g for 1 hour in 4°C to precipitate MVs. After being washed in phosphate-buffered saline, MVs were resuspended in phosphate-buffered saline and stored in 4°C for characterization.
The normal bone marrow stromal cell line (HS-5) and primary CLL B cells were cultured in appropriate growth media.11 Primary bone marrow stromal cells (BMSCs) were isolated from the bone biopsy materials and maintained in vitro as we have previously described.12 For the MV stimulation experiments, serum-starved BMSCs were stimulated with 30 μg/mL MVs for various periods of time as indicated and used for subsequent experiments. Conditioned media were analyzed for cytokines by the use of appropriate enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems.
Reagents
All the antibodies and inhibitors were purchased from Cell Signaling Technologies with the exception of antibodies to vascular endothelial growth factor (VEGF; Santa Cruz Biotechnology); VEGF165b, phospho-Axl (R&D Systems); c-myc; phycoerythrocyanin-conjugated antibodies to CD61, CD31, and CD19; and fluorescence-labeled isothiocyanate-conjugated annexin V (BD Biosciences). Src/Abl kinase inhibitor SKI-606 was purchased from ChemieTek and 1-μm beads from Sigma-Aldrich.
Flow cytometric analysis
MVs were double stained with annexin V and antibody to the platelet/megakaryocyte marker CD61, B-lymphocyte marker CD19, CD31, CD52, or CD20 and analyzed by flow cytometry with the use of 1-μm beads (Sigma-Aldrich) for size.13 Thus, we termed “platelet-derived MV” on the basis of the presence of platelet/megakaryocyte marker CD61 and “B-lymphocyte–derived” on the basis of the presence of CD19 cell-surface marker.
Electron microscopy
MVs were mixed 1:1 with 1% phosphotungstic acid and adsorbed directly onto parlodium-coated 300-mesh copper grids. After 3 brief washes with water, a layer of 1% phosphotungstic acid was added to the grid and allowed to dry before microscopy. Micrographs were acquired by the use of a Technai G2 12 Transmission Electron Microscope (FEI Inc), equipped with an AMT CCD camera system (Advanced Microscopy Techniques).
Labeling of MVs with PKH67 and confocal microscopy
MVs were labeled with a PKH67 green fluorescent labeling kit (Sigma-Aldrich) following the manufacturer's instructions. BMSCs were incubated with the PKH67-labeled MVs for the indicated time. Cells were washed, counterstained with DAPI (VectorShield; Vector Laboratories), and subjected to confocal microscopy (Zeiss LSM 510 confocal laser-scanning microscope). For staining with β-catenin, serum-starved CLL-BMSCs were stimulated with MVs for 48 hours and immunostained with a specific antibody to β-catenin, followed by Alexa-555–conjugated anti–rabbit IgG (Molecular Probes). Cells were counterstained with DAPI and visualized under a confocal microscope.
Immunoblotting
Cells were lysed directly in sodium dodecyl sulfate–Laemmli sample buffer and subjected to Western blot analyses as described previously.14
Phospho-receptor tyrosine kinase antibody array
Equal amounts of cell lysates prepared as described14 from primary CLL-BMSCs stimulated with MVs or left untreated for 24 hours were added to the human phospho-receptor tyrosine kinase (RTK) antibody arrays (R&D Systems), following the manufacturer's instructions. The arrays were scanned and analyzed by densitometry. Relative pixel density was calculated and represented as fold activation.
Statistics
Statistical analysis was performed by the use of a 2-tailed t test.
Results
Characterization of the plasma MVs in CLL patients
We isolated MVs from the plasma of previously untreated CLL patients in various disease stages (Rai 0-IV) as well as healthy human subjects. Flow cytometric analyses demonstrated that the majority of MVs isolated from CLL plasma were positive for annexin V binding (typically, >90%; Figure 1A), and sizes were within 1.0 μm, typical characteristics of MVs,2 on the basis of forward/side scatter with the use of 1.0-μm standard beads.13 These were heterogeneous, membrane-encapsulated small vesicles ranging in size from 0.1 to 1.0 μm as revealed under electron microscopy (Figure 1B), also typical features of MV. Although significantly greater levels (P < .006) of MVs were demonstrable in the majority of CLL plasma (Figure 1C), we could not find a significant association of MV levels with disease stage or other prognostic factors (data not shown). These initial results revealed that CLL patients have increased levels of MVs in their circulation and that they are typical of MVs with heterogeneous size and are positive for annexin V binding.
Identification and characterization of MVs. (A) MVs were identified by evaluating their ability to bind annexin V by flow cytometry. Annexin positivity is shown by histogram from 2 representative MV preparations from CLL plasma. (B) Heterogeneity and sizes of the MVs were determined by electron microscopy after negative membrane staining with phosphotungstic acid (magnification level is indicated by the horizontal bar). (C) Plasma levels of MVs were determined by measuring the total protein content and presented as micrograms of MV per milliliter of plasma (isolated from CLL patients [n = 58] or healthy human subjects [n = 5]). (D-E) MVs isolated from CLL patients at various disease stages as indicated were phenotypically characterized by the use of CD61 for platelet-derived or CD19 for B lymphocyte–derived cell-surface marker by flow cytometry. Results from individual patients are presented with mean values.
Identification and characterization of MVs. (A) MVs were identified by evaluating their ability to bind annexin V by flow cytometry. Annexin positivity is shown by histogram from 2 representative MV preparations from CLL plasma. (B) Heterogeneity and sizes of the MVs were determined by electron microscopy after negative membrane staining with phosphotungstic acid (magnification level is indicated by the horizontal bar). (C) Plasma levels of MVs were determined by measuring the total protein content and presented as micrograms of MV per milliliter of plasma (isolated from CLL patients [n = 58] or healthy human subjects [n = 5]). (D-E) MVs isolated from CLL patients at various disease stages as indicated were phenotypically characterized by the use of CD61 for platelet-derived or CD19 for B lymphocyte–derived cell-surface marker by flow cytometry. Results from individual patients are presented with mean values.
In normal plasma, the majority of MVs have been shown to originate from platelets (80%), whereas the remainder come from endothelium (10%) and leukocytes (10%).15,16 To this end, although we observed a pattern of detecting more platelet-derived (CD61+, a platelet/megakaryocyte antigen) MVs (∼ 84%) in the earliest stage (Rai 0), a wide range of CD61-bearing MVs were demonstrable in the plasma of intermediate stage (Rai I/II) of CLL (Figure 1D). MVs from more advanced stages (Rai III/IV) displayed an average CD61+ level of approximately 50% (Figure 1D). Importantly, we detected relatively high levels (P = .068) of CD19+ MVs in intermediate and significantly greater levels (P < .001) in advanced stages of the disease compared with early stage (Figure 1E). Because the major source of CD19 antigen is the B lymphocyte, and the overwhelming B-cell population in advanced-stage CLL is the leukemic B cell, which expresses CD19 membrane antigen, we assume that the source of the CD19+ MVs is the leukemic B cells (CD19+ MVs also are referred to as “B lymphocyte–derived” in our text). However, we did not observe any clear association between total absolute lymphocyte counts and the levels of plasma MVs (data not shown), suggesting the generation of leukemic cell-derived MVs may depend more on the disease stage as determined by the use of the Rai staging system,17 which incorporates lymph node and spleen involvement with CLL rather than total blood lymphocyte counts. In total, these observations suggested a possible link between MV phenotypes and the progression of disease.
MVs can integrate into leukemic B cells and bone marrow stromal cells
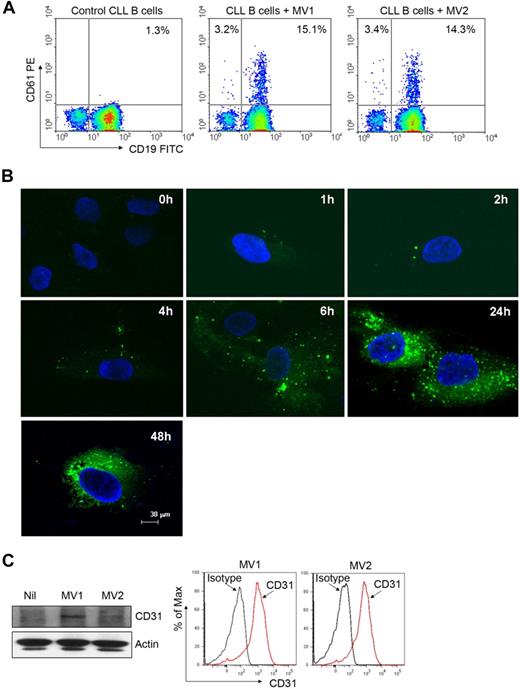
MVs are able to transfer various components of their contents into the membranes of target cells, triggering a variety of biologic responses.2 We first examined whether CLL-MVs could transfer MV-associated surface proteins into target cells. Interestingly, we detected CD61 surface marker on the leukemic B cells after incubation with CD61+ MVs, suggesting transfer of CD61 from MVs into the CLL B cells because these leukemic cells do not express CD61 (Figure 2A). To further examine whether MVs are internalized by target cells, we incubated BMSCs with the fluorescent dye PKH67-labeled MV. Binding of PKH67-MVs to the BMSC surface was detected within an hour, and they were internalized within 1 to 2 hours (Figure 2B). Increased binding and internalization of more PKH67-MVs into the target cells was detected with time (Figure 2B). In addition, we detected CD31 in MV-exposed BMSCs delivered by the MVs (Figure 2C) because BMSCs do not express CD31.18 These results suggest that the plasma CLL-MVs possess the ability to bind and internalize into various target cells and transfer their contents to them.
MVs can integrate into various target cells. (A) Freshly isolated primary CLL B cells were incubated with MVs isolated from different CLL patients for 48 hours. Transfer of CD61 marker from MV to CLL B cells was analyzed by flow cytometry. (B) Purified MVs were labeled with the membrane dye PKH67 (green) and incubated with CLL-BMSCs for the indicated periods. Binding and internalization of MVs into BMSCs was visualized after staining with DAPI (nuclear stain) under confocal microscope (magnification level is indicated by the horizontal bar). (C) MV-mediated transfer of message into BMSCs was examined by Western blot after a 24-hour incubation by the use of an antibody to CD31. Expression of CD31 on MVs was verified by flow cytometry.
MVs can integrate into various target cells. (A) Freshly isolated primary CLL B cells were incubated with MVs isolated from different CLL patients for 48 hours. Transfer of CD61 marker from MV to CLL B cells was analyzed by flow cytometry. (B) Purified MVs were labeled with the membrane dye PKH67 (green) and incubated with CLL-BMSCs for the indicated periods. Binding and internalization of MVs into BMSCs was visualized after staining with DAPI (nuclear stain) under confocal microscope (magnification level is indicated by the horizontal bar). (C) MV-mediated transfer of message into BMSCs was examined by Western blot after a 24-hour incubation by the use of an antibody to CD31. Expression of CD31 on MVs was verified by flow cytometry.
MVs activate bone marrow stromal cells
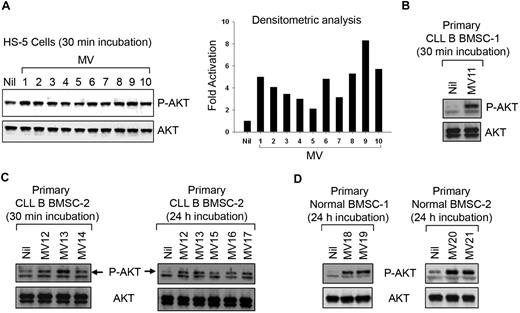
Next, we interrogated the functional implication of the MV-BMSC interaction because of the well-recognized nurturing interaction between stromal cells and leukemic CLL B cells as well as the finding that these MVs can transfer material to the BMSCs. To address this, the normal human BMSC line (HS-5)19 was analyzed for the activation of various survival pathways after incubation with CLL-MVs. Although we could not detect any activation of extracellular signal–regulated kinase 1/2 (ERK1/2), nuclear factor-κB, or signal transducer and activator of transcription 3 (data not shown), a 30-minute exposure to MV resulted in AKT phosphorylation at variable degrees (2- to 8-fold) in HS-5 cells (Figure 3A). We found primary BMSCs isolated from CLL patients or healthy human subjects responded to MVs in a similar fashion (Figure 3B-D). MV-exposed primary BMSCs maintained AKT in a sustained activated state for at least 24 hours (Figure 3C-D). Thus, it appears that CLL-MVs not only interact or are internalized into target cells, but they can keep the AKT signaling pathway in a sustained state of activation in bone marrow stromal cells.
MVs activate human bone marrow stromal cells. (A) MVs isolated from different CLL patients' plasma exhibiting variable degrees of AKT activation in HS-5 cell line as determined by densitometric analysis of the results from Western blot. (B-D) MVs activate primary BMSCs isolated from CLL or healthy human subjects as analyzed by Western blot. A sustained level of AKT activation is detected at least up to 24 hours after incubation with MVs.
MVs activate human bone marrow stromal cells. (A) MVs isolated from different CLL patients' plasma exhibiting variable degrees of AKT activation in HS-5 cell line as determined by densitometric analysis of the results from Western blot. (B-D) MVs activate primary BMSCs isolated from CLL or healthy human subjects as analyzed by Western blot. A sustained level of AKT activation is detected at least up to 24 hours after incubation with MVs.
MVs activate the AKT/mammalian target of rapamycin/p70S6K/hypoxia-inducible factor-1α axis and induce VEGF expression in CLL-BMSCs
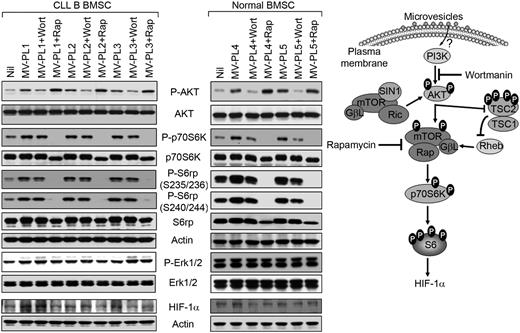
Phosphatidylinositol 3-kinase (PI3K)/AKT is the central regulator of a number of signaling pathways involved in cell survival, proliferation, and angiogenesis.20 The mammalian target of rapamycin (mTOR)/p70S6K is activated by cytokines and growth factors through the activation of its upstream pathways, such as PI3K/AKT and mitogen-activated protein kinase/ERK pathways (see model, Figure 4) and somatic mutations or deletions of the tumor suppressor phosphatase and tensin homolog.21,22 We examined whether the mTOR/p70S6K-pathway was activated in MV-exposed primary BMSCs (Figure 4). A 3-fold activation of AKT was detected in MV-exposed CLL-BMSCs compared with MV-unexposed cells. Treatment with wortmannin (a PI3K inhibitor) inhibited MV-mediated AKT phosphorylation in BMSCs, as expected. However, rapamycin (an mTOR inhibitor) treatment further increased the levels of AKT phosphorylation compared with the cells incubated with MV alone or MV-unexposed cells (∼ 5-fold; Figure 4 top). This finding is consistent with a report that the effects on AKT signaling by rapamycin are complicated by positive and negative feedback loops from mTOR to AKT in different cellular components of the tumor microenvironment.23
MVs activate the AKT/p70S6K/HIF-1α signaling axis in BMSCs. CLL or healthy BMSCs (“normal”) were exposed to MVs with or without presence of wortmannin or rapamycin for 24 hours. BMSC lysates were analyzed for the activation of the AKT/p70S6K signaling pathway as shown by Western blots by the use of specific antibodies. Expression of HIF-1α, a downstream target of AKT/p70S6K axis, also is shown. Actin was used as loading control. MV-mediated modulation of the AKT/p70S6K/HIF-1α axis is shown for better understanding (right).
MVs activate the AKT/p70S6K/HIF-1α signaling axis in BMSCs. CLL or healthy BMSCs (“normal”) were exposed to MVs with or without presence of wortmannin or rapamycin for 24 hours. BMSC lysates were analyzed for the activation of the AKT/p70S6K signaling pathway as shown by Western blots by the use of specific antibodies. Expression of HIF-1α, a downstream target of AKT/p70S6K axis, also is shown. Actin was used as loading control. MV-mediated modulation of the AKT/p70S6K/HIF-1α axis is shown for better understanding (right).
We also detected that MV-exposed CLL-BMSCs displayed phosphorylation of p70S6K at the threonine residue (Thr389) that was not inhibited by wortmannin, suggesting possible cross-talk with other signaling pathways (Figure 4). Although activation of p70S6K involves phosphorylation of multiple serine and threonine residues, phosphorylation of Thr389 by mTOR is critical for p70S6K activation and serves as a marker for mTOR activity.24,25 Importantly, rapamycin completely inhibited MV-mediated activation of p70S6K and phosphorylation at all the serine residues (235/236/240/244) of its downstream target 40S ribosomal protein S6 (S6rp), which were not inhibited by wortmannin (Figure 4). Similarly, we observed activation of AKT in normal BMSCs when exposed to MV, which was inhibited to basal levels by wortmannin (Figure 4). Rapamycin treatment further enhanced AKT activation in healthy BMSCs similar to CLL-BMSC; however, in contrast to CLL-BMSC, wortmannin was able to inhibit AKT-mediated activation of the p70S6K and its downstream targets in healthy BMSCs, whereas rapamycin completely inhibited p70S6K, as expected (Figure 4 right). These findings suggest that MV-mediated modulation of AKT intracellular signaling pathway in healthy BMSCs is different from CLL-BMSCs, which could reflect an inherent functional difference in the 2 types of BMSCs.
To further examine this possibility, we examined ERK1/2 activation in both normal and CLL-BMSCs. ERK is known to activate mTOR through inhibition of tuberin, an essential component of the AKT/mTOR signaling pathway in embryonic kidney cells,26 and induces p70S6K activation.27 We explored whether the p42/44 mitogen-activated protein kinase (ERK1/2) pathway was activated by MVs in primary BMSCs. We found sustained activation of ERK1/2 in MV-exposed CLL-BMSCs from their basal levels but not in normal BMSCs, which remained unaffected after treatment with wortmannin (Figure 4). This finding may explain why activation of p70S6K was not inhibited in MV-exposed CLL-BMSC as a result of AKT inhibition by wortmannin but was in healthy BMSCs.
p70S6K regulates the translation of a group of mRNAs possessing a 5′-terminal oligopyrimidine tract (5′TOP), a stretch of 4 to 14 pyrimidines found at the extreme 5′ terminus of certain mRNAs through phosphorylation of the S6 protein of the 40S ribosomal unit28 that are predicted to account for 15% to 20% of total cellular mRNA. p70S6K has been found to up-regulate general translation capacity of the cells via increased translation of 5′TOP mRNAs.29 The 5′ untranslated region of hypoxia-inducible factor-1α (HIF-1α) mRNA contains 5′TOP tracts, and the translation is dependent on the mTOR/p70S6K pathway in certain cellular contexts.22 Indeed, we detected increased levels of HIF-1α protein synthesis in MV-mediated p70S6K activation in CLL-BMSCs; however, only a subtle, if any, increase in HIF-1α protein was detected in healthy BMSCs after exposure to MVs under similar experimental conditions (Figure 4 bottom). Consistent with this observation, we found that wortmannin had no detectable effect on HIF-1α expression in CLL-BMSCs. However, rapamycin inhibited HIF-1α translation close to the basal levels, suggesting that activation of the mTOR/p70S6K pathway resulted in the production of more HIF-1α in CLL-BMSC even under normoxic conditions.
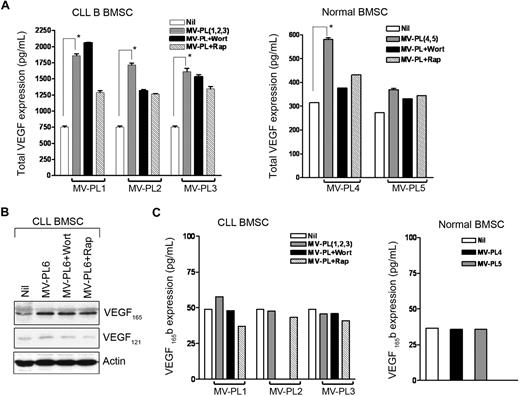
HIF-1α is considered the master regulator of VEGF (VEGF-A), a potent proangiogenic and survival factor for CLL B cells.14 To examine the functionality of HIF-1α expression in CLL-BMSCs under normoxia, we measured the total VEGF production in conditioned media of MV-exposed CLL-BMSCs by ELISA. VEGF production was elevated in the conditioned media but was inhibited by rapamycin, although it was not found to completely inhibit VEGF levels (Figure 5A). This result is probably attributable to the positive regulatory effects of activated AKT on VEGF transcription through Sp1, which is independent of HIF-mediated transcription.30 Although we found an approximately 2-fold increase in total VEGF production in healthy BMSCs exposed to MVs (Figure 5A), it was well below the basal level production of VEGF by the unstimulated CLL-BMSCs (Figure 5A left). We believe this finding is important in CLL B-cell disease progression in that the in vivo programming of CLL-BMSCs to produce more VEGF may result in making them more prone to subsequent MV-mediated regulation than healthy BMSCs. The exact reason why CLL-BMSCs have inherent functional signaling differences than healthy BMSCs remains unclear but could be related to previous in vivo MV exposure.
MVs induce expression of VEGF in CLL-BMSCs. (A) Production of total VEGF in the conditioned media of BMSCs stimulated with MVs as described previously was measured by ELISA and mean values are presented with SDs (pg/mL per 105 cells). *Statistical significance (P < .001) compared with the unstimulated controls. (B) CLL-BMSCs were exposed to MVs with or without presence of wortmannin or rapamycin for 24 hours. Cell lysates were analyzed for the expression of different isoforms of VEGF by Western blot by the use of an antibody to human VEGF. Results indicate that MVs induce expression predominantly of the VEGF165 isoform in CLL-BMSC. Actin was used as loading control. (C) Production of the antiangiogenic isoform VEGF165b in the aforementioned (B) conditioned media of BMSCs stimulated with MVs was measured by ELISA and mean values are presented (pg/mL per 105 cells).
MVs induce expression of VEGF in CLL-BMSCs. (A) Production of total VEGF in the conditioned media of BMSCs stimulated with MVs as described previously was measured by ELISA and mean values are presented with SDs (pg/mL per 105 cells). *Statistical significance (P < .001) compared with the unstimulated controls. (B) CLL-BMSCs were exposed to MVs with or without presence of wortmannin or rapamycin for 24 hours. Cell lysates were analyzed for the expression of different isoforms of VEGF by Western blot by the use of an antibody to human VEGF. Results indicate that MVs induce expression predominantly of the VEGF165 isoform in CLL-BMSC. Actin was used as loading control. (C) Production of the antiangiogenic isoform VEGF165b in the aforementioned (B) conditioned media of BMSCs stimulated with MVs was measured by ELISA and mean values are presented (pg/mL per 105 cells).
The gene for human VEGF is organized into 8 exons.31 As a result of alternative splicing, at least 4 transcripts encoding mature monomeric VEGF containing 121, 165, 189, and 206 amino acid residues (VEGF121, VEGF165, VEGF189, and VEGF206) have been detected. The secretion pattern of the 4 isoforms differs markedly. VEGF121 and VEGF165 are the diffusible proteins secreted by various normal and transformed cells, whereas VEGF165 is the predominant secreted isoform. The VEGF189 and VEGF206, however, are not secreted freely but instead remain predominantly bound to the cell surface and/or extracellular matrix.32 Thus, to identify the predominant VEGF isoform(s) expressed in MV-exposed CLL-BMSCs, we analyzed the lysates of CLL-BMSCs exposed to MV with or without treatment with wortmannin or rapamycin by Western blot by the use of an antibody to human VEGF (Figure 5B). We detected greater expression level of VEGF165 in the MV-exposed CLL-BMSCs compared with that in unexposed control BMSC cells. Importantly, MV-mediated up-regulation of VEGF165 was found to be the predominant isoform compared with the VEGF121 isoform, which was in notably low levels in MV-exposed CLL-BMSCs (Figure 5B). Nonetheless, we could not detect VEGF189 or VEGF206 isoform in these cells under similar experimental conditions. Together, these results suggest that MVs induce expression predominantly of the VEGF165 isoform in CLL-BMSCs.
To further evaluate the nature of the VEGF molecule secreted by the CLL-BMSCs, we looked at 2 variants of VEGF165. VEGF165 is alternatively spliced to form proangiogenic VEGF165 and antiangiogenic VEGF165b; the latter molecule has identical length but a differing C-terminus amino acid residue. The functional consequences of this altered C-terminus are that the VEGF165b homodimer competes with the proangiogenic VEGF165 homodimer for binding to its receptors and thereby inhibits its function.33 Importantly, we could not detect any increase in VEGF165b production in the conditioned media of MV-exposed BMSCs (both CLL and normal) from the basal levels (Figure 5C), suggesting that MV only induce production of the proangiogenic VEGF165.
MV-mediated AKT activation in BMSCs modulates the glycogen synthase kinase 3β/β-catenin pathway
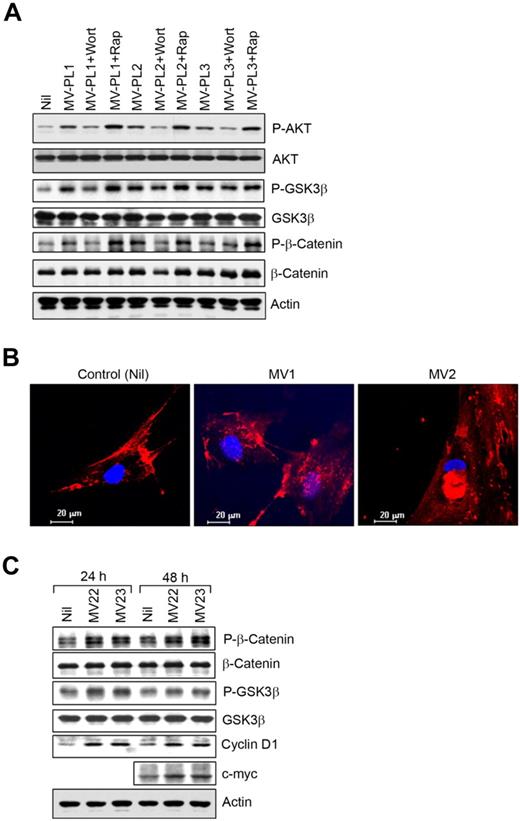
Phosphorylation of glycogen synthase kinase 3 β (GSK3β) at Ser9 residue by AKT results in the autoinhibition of GSK3β.34 We found that AKT mediated the inhibitory phosphorylation of GSK3β at Ser9 in CLL-BMSCs exposed to MVs (Figure 6A). As expected, wortmannin, not rapamycin, inhibited AKT-mediated GSK3β-phosphorylation. GSK3β constitutively phosphorylates β-catenin at both serine and threonine residues in the amino-terminal region, resulting in ubiquitination and subsequent proteasome-mediated degradation of the protein.35 We did not observe any significant accumulation of β-catenin in BMSCs as a consequence of AKT-mediated inhibition of GSK3β (Figure 6A); instead, we found AKT mediated direct phosphorylation of β-catenin (Ser552) in MV-exposed BMSCs (Figure 6A), which was reported to promote β-catenin transcriptional activity.36
MVs modulate GSK3β/β-catenin signaling in BMSCs. (A) The BMSC lysates stimulated with MVs described in Figure 4A were analyzed for AKT-mediated phosphorylation of GSK3β and β-catenin at Ser552 by Western blot by the use of specific antibodies. Phosphorylation of AKT is also shown for comparison. (B) Translocation of β-catenin was visualized in BMSCs stimulated with MVs from CLL patients (MV1 and MV2) by the use of a specific antibody to β-catenin and nuclear stain, DAPI under confocal microscope (magnification level is indicated by the horizontal bar). (C) AKT-mediated phosphorylation of β-catenin at Ser552 and GSK3β was further examined in BMSCs stimulated with MVs for 24 or 48 hours by Western blotting. Expression of cyclin D1 and c-myc, downstream targets of GSK3β/β-catenin, was shown in BMSCs after stimulation with MVs (MV22 and MV23) compared with the unstimulated controls in Western blot analysis.
MVs modulate GSK3β/β-catenin signaling in BMSCs. (A) The BMSC lysates stimulated with MVs described in Figure 4A were analyzed for AKT-mediated phosphorylation of GSK3β and β-catenin at Ser552 by Western blot by the use of specific antibodies. Phosphorylation of AKT is also shown for comparison. (B) Translocation of β-catenin was visualized in BMSCs stimulated with MVs from CLL patients (MV1 and MV2) by the use of a specific antibody to β-catenin and nuclear stain, DAPI under confocal microscope (magnification level is indicated by the horizontal bar). (C) AKT-mediated phosphorylation of β-catenin at Ser552 and GSK3β was further examined in BMSCs stimulated with MVs for 24 or 48 hours by Western blotting. Expression of cyclin D1 and c-myc, downstream targets of GSK3β/β-catenin, was shown in BMSCs after stimulation with MVs (MV22 and MV23) compared with the unstimulated controls in Western blot analysis.
Although wortmannin inhibited AKT-mediated phosphorylation of β-catenin, rapamycin increased levels of β-catenin phosphorylation because of enhanced AKT activation (Figure 6A). We also detected translocation of β-catenin to the nuclei of MV-exposed BMSCs, which is consistent with the phosphorylation of β-catenin at Ser552 and the inhibition of GSK3β (Figure 6B). Importantly, the downstream targets of β-catenin, cyclin D1, and c-myc were up-regulated in BMSCs exposed to MVs (Figure 6C), further suggesting β-catenin was transcriptionally active in MV-exposed BMSCs. Because cyclin D1 is the downstream target of both β-catenin and GSK3β pathways,37 up-regulation of cyclin D1 in MV-exposed BMSCs could be a result of a combined effect from these 2 pathways. These results indicate that MVs not only activate the AKT/mTOR/p70S6K/HIF-1α axis but that they can potentially modulate the AKT/GSK3β or AKT/β-catenin signaling pathways in BMSCs.
MVs activate RTK activity in BMSCs
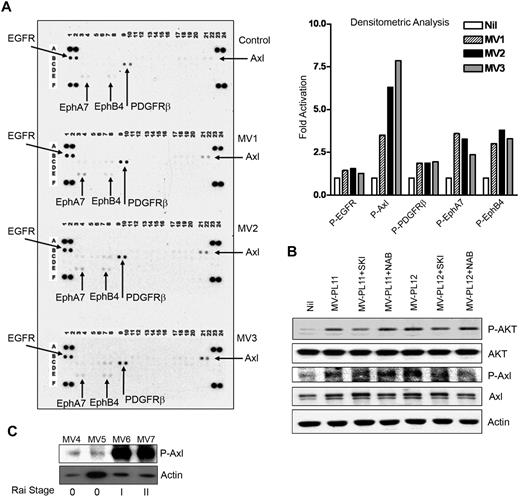
Finally, we interrogated whether MVs interact with specific receptors to initiate and generate signaling pathways which in turn activate AKT. To address this, we analyzed MV-exposed CLL-BMSCs for the activation of any RTK by using a phospho-RTK antibody array blots. Interestingly, MVs from different CLL patients displayed similar patterns of activating epidermal growth factor receptor, platelet-derived growth factor receptorβ, EphA7, and EphB4, with Axl-activation (∼ 4- to 7.5-fold) being the greatest in CLL-BMSCs compared with the unstimulated control (Figure 7A). Axl overexpression has been reported in several types of human cancers, including colon, prostatic, thyroid, breast, gastric, renal, and lung.38 Activation of Axl upon binding its ligand Gas6 (growth arrest-specific gene) results in activation of PI3K and has been implicated in regulating cell survival, proliferation, and migration.39 However, we did not detect any significant expression of Gas6 in the MV-exposed BMSCs (data not shown).
MVs activate RTKs in BMSCs. (A) Activation of RTKs in BMSCs stimulated with MVs obtained from CLL patients at Rai stages 0 (MV1), I (MV2), or II (MV3) was analyzed on human phospho-RTK antibody array blots and the level of activation compared with the unstimulated control BMSCs. Activation of various RTKs was analyzed and presented as fold-activation by bar diagrams. (B) Lysates from BMSCs stimulated with MVs after treatment with the Axl-inhibitor, SKI-606 (SKI) or anti-VEGF neutralizing antibody (NAB) or left unstimulated or untreated were examined for the phosphorylation of AKT by Western blot. Treatment with SKI-606 showed substantial inhibition of MV-mediated activation of AKT; however, anti-VEGF antibody did not show any effect. Phosphorylation of Axl is also shown for comparison. Actin was used as loading control. (C) MVs obtained from CLL patients at various Rai stages (0, I, II) were analyzed for the presence of constitutively active Axl by Western blot with the use of a phospho-specific antibody to Axl. Actin was used as loading control.
MVs activate RTKs in BMSCs. (A) Activation of RTKs in BMSCs stimulated with MVs obtained from CLL patients at Rai stages 0 (MV1), I (MV2), or II (MV3) was analyzed on human phospho-RTK antibody array blots and the level of activation compared with the unstimulated control BMSCs. Activation of various RTKs was analyzed and presented as fold-activation by bar diagrams. (B) Lysates from BMSCs stimulated with MVs after treatment with the Axl-inhibitor, SKI-606 (SKI) or anti-VEGF neutralizing antibody (NAB) or left unstimulated or untreated were examined for the phosphorylation of AKT by Western blot. Treatment with SKI-606 showed substantial inhibition of MV-mediated activation of AKT; however, anti-VEGF antibody did not show any effect. Phosphorylation of Axl is also shown for comparison. Actin was used as loading control. (C) MVs obtained from CLL patients at various Rai stages (0, I, II) were analyzed for the presence of constitutively active Axl by Western blot with the use of a phospho-specific antibody to Axl. Actin was used as loading control.
Recently, it has been shown that Axl is a target of the Src/Abl inhibitor SKI-606, a 3-quinolinecarbonitrile compound.40 We explored the possibility of whether MV-mediated Axl-activation was an upstream event responsible for the activation of AKT in BMSCs by using the inhibitor SKI-606. We found reduction of MV-mediated Axl-phosphorylation levels by SKI-606 with concomitant inhibition of AKT-phosphorylation in BMSC (Figure 7B), suggesting Axl-activation by MV may result in activation of AKT. We did not observe any significant expression of Gas6 in the BMSC lysates with or without stimulation by MV (data not shown). In contrast to a previous report,41 use of anti-VEGF antibody did not exhibit any effect on Axl-phosphorylation in MV-stimulated BMSCs, thus raising the possibility that MVs delivered a constitutively active Axl directly to the BMSCs. We detected low levels of phosphorylated Axl in MVs isolated from early stage/low risk (Rai 0) and greater levels from Rai intermediate stages I/II (Figure 7C). These latter findings suggest that the phosphorylated Axl is likely to be delivered to BMSCs by MVs and seems to be one of the upstream events of AKT activation. Our preliminary work on CLL B cells does in fact show easily detectable constitutively phosphorylated Axl receptor by immunoblot, adding to the possibility that B-cell–derived MVs in CLL plasma may contain Axl (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Discussion
In this study, we found elevated levels of plasma MVs that appear to be predominantly platelet derived in early-stage disease but, with the progression of disease, become more B-lymphocyte–derived. These MVs appear to have potent functional capacity in terms of modulating stromal cells. Thus, we have detected a very complex array of altered signaling pathways of the MV-targeted BMSCs. These results indicate that MVs not only activate the AKT/mTOR/p70S6K/HIF-1α axis but can modulate the AKT/GSK3β or AKT/β-catenin signaling pathways in bone marrow stromal cells. Supplemental Figure 2 is our attempt to summarize these events detailing more of our findings in this model.
Previous work has shown that interaction of MVs with target cells initiates various biologic responses depending on the nature of this interaction.2 When MVs fuse to their target cells, they can transfer important membrane components such as receptors and ligands (supplemental Figure 2B). Thus, MV-mediated transfer of the truncated epidermal growth factor receptor (ie, EGFRvIII) into U373 glioma cells resulted in a consistent increase in ERK1/2 phosphorylation.42 In our studies, CLL-MV–mediated transfer of constitutively active Axl into BMSCs could potentially alter normal cellular function by triggering/reprogramming other signaling cascades that may modulate the tumor microenvironment in favor of disease progression. For example, extracellular VEGFs produced by the MV-stimulated BMSCs may bind and activate platelet-derived growth factor receptors (as reported earlier41 ) on MV-exposed or MV-unexposed neighboring BMSCs or endothelial cells to keep AKT in a state of activation (supplemental Figure 2C). An interesting and important finding was that we detected increased levels of phosphorylated Axl in CLL-MV from higher stages, which suggests the critical involvement of MV in the disease process. Thus, continuous and systemic circulation of MV with interaction via fusion to the BMSC membrane and subsequent transfer of critical signaling moieties to these stromal cells in various tissue sites is likely to favor disease progression.
The consequence of the CLL-MV interaction with BMSCs is that certain key regulators of VEGF production are increased in these cells, including HIF-1α. Angiogenesis likely plays an important role in the pathogenesis of CLL because of 2 known phenomenon: (1) increased neovascularization in the bone marrow and extramedullary tissues43 and (2) the VEGF-based autocrine pathways for the accumulation of leukemic B cells.14 Interestingly, both the microvessel density and hotspot density in bone marrow trephine biopsy sections correlate positively with the clinical stage of the CLL patients.43 We found that CLL-BMSCs are capable of producing more VEGF than their normal counterparts, and MVs preferentially induce production of more VEGF from CLL-BMSCs, which may modulate the CLL microenvironment in favor of CLL survival and resistance to chemotherapy. The study of VEGF secretion here has revealed important differences between the CLL and healthy control sources of BMSC, and we believe these findings imply that there are either some endogenous differences between normal and CLL-BMSCs or these cells have acquired modified signaling pathways that at least relate to VEGF production. Because we see heavily sustained activation of AKT in BMSCs after MV exposure, we postulate that in vivo exposure to increased levels of MV may ultimately result in reprogramming of the BMSCs with these qualities, and this change may in turn facilitate CLL disease progression.
MVs from CLL plasma also may influence disease progression or drug resistance in other ways. Elevated levels of soluble CD52 (alemtuzumab target) and CD20 (rituximab target) have been previously reported in CLL plasma.44,45 Interestingly, we detected that CD52 molecules were bound to the CLL-MVs, and the levels were increased as the disease progressed (supplemental Figure 3A). Although low, CD20 molecules also were detected on circulating CLL-MVs (supplemental Figure 3B). These findings suggest that soluble CD52 and CD20 molecules, known to be present in CLL plasma, are likely to be MV bound. Thus, accumulation of CD52- and CD20-expressing MVs in CLL circulation may potentially bind the specific monoclonal antibodies and contribute to the process of drug resistance to alemtuzumab- and rituximab-based therapies, respectively.
In summary, MVs, regardless of tumor types, can influence the tumor microenvironment in favor of disease progression. Our studies have strongly implicated MVs in the modulation of human stromal cells in B-CLL, in particular with respect to VEGF production. We also have found that because MVs express target receptors for 2 commonly used antibodies for treatment of CLL, these MVs may blunt treatment efficacy. Importantly, we have uncovered several unique aspects of the MV in terms of their inherent ability to modify the stromal cell environment that we believe can be exploited to both better understand disease progression in this currently incurable disease and also suggest novel therapies for these patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Donner Family Foundation for their philanthropic support and Susan McLean (Mayo Clinic) for manuscript preparation. We also thank our CLL patients for their cooperation and help in providing blood specimens for this work.
This work was supported by the National Institutes of Health–National Cancer Institute grant R01 CA116237 (N.E.K.).
National Institutes of Health
Authorship
Contribution: A.K.G., D.M., and N.E.K. designed the experiments and analyzed the data; A.K.G., C.R.S., T.R.K., and W.D. performed the experiments; A.K.G. wrote the paper; and D.M. and N.E.K. edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Neil E. Kay, MD, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: kay.neil@mayo.edu; or Asish K. Ghosh, PhD, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: ghosh.asish@mayo.edu.

![Figure 1. Identification and characterization of MVs. (A) MVs were identified by evaluating their ability to bind annexin V by flow cytometry. Annexin positivity is shown by histogram from 2 representative MV preparations from CLL plasma. (B) Heterogeneity and sizes of the MVs were determined by electron microscopy after negative membrane staining with phosphotungstic acid (magnification level is indicated by the horizontal bar). (C) Plasma levels of MVs were determined by measuring the total protein content and presented as micrograms of MV per milliliter of plasma (isolated from CLL patients [n = 58] or healthy human subjects [n = 5]). (D-E) MVs isolated from CLL patients at various disease stages as indicated were phenotypically characterized by the use of CD61 for platelet-derived or CD19 for B lymphocyte–derived cell-surface marker by flow cytometry. Results from individual patients are presented with mean values.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/9/10.1182_blood-2009-09-242719/4/m_zh89990949200001.jpeg?Expires=1763568750&Signature=0YFG2C4YzjE2ssnJ2b0N8kjRx~ZMkzfXLDH3~-5AExyHjFwXXRyWrzuqnoTh-N6Db5wW6MK8gd~gqx8h9-2AIS3DW1vKT-nVnTkDhF3h35AEuukUiKRLpVktekI7u~YUSiibcJTepZjTrFGaq0iuEYTFGJqhCQQIcdPCZXdrUm~ptxFdF~vOurKTtQjEnXAUaKXwboZrRJInB7oGqeNifOuvcOfUx9h1xEVFjBD8yz8fEWST9ZqH9pKUBVNZ8M7XgnP8BlfgsBYxKEuTXAhuvTTznJeDHN5I9dH1LL9IuBWjHVE6g9cN4tkOwbr6EZ53Qai4JrrszxwUOvIHnJDkMg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal