Abstract
Several clinical studies done with intravenous immunoglobulin (IVIg)–treated autoimmune patients as well as several in vitro studies have revealed that IVIg can reduce polyclonal T-cell activation and modify their cytokine secretion pattern. However, their effect on (auto)antigen-specific T-cell responses has never been addressed directly. In the present work, we used an in vivo model of induction of antigen-specific T-cell responses and an in vitro antigen presentation system to study the effects of IVIg on T-cell responses. The results obtained showed that IVIg inhibited both the in vivo and in vitro antigen-specific T-cell responses but that this effect was the indirect consequence of a reduction in the antigen presentation ability of antigen-presenting cells. The inhibitory effect of IVIg was FcγRIIb-independent, suggesting that IVIg must interfere with activating FcγRs expressed on antigen-presenting cells to reduce their ability to present antigens. Such inhibition of T-cell responses by reducing antigen presentation may therefore contribute to the well-known anti-inflammatory effects of IVIg in autoimmune diseases.
Introduction
Adaptive immune responses are initiated after recognition by T cells of peptides bound to major histocompatibility complex (MHC) molecules expressed on the surface of antigen-presenting cells (APCs).1 This antigen-mediated T-cell activation triggers the differentiation of naive T cells into effector cells, their expansion, and the subsequent activation of other cells of the immune system. In autoimmune diseases, self-reactive T cells become activated after presentation of autoantigens by APCs.2 This activation may be subsequently amplified, as proposed several years ago for immune thrombocytopenia,3-5 by the continuous secretion of autoantibodies that form immune complexes (ICs) with self-antigens, leading to FcγR-mediated internalization by APCs of these ICs and restimulation of autoreactive T cells. Therapeutic approaches that could interfere with this continuous positive feedback loop may therefore lead to rapid clinical improvement in patients affected by acute episodes of autoimmune diseases.
Intravenous immunoglobulins (IVIg) are pharmaceutical preparations of plasma-derived human IgG that have been proven to be beneficial in the treatment of a variety of autoimmune and inflammatory conditions.6,7 Several hypotheses have been proposed to explain their anti-inflammatory effects, including FcγR blockade, neutralization of pathogenic autoantibodies, inhibition of B- and T-cell proliferation, priming of dendritic cell (DC) regulatory activity, and modulation of cytokine expression (reviewed in Nimmerjahn and Ravetch8 ). Although most of these mechanisms were derived from in vitro work or animal models, some clinical studies confirmed that IVIg could decrease the Th1/Th2 ratio in the CD4+ T-cell population9-12 of treated patients or promote induction of Th0 or Th2 anti-inflammatory responses.13-15 Such modulations of T-cell populations and of their cytokine secretion pattern could therefore contribute to the overall anti-inflammatory effects of IVIg by interfering with the pathogenic loop of continuous stimulation occurring in autoimmune diseases.
Several groups of investigators have studied the direct effects of IVIg on T-cell activation, proliferation, and survival using in vitro assays. These studies showed that IVIg inhibited T-cell proliferation or induced apoptosis.16-22 However, other studies reported the absence of effect of IVIg on T cells using alternative experimental conditions, making it difficult to derive strong conclusions on the role of IVIg on T-cell activation, proliferation, and survival.16,23-25 One possible explanation for the discrepant results could be that ex vivo allogeneic or polyclonal activation of purified cells by lectins, mitogens, or anti-CD3 antibodies is different from the physiologic context, leading to in vivo T-cell activation in response to an (auto)antigen. Therefore, to better reflect physiologic conditions, we used an in vivo murine model of ovalbumin (OVA) immunization to determine the effects of IVIg on the induction of antigen-specific T-cell responses. Our results showed that IVIg interfered with the generation of antigen-specific T cells in mice, leading to a significant inhibition of the OVA-specific humoral immune response. Further in vitro experiments revealed that IVIg inhibited T-cell activation, proliferation, and cytokine secretion without directly interacting with these cells. The effect of IVIg was rather mediated through APCs, resulting in inhibition of upstream signals provided by APCs that are required for antigen-specific T-cell activation.
Methods
Animals and cells
Wild-type female BALB/c mice were obtained from Charles River. BALB/c fcgr2b−/− (C.129S4(B6)-Fcgr2btm1TtKN12) and fcrg−/− (C.129P2(B6)-Fcer1gtm1RavN12) mice were obtained from Taconic Farms. DO11.10 (BALB/c-Tg(DO11.10)10Loh/J) mice bearing an OVA-reactive transgenic T-cell receptor (TCR) were purchased from The Jackson Laboratory. Mice were kept at the animal facility at Laval University, and all procedures were approved by the Laval University Animal Care Committee.
The I-Ad–restricted anti-OVA T-cell hybridoma (DO-11.10) was obtained from the European Collection of Cell Cultures. The P388D1 macrophage-like cell line expressing MHC II (H-2 I-Ad)26 was obtained from ATCC. Bone marrow–derived dendritic cells (BMDCs) expressing MHC II (H-2 I-Ad) were generated as described by Lutz et al27 with some modifications. Briefly, femurs and tibiae of wild-type or fcgr2b−/− BALB/c mice were removed, and bone marrow was extracted. Red blood cells were lysed in cold hypotonic ammonium chloride buffer, and 2 to 4 × 106 bone marrow leukocytes were cultured for 7 days in culture medium containing 20 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (PeproTech). BMDCs were purified from the cultures by CD11c+ selection with the MACS Cell Separation system (Miltenyi Biotec). Purity was more than 95% according to CD11c and MHC II expression analyzed using a FACSCalibur flow cytometer and the CellQuest Pro software (BD Biosciences). Fluorescein isothiocyanate–conjugated anti–mouse I-A/I-E (MHC-II) and phycoerythrin-conjugated anti–mouse CD11c were from BD Biosciences. The 2.4G2 antibody was purified by chromatography on protein G-Sepharose (GE Healthcare Bio Sciences) from culture supernatants of 2.4G2 hybridoma cells (obtained from ATCC).
IVIg
IVIg (Gamunex; Talecris Biotherapeutics) were dialyzed extensively before use against RPMI 1640 medium (Invitrogen Canada) at 4°C to remove stabilizing agents. F(ab′)2 fragments of IVIg were prepared by immobilized pepsin digestion according to the manufacturer's instructions (Pierce Protein Research Products; Thermo Fisher Scientific). The recovered F(ab′)2 fragments were free of intact IgG or Fc fragments as evaluated by polyacrylamide gel electrophoresis followed by Coomassie blue staining.28
OVA immunization and antigenic recall
BALB/c mice received 2 subcutaneous injections (day 1 and day 14) of 100 μg of OVA (MP Biomedicals) emulsified in incomplete Freund adjuvant (Sigma-Aldrich Canada) supplemented with 10 μg of CpG B-class ODN (5′ TCGTCGTTTTGTCGTTTTGTCGTT 3′; Integrated DNA Technologies) as described.29 IVIg (50 mg per mouse,30 corresponding to 2.5 g/kg) were injected intraperitoneally on days 0, 1, 2, and 3 and on days 13, 14, 15, and 16. Twenty-eight days after the first immunization, the animals were killed. The OVA-specific antibody titer was determined in the serum by enzyme-linked immunosorbent assay (ELISA) using OVA as capture antigen and a goat antimouse IgG (H+L) horseradish peroxidase–conjugated (Jackson ImmunoResearch Laboratories) to reveal the presence of OVA-specific mouse IgG. The spleens of the animals were recovered to measure interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-10 secretion after in vitro T-cell stimulation with OVA immune complexes (OVA-IC). For OVA-IC preparation, the IgG fraction of whole antiserum from OVA-immunized rabbits (Sigma-Aldrich) was purified on Hi-trap protein G-Sepharose (GE Healthcare Bio-Sciences). A total of 5 μg/mL OVA was then mixed with 100 μg/mL of the purified IgG fraction in culture medium and incubated for 30 minutes at 37°C, as previously described.31 For the in vitro antigenic recall, 1.5 × 106 splenocytes were incubated in culture medium in the presence of 1.66 μg/mL OVA-IC for 3 days at 37°C in a humidified atmosphere containing 10% CO2. IFN-γ, IL-4, and IL-10 secretion was evaluated by ELISA using matched antibody pairs from R&D Systems. In some experiments, OVA was first biotinylated before formation of immune complexes, using EZ-Link Sulfo-NHS-LC-Biotin (Pierce Protein Research Products).
Adoptive transfer experiments
Total splenocytes from DO11.10 mice were stained with 5 μM carboxyfluorescein succinimidyl ester (CFSE; Invitrogen Canada) and were transferred by intravenous injection to naive BALB/c mice. In vivo stimulation of OVA-specific CD4+ T cells from DO11.10 mice was performed 16 hours after cell transfer, by intravenous injection of 0.1 μg of OVA-IC in recipient BALB/c mice. Two prophylactic injections of IVIg were performed at 16 hours (2.5 g/kg IVIg) and 2 hours (0.75 g/kg IVIg) before OVA-IC injection. Three days later, spleens of recipient BALB/c mice were recovered and proliferation of OVA-specific CD4+ T cells was assessed by gating on CD4+ cells using an anti–mouse CD4-APC conjugate (BD Biosciences) and evaluating the extent of CFSE dilution by flow cytometry. Alternatively, the KJ1-26+ cell numbers were measured by flow cytometry using the KJ1-26 monoclonal antibody (mAb) conjugated to phycoerythrin (BD Biosciences).
Antigen presentation assay
P388D1 cells were activated with 100 IU/mL IFN-γ, 24 hours before the presentation assay. A total of 5 × 104 IFN-γ–activated P388D1 cells or BMDCs were incubated with 105 DO-11.10 hybridoma cells in the presence of 1.66 μg/mL OVA-IC and dialyzed IVIg for 24 hours at 37°C in a humidified atmosphere containing 10% CO2. IL-2 production by DO-11.10 cells was measured in supernatants by ELISA, using matched antibody pairs (R&D Systems). In some assays, OVA-specific CD4+ T cells were purified from spleen of DO11.10 mice by negative selection using the EasySep separation system (StemCell Technologies) and stained with 5μM CFSE. The purified CD4+ T cells (105) were then added to 5 × 104 APC in the presence of OVA-IC and IVIg. OVA-specific T-cell proliferation was evaluated 72 hours later by measuring the extent of CFSE dilution. In other assays, APCs were first incubated with 1.66 μg/mL OVA-IC during 24 hours followed by fixation fixed with paraformaldehyde diluted to 1% in RPMI 1640 medium for 10 minutes and washed with 3.6% D-lysine-RPMI buffer before the presentation assay.
CD3/CD28 stimulation
Splenocytes (106 cells) from BALB/c mice were stained with 5 μM CFSE and activated with Dynabeads Mouse CD3/CD28 T cells expander (Invitrogen; ratio of 1 bead per T cell) for 24 to 72 hours. IL-2 secretion was measured after 24 hours by ELISA and CD4+ T-cell expansion was measured after 72 hours by evaluating the extent of CFSE dilution by ELISA.
Cell-surface expression of MHC II, CD80, and CD86
BMDCs (105 cells) were incubated with 10 mg/mL IVIg or RPMI 1640 medium (negative control) for 48 hours. The cell-surface expression of MHC II, CD80, and CD86 was evaluated by flow cytometry using fluorescein isothiocyanate–conjugated anti–mouse I-A/I-E, CD80, and CD86 antibodies (BD Biosciences).
Statistical analysis
An unpaired Student t test was used to determine the statistical significance of the data. The level of significance was set at P values less than .05. Some data were also analyzed using the Wilcoxon-Mann-Whitney test.
Results
Effect of IVIg on the in vivo generation of antigen-specific T cells
The effect of IVIg on the in vivo generation of antigen-specific T cells was evaluated using naive BALB/c mice immunized with OVA and treated or not with IVIg. The presence of OVA-specific T cells 28 days after immunization was evaluated by performing an in vitro antigenic recall with OVA-IC on spleen cells and by measuring the amount of IFN-γ (Th1 cytokine) and IL-4 and IL-10 (Th2 cytokines) secreted after 3 days. The results (Figure 1A) showed the secretion of both Th1 and Th2 cytokines in control animals, indicating the presence of OVA-specific T cells. Cytokine secretion was at least 2 times lower for T cells recovered from IVIg-treated mice, suggesting that the presence of IVIg during the immunization phase interfered with the generation of OVA-specific T cells.
Effect of IVIg on the generation and activation of OVA-specific T cells. (A) Groups of 4 mice were immunized with OVA and treated or not with IVIg, as described in “OVA immunization and antigenic recall.” Spleens were recovered after 28 days, and the splenocytes (10 × 106 cells/mL for the secretion of IFN-γ and IL-4 and 5 × 106 cells/mL for IL-10) were stimulated in vitro with OVA-IC. After 3 days, the presence of OVA-specific T cells was evaluated by measuring the secretion of IFN-γ, IL-4, and IL-10 using ELISA. (B) Groups of 4 mice were immunized with OVA in the absence of IVIg. Spleens were recovered after 28 days, and the splenocytes were stimulated in vitro for 3 days with OVA-ICs, in the presence or not of 10 mg/mL IVIg. OVA-specific T-cell activation was evaluated by measuring cytokine secretion by ELISA. The results are representative of 2 independent experiments. *P < .001.
Effect of IVIg on the generation and activation of OVA-specific T cells. (A) Groups of 4 mice were immunized with OVA and treated or not with IVIg, as described in “OVA immunization and antigenic recall.” Spleens were recovered after 28 days, and the splenocytes (10 × 106 cells/mL for the secretion of IFN-γ and IL-4 and 5 × 106 cells/mL for IL-10) were stimulated in vitro with OVA-IC. After 3 days, the presence of OVA-specific T cells was evaluated by measuring the secretion of IFN-γ, IL-4, and IL-10 using ELISA. (B) Groups of 4 mice were immunized with OVA in the absence of IVIg. Spleens were recovered after 28 days, and the splenocytes were stimulated in vitro for 3 days with OVA-ICs, in the presence or not of 10 mg/mL IVIg. OVA-specific T-cell activation was evaluated by measuring cytokine secretion by ELISA. The results are representative of 2 independent experiments. *P < .001.
The effect of IVIg on antigen-mediated T-cell activation was next studied using total splenocytes recovered from mice 28 days after OVA immunization and incubated with OVA-IC in the presence or absence of 10 mg/mL IVIg for 72 hours. The 10 mg/mL dose of IVIg was selected because it is equivalent to the therapeutic dose used for the treatment of autoimmune diseases in humans and also corresponds to the dose used in the in vivo experiments presented in Figure 1A (1-2 g/kg). The results obtained showed that the presence of IVIg during in vitro OVA-IC stimulation of splenocytes from OVA-immunized mice resulted in a decrease in cytokine secretion of approximately 2 to 3 times (Figure 1B), indicating that IVIg inhibited the activation of antigen-specific T cells.
Effect of IVIg on the in vivo antigen-specific T-cell proliferation
To study the effects of IVIg on the in vivo proliferation of antigen-specific T cells, spleen cells from DO11.10 transgenic mice were used. All CD4+ T cells from these transgenic mice are responsive to OVA presentation in an MHC II–restricted context. After staining with CFSE, 3 to 4 × 106 DO11.10 spleen cells were injected intravenously into H2d-compatible BALB/c mice (each mouse received the same number of cells), 16 hours before in vivo stimulation with 0.1 μg of OVA-IC. The mice also received injections of IVIg before OVA-IC stimulation, as described in “Adoptive transfer experiments.” Proliferation of the transferred CFSE-stained CD4+ T cells was evaluated in the spleen of recipient mice, 3 days after OVA-IC stimulation. The results (Figure 2A) showed that CFSE fluorescence was much reduced in CD4+ T cells obtained from OVA-stimulated control (mean fluorescence intensity [MFI] of 62) compared with unstimulated mice (MFI of 225), indicating that extensive cell division occurred after OVA-IC stimulation. However, the decrease in CFSE fluorescence in OVA-stimulated cells from IVIg-treated mice was significantly less extensive (MFI of 93), indicating that the proliferation of OVA-specific CD4+ T cells was reduced in IVIg-treated animals. The extent of OVA-specific T-cell proliferation was also determined using a mAb (KJ1-26) specifically reacting with the TCR expressed by CD4+ T cells of DO11.10 mice.32 The number of KJ1-26+ cells was approximately 2 times lower in spleens of OVA-stimulated IVIg-treated mice compared with OVA-stimulated control mice (Figure 2B), in agreement with the results obtained for CD4+ cells by CFSE dilution analysis. When human serum albumin (2.5 g/kg) was used as protein control, there was no reduction in OVA-mediated T-cell proliferation in the spleens of adoptively transferred mice as evaluated using the KJ1-26 mAb (data not shown), indicating that the effect observed was specific to IVIg.
Effect of IVIg on the in vivo OVA-specific T-cell proliferation. A total of 5 × 106 CFSE-stained spleen cells from DO11.10 transgenic mice were transferred to naive BALB/c mice. Mice (n = 4) were treated or not with IVIg before stimulation with OVA-ICs. OVA-specific T-cell expansion in the spleen of recipient BALB/c mice was determined 3 days later (A) by measuring the CFSE fluorescence intensity of splenic CD4+ cells by flow cytometry and (B) by evaluating of the number of OVA-specific T cells using the KJ1-26 mAb specific for the TCR expressed by DO11.10 mice. Results are representative of 3 independent experiments. *P < .001.
Effect of IVIg on the in vivo OVA-specific T-cell proliferation. A total of 5 × 106 CFSE-stained spleen cells from DO11.10 transgenic mice were transferred to naive BALB/c mice. Mice (n = 4) were treated or not with IVIg before stimulation with OVA-ICs. OVA-specific T-cell expansion in the spleen of recipient BALB/c mice was determined 3 days later (A) by measuring the CFSE fluorescence intensity of splenic CD4+ cells by flow cytometry and (B) by evaluating of the number of OVA-specific T cells using the KJ1-26 mAb specific for the TCR expressed by DO11.10 mice. Results are representative of 3 independent experiments. *P < .001.
Effect of IVIg on the in vivo antigen-specific B-cell responses
We next evaluated the effect of IVIg on the production of antigen-specific antibodies in OVA-immunized mice, 28 days after the first immunization. The mean anti-OVA antibody titer was significantly higher in control mice compared with mice treated with IVIg during the immunization period (Figure 3). More precisely, 4 of 7 control mice, but none of the 7 IVIg-treated mice, had anti-OVA titers higher than 120 000. The decrease in OVA-specific antibody secretion in IVIg-treated immunized animals was therefore consistent with the effects of IVIg on antigen-specific T-cell generation and activation. Altogether, these results indicated that IVIg could decrease the in vivo antigen-specific T-cell response and the subsequent production of antigen-specific antibodies.
Effect of IVIg on the in vivo antigen-specific B-cell response. Anti-OVA IgG titers were measured in the serum of OVA-immunized mice treated (n = 7) or not (n = 7) with IVIg, 28 days after the first immunization. The results were compared using the Wilcoxon-Mann-Whitney 2-sample rank-sum test. The median values in the IVIg-treated and control groups were 62 424 and 119 258, respectively. The analysis confirmed that IVIg treatment resulted in significantly lower OVA-specific antibody titers compared with the control group (Mann-Whitney U = 14, n1 = n2 = 7, α = 0.05). The anti–human IgG response was also determined in a similar ELISA, using IVIg instead of OVA as capture antigen and revealed the absence of a significant anti–human IgG response in all mice treated with IVIg at the dilutions tested (titers < 1000).
Effect of IVIg on the in vivo antigen-specific B-cell response. Anti-OVA IgG titers were measured in the serum of OVA-immunized mice treated (n = 7) or not (n = 7) with IVIg, 28 days after the first immunization. The results were compared using the Wilcoxon-Mann-Whitney 2-sample rank-sum test. The median values in the IVIg-treated and control groups were 62 424 and 119 258, respectively. The analysis confirmed that IVIg treatment resulted in significantly lower OVA-specific antibody titers compared with the control group (Mann-Whitney U = 14, n1 = n2 = 7, α = 0.05). The anti–human IgG response was also determined in a similar ELISA, using IVIg instead of OVA as capture antigen and revealed the absence of a significant anti–human IgG response in all mice treated with IVIg at the dilutions tested (titers < 1000).
Mechanism of inhibition of antigen-specific T-cell responses by IVIg
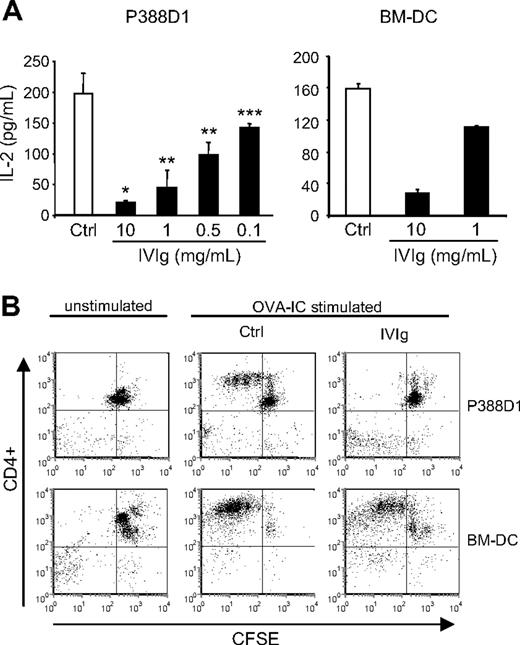
To better study the mechanisms by which IVIg can affect the in vivo antigen-specific T-cell responses, we used an in vitro assay of antigen presentation. The assay was done using the OVA-specific T-cell hybridoma (DO-11.10) and IFN-γ–stimulated P388D1 cells or BMDCs as APCs. DO-11.10 cells were incubated for 24 hours with APC and OVA-IC in the presence or absence of IVIg, and IL-2 secretion was evaluated as a measure of T-cell activation. The results first showed the secretion of significant levels of IL-2 in controls done in the presence of OVA-IC for both types of APCs (Figure 4A). In the absence of OVA-IC, IL-2 secretion did not reach measurable levels (the detection limit of the assay is 4 pg/mL; data not shown). The results further revealed a dose-dependent reduction in IL-2 secretion in the presence of IVIg for both types of APCs. The reduction was already apparent at a dose of 0.1 mg/mL IVIg for P388D1 cells, whereas a dose of 10 mg/mL IVIg resulted in a reduction in IL-2 secretion of more than 95% for P388D1 cells and of approximately 80% for BMDCs. The effect of IVIg on the in vitro antigen-specific T-cell activation was alternatively studied using CFSE-stained OVA-specific CD4+ T cells isolated from spleens of transgenic DO11.10 mice in coculture with APCs and OVA-IC, in the presence or absence of 10 mg/mL IVIg. T-cell proliferation was evaluated by flow cytometry analysis of CFSE dilution, after 3 days of culture. The results (Figure 4B) showed a more important dilution of CFSE staining for the control condition (upper quadrants, from right to left) compared with IVIg-treated cells, indicating that IVIg interfered with the OVA-specific activation of CD4+ T cells by both types of APCs. Therefore, IVIg inhibited the in vitro antigen-mediated T-cell activation of normal (decreased proliferation) and transformed (decreased IL-2 secretion) cells, in agreement with our previous in vivo observations.
Effect of IVIg on the in vitro antigen-specific T-cell activation. (A) OVA-specific DO-11.10 hybridoma cells were cultured for 24 hours with IFN-γ–stimulated P388D1 cells or BMDCs in the presence of absence of IVIg and OVA-IC. DO-11.10 cell activation was evaluated by measuring the IL-2 secretion using ELISA. (B) CD4+ T cells isolated from DO11.10 transgenic mice were stained with CFSE and cultured with IFN-γ–stimulated P388D1 cells or BMDCs in the presence or absence of IVIg (10 mg/mL) and OVA-ICs. Proliferation of OVA-specific CD4+ T cells was evaluated after 3 days, by flow cytometric analysis of CFSE dilution. The percentage of proliferating CD4+ T cells is shown in the upper left quadrant. Results are representative of 3 independent experiments. *P < .001; **P < .01; ***P < .05.
Effect of IVIg on the in vitro antigen-specific T-cell activation. (A) OVA-specific DO-11.10 hybridoma cells were cultured for 24 hours with IFN-γ–stimulated P388D1 cells or BMDCs in the presence of absence of IVIg and OVA-IC. DO-11.10 cell activation was evaluated by measuring the IL-2 secretion using ELISA. (B) CD4+ T cells isolated from DO11.10 transgenic mice were stained with CFSE and cultured with IFN-γ–stimulated P388D1 cells or BMDCs in the presence or absence of IVIg (10 mg/mL) and OVA-ICs. Proliferation of OVA-specific CD4+ T cells was evaluated after 3 days, by flow cytometric analysis of CFSE dilution. The percentage of proliferating CD4+ T cells is shown in the upper left quadrant. Results are representative of 3 independent experiments. *P < .001; **P < .01; ***P < .05.
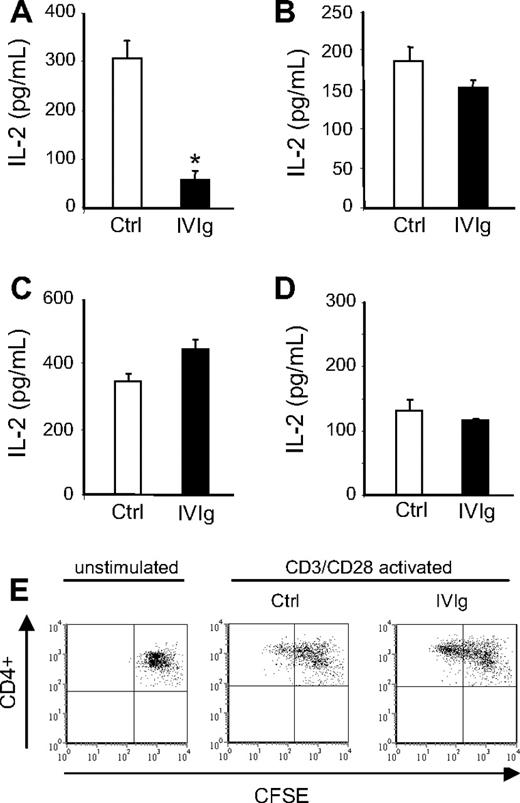
We next determined whether IVIg inhibited T-cell activation by a direct interaction with T cells. IFN-γ–stimulated P388D1 cells were first incubated with IVIg (10 mg/mL) or with RPMI 1640 medium for 24 hours. IVIg (or RPMI) were then removed before addition of OVA-IC and DO-11.10 hybridoma cells and incubation for an additional 24 hours. The pretreatment of P388D1 cells with IVIg, but not with RPMI 1640 medium, inhibited T-cell activation as indicated by a 5-fold reduction in IL-2 secretion level (Figure 5A), suggesting that the inhibitory effect of IVIg was not a consequence of their direct interaction with T cells. To confirm the absence of a direct effect of IVIg on T cells, IFN-γ–stimulated P388D1 cells were first incubated with OVA-IC for 24 hours to allow antigen processing and were then fixed with paraformaldehyde. DO-11.10 hybridoma cells were then added to fixed OVA-loaded P388D1 cells, in the presence or not of IVIg and incubated for an additional 24 hours. As shown in Figure 5B, fixed OVA-loaded P388D1 cells efficiently activated DO-11.10 cells. Addition of IVIg did not significantly reduce IL-2 secretion by DO-11.10 cells (P = .06), indicating that DO-11.10 cells could be activated in the presence of IVIg. This result was confirmed in a similar experiment using primary CD4+ T cells recovered from the spleen of DO11.10 mice. IL-2 secretion by OVA-specific CD4+ T cells after stimulation with fixed OVA-loaded P388D1 cells was comparable in the presence or absence of IVIg (and even slightly higher in the presence of IVIg; Figure 5C).
IVIg did not inhibit T-cell responses by direct interaction with T cells. (A) IFN-γ–stimulated P388D1 cells were incubated in the presence of IVIg (10 mg/mL) or the corresponding volume of RPMI 1640 medium (control), for 24 hours. IVIg (or RPMI) was then removed and DO-11.10 hybridoma cells were added with OVA-IC for an additional 24 hours. DO-11.10 cell activation was measured by IL-2 secretion. (B) IFN-γ–stimulated P388D1 cells were pulsed with OVA-ICs for 24 hours and fixed with paraformaldehyde. DO-11.10 cells were then added in the presence or not of IVIg (10 mg/mL), and IL-2 secretion was measured 24 hours later. (C) OVA-specific CD4+ T cells isolated from spleens of DO11.10 mice were cultured with OVA-IC—pulsed, P388D1-fixed cells in the presence or not of IVIG (10 mg/mL). IL-2 secretion was measured 24 hours later. (D-E) CFSE-stained spleen cells from naive BALB/c mice were incubated with or without Dynabeads Mouse CD3/CD28 T-cell expander in the presence or absence of IVIg (10 mg/mL): (D) polyclonal T-cell activation was evaluated by measuring IL-2 secretion 24 hours later, and (E) T-cell proliferation was evaluated by analysis of CFSE dilution by flow cytometry after 72 hours of culture. All results are representative of 2 independent experiments. *P < .001.
IVIg did not inhibit T-cell responses by direct interaction with T cells. (A) IFN-γ–stimulated P388D1 cells were incubated in the presence of IVIg (10 mg/mL) or the corresponding volume of RPMI 1640 medium (control), for 24 hours. IVIg (or RPMI) was then removed and DO-11.10 hybridoma cells were added with OVA-IC for an additional 24 hours. DO-11.10 cell activation was measured by IL-2 secretion. (B) IFN-γ–stimulated P388D1 cells were pulsed with OVA-ICs for 24 hours and fixed with paraformaldehyde. DO-11.10 cells were then added in the presence or not of IVIg (10 mg/mL), and IL-2 secretion was measured 24 hours later. (C) OVA-specific CD4+ T cells isolated from spleens of DO11.10 mice were cultured with OVA-IC—pulsed, P388D1-fixed cells in the presence or not of IVIG (10 mg/mL). IL-2 secretion was measured 24 hours later. (D-E) CFSE-stained spleen cells from naive BALB/c mice were incubated with or without Dynabeads Mouse CD3/CD28 T-cell expander in the presence or absence of IVIg (10 mg/mL): (D) polyclonal T-cell activation was evaluated by measuring IL-2 secretion 24 hours later, and (E) T-cell proliferation was evaluated by analysis of CFSE dilution by flow cytometry after 72 hours of culture. All results are representative of 2 independent experiments. *P < .001.
To determine whether the absence of a direct effect of IVIg on T cells was restricted to antigen-specific T-cell activation or could be extended to polyclonal T-cell activation, we used CFSE-stained total splenocytes from normal BALB/c mice in which T cells were activated after addition of anti-CD3/CD28 beads in the presence or absence of IVIg. The results obtained first showed that the amount of IL-2 secreted was similar in the presence or absence of IVIg (Figure 5D), indicating that IVIg did not inhibit polyclonal T-cell activation by anti-CD3/CD28 beads. Similarly, CFSE dilution of T cells activated in the presence of IVIg was at least as important as the one observed in the control condition (Figure 5E), confirming that IVIg did not interfere with the polyclonal T-cell activation.
Costimulatory molecules and inhibitory FcγRIIb are not involved in the inhibition of OVA-IC presentation
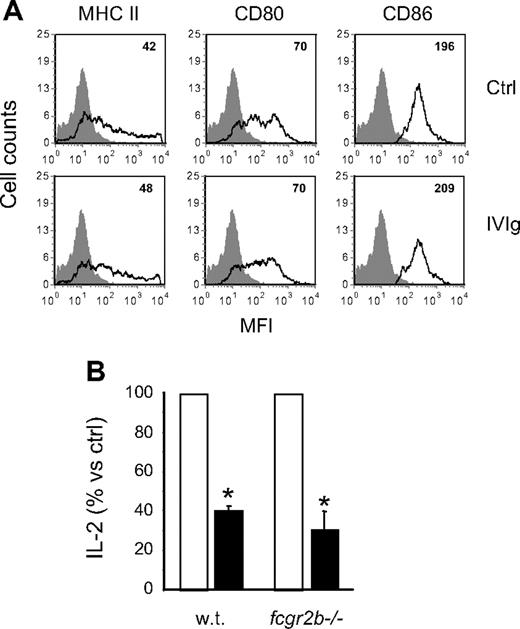
The absence of a direct effect on T cells suggested that IVIg inhibited antigen-specific T-cell activation by acting on the antigen-presenting ability of APCs. It was previously reported that IVIg modulated the cell surface expression of MHC II and of the CD80/CD86 costimulatory molecules,33 which are very important for antigen presentation. We thus analyzed the expression of these molecules on BMDCs to determine whether a similar modulation could explain the results obtained in the present study. BMDCs were incubated in the presence or absence of IVIg, labeled with MHC II, CD80, or CD86-specific fluorescent antibodies and analyzed by flow cytometry. The results obtained (Figure 6A) showed no significant differences in MFI between control and IVIg-treated cells, indicating that IVIg did not modulate the expression of MHC II or costimulatory molecules involved in antigen presentation.
Costimulatory molecules and inhibitory FcγRIIb are not involved in the inhibition of OVA-IC presentation. (A) The cell-surface expression of MHC II, CD80, and CD86 was measured on BMDCs cultured for 48 hours in the presence or absence of IVIg (10 mg/mL). The MFIs are indicated in the upper right corner of the histograms. (B) BMDCs from wild-type or fcgr2b−/− BALB/c mice were used in the OVA-IC antigen presentation assay done with DO-11.10 cells in the presence (■) or absence (□) of IVIg (10 mg/mL). The results are expressed as the percentage of the amount of IL-2 secreted in the presence of IVIg compared with the control condition in the absence of IVIg. Results shown are representative of 2 independent experiments. *P < .001.
Costimulatory molecules and inhibitory FcγRIIb are not involved in the inhibition of OVA-IC presentation. (A) The cell-surface expression of MHC II, CD80, and CD86 was measured on BMDCs cultured for 48 hours in the presence or absence of IVIg (10 mg/mL). The MFIs are indicated in the upper right corner of the histograms. (B) BMDCs from wild-type or fcgr2b−/− BALB/c mice were used in the OVA-IC antigen presentation assay done with DO-11.10 cells in the presence (■) or absence (□) of IVIg (10 mg/mL). The results are expressed as the percentage of the amount of IL-2 secreted in the presence of IVIg compared with the control condition in the absence of IVIg. Results shown are representative of 2 independent experiments. *P < .001.
APCs are known to express several classes of FcγR, including the inhibitory FcγRIIb that was shown to play a central role in the anti-inflammatory effects of IVIg in other experimental systems.8,34 To determine whether FcγRIIb were required for the inhibitory effect of IVIg observed here, BMDCs from wild-type or FcγRIIb−/− mice were used as APCs. BMDCs were incubated with OVA-IC and DO-11.10 hybridoma cells in the presence or absence of 10 mg/mL IVIg, and IL-2 secretion was measured 24 hours later. The results showed a similar decrease in IL-2 secretion for both wild-type and FcγRIIb−/− BMDCs in the presence of 10 mg/mL IVIg (Figure 6B), indicating that IVIg-mediated inhibition of OVA presentation occurred by an FcγRIIb-independent mechanism.
IVIg compete with immune complexes for binding to activating FcγR
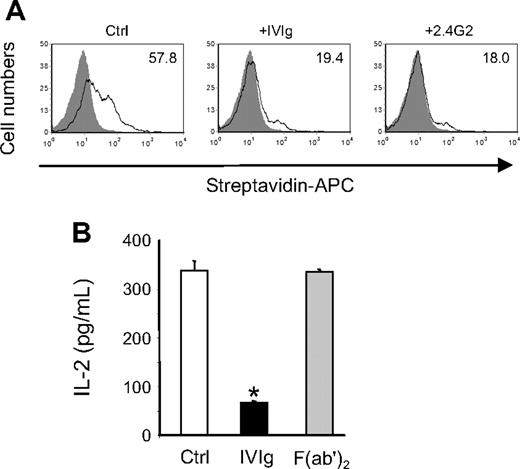
Antigen presentation of immune complexes is known to be mediated by activating FcγR, and a predominant role for the low-affinity FcγRIII has been proposed in mice.35 Accordingly, we observed that BMDCs from γ-chain–deficient mice failed to activate DO-11.10 cells in the presence of OVA-IC (IL-2 secretion was below detection level; data not shown). Therefore, to determine the contribution of these receptors to the effect of IVIg, biotinylated OVA-ICs were incubated with P388D1 cells in the presence or absence of IVIg or FcγRII/III-specific monoclonal antibody 2.4G2,36 and binding of the complexes to the cell surface was monitored by flow cytometry. The results obtained (Figure 7A) showed that IVIg and 2.4G2 inhibited similarly the binding of biotin-OVA-ICs (3-fold reduction of MFI). Similar results were obtained with BMDCs (data not shown). In addition, the inhibitory potential of F(ab′)2 fragments prepared from IVIg was compared with that of intact IgG in the OVA-IC presentation assay. The results obtained (Figure 7B) showed that F(ab′)2 fragments did not decrease IL-2 secretion by DO-11.10 cells compared with the control condition, whereas addition of IVIg resulted in approximately 80% inhibition of IL-2 secretion, indicating that interaction between IVIg and FcγR was required for inhibition of OVA-IC presentation. Altogether, these results strongly suggest that a competition between IVIg and immune complexes for binding to activating FcγR is responsible for the IVIg-mediated inhibition of antigen presentation.
Interaction between IVIg and low-affinity FcγR is required for the inhibition of OVA-IC presentation. (A) Biotin-OVA-IC (1.66 μg/mL) was incubated with IFN-γ-activated P388D1 cells (1 × 105 cells) for 1 hour at 4°C in the presence or not of IVIg (10 mg/mL) or 2.4G2 mAb (30 μg/mL). Binding of biotin–OVA-ICs was monitored by flow cytometry using allophycocyanin-conjugated streptavidin. The MFIs are indicated in the histograms. (B) IVIg and F(ab′)2 fragments of IVIg were used at 10 mg/mL in the OVA-IC presentation assay. IL-2 secretion by DO-11.10 cells was measured as before. Results are representative of 3 independent experiments. *P < .001.
Interaction between IVIg and low-affinity FcγR is required for the inhibition of OVA-IC presentation. (A) Biotin-OVA-IC (1.66 μg/mL) was incubated with IFN-γ-activated P388D1 cells (1 × 105 cells) for 1 hour at 4°C in the presence or not of IVIg (10 mg/mL) or 2.4G2 mAb (30 μg/mL). Binding of biotin–OVA-ICs was monitored by flow cytometry using allophycocyanin-conjugated streptavidin. The MFIs are indicated in the histograms. (B) IVIg and F(ab′)2 fragments of IVIg were used at 10 mg/mL in the OVA-IC presentation assay. IL-2 secretion by DO-11.10 cells was measured as before. Results are representative of 3 independent experiments. *P < .001.
Discussion
In the present report, we show that IVIg inhibit the in vivo and in vitro antigen-dependent T-cell responses by decreasing the ability of APCs to present antigens in an MHC II-restricted manner. This effect was first documented during primary immunization of mice with OVA done in the presence of IVIg. We showed that the number of antigen-specific T cells generated was significantly lower in the spleens of mice immunized in the presence of IVIg as evaluated by the reduction in helper T-cell cytokine secretion after in vitro stimulation with OVA-ICs. Our results also showed that IVIg inhibited the in vitro activation of antigen-specific T cells from OVA-immunized mice and the in vivo proliferation of adoptively transferred T cells from transgenic mice that carry an MHC II–restricted rearranged TCR transgene reacting specifically with OVA. Finally, we showed that treatment with IVIg during immunization negatively influenced the B-cell responses, leading to a reduction in OVA-specific antibody titers in immunized animals. This latter effect on B cells is probably a consequence of the reduction in antigen-specific T-cell response rather than a direct effect of IVIg on B cells, although we cannot rule out at present the possibility that IVIg could also affect directly the activation of antigen-specific B cells.
Although the experimental system used in the present work was not autoimmune, our observations are of particular interest in the context of the existence of a pathogenic loop that maintains the ongoing autoantibody production in autoimmune diseases.37,38 Central to this pathogenic loop are the APCs that phagocytose opsonized self-antigens and present them to CD4+ T cells, which in turn provide the necessary helper activity to sustain the autoantibody production. Recently, Siragam et al showed that the short-term effects of IVIg in a mouse model of immune thrombocytopenia (prevention of opsonized platelet phagocytosis) could be recapitulated by triggering the activating FcγR on DCs.30 Our results suggest that, in addition to these short-term effects, interaction of IVIg with DCs may also induce long-term immunomodulatory effects. Indeed, IVIg have been shown to decrease autoantibody production in autoimmune diseases,39-41 a phenomenon that may be related to the effects of IVIg on antigen presentation reported here.
Previous reports on the effects of IVIg on T-cell activation suggested that IVIg inhibited T-cell activation by blocking or interacting with cell-surface receptors.21,22,42 We rather showed here that T cells could be efficiently activated in the presence of IVIg, using paraformaldehyde-fixed OVA-loaded APCs or polyclonal T-cell activation after CD3/CD28 stimulation. These experiments led to the hypothesis that IVIg inhibited T-cell responses by decreasing the ability of APCs to present antigens in an MHC II–restricted manner. Indeed, treatment of APCs with IVIg during the antigen-loading phase, followed by extensive washing to remove IVIg before T-cell stimulation, led to an almost complete inhibition of T-cell activation, confirming that the effects of IVIg were directed against APC rather than T cells. Additional evidence for the absence of a direct effect of IVIg on T-cell proliferation was recently obtained in our laboratory using phytohemagglutinin-activated human T cells (L. Padet, I. St-Amour, E.A., R.L., and R.B., manuscript in preparation).
It was previously reported that treatment with IVIg decreased the cell surface expression of MHC II and CD80/CD86 on human DCs, resulting in a decreased ability to activate autologous and allogenic T cells.33 In contrast, Ohkuma et al reported that the expression of CD86 remained high in IVIg-treated human DCs,43 in agreement with our results obtained with murine BMDCs. The discrepancies between these results may be explained by differences in culture and other experimental conditions used to derive and treat DCs. In some experiments, we used P388D1 cells stimulated with IFN-γ (to increase MHC II expression on the surface of these cells44 ) as APCs. As observed with BMDCs, the MHC II and CD80/CD86 expression was not modified in IFN-γ–treated P388D1 cells (data not shown) in the presence of IVIg, although it was previously reported that IVIg inhibited the IFN-γ–mediated macrophage cell functions.45 We therefore conclude that, even if IVIg can modulate the expression of costimulatory molecules under certain experimental conditions, the effects reported here could not be explained by such modulations.
It is well recognized that the inhibitory FcγRIIb are important mediators of the anti-inflammatory effects of IVIg. However, in the present work, the effect of IVIg was similar whether BMDCs were derived from fcr2b−/− or wild-type mice, indicating that FcγRIIb does not play an important role in the IVIg-mediated inhibition of antigen presentation. In contrast, our results strongly suggest that a competition between IVIg and immune complexes for binding to activating low-affinity FcγR is responsible for the IVIg-mediated inhibition of antigen presentation. In the present study, the maximal inhibitory effect of IVIg was observed at a dose of IVIg approximately 6000 times higher than the dose of OVA-ICs used in the presentation assay (10 mg/mL IVIg vs 1.66 μg/mL OVA-ICs). This observation correlates well with the known lower affinity of monomeric IgG for low-affinity FcγRs compared with immune complexes and is in agreement with a previous report by van Mirre et al who showed that monomeric IgG have sufficient affinity to compete for the binding of dimeric or aggregated IgG to low-affinity FcγRs expressed on human neutrophils.46 Finally, the observations reported here may be physiologically relevant because the doses of IVIg needed to produce the inhibitory effect were comparable with the therapeutic doses used for treatment of autoimmune patients and were also shown to be effective in vivo in mice.
In conclusion, we showed that IVIg inhibit the in vivo and in vitro antigen-dependent T-cell responses by decreasing the ability of APCs to present antigens in an MHC II-restricted manner. In the context of an autoimmune disease, such inhibition would result in a decreased presentation of self-antigens to autoreactive T cells and could thus contribute to reduce their subsequent restimulation and the concurrent autoantibody secretion. The observations reported here could therefore contribute to explain the sustained anti-inflammatory effects of IVIg in autoimmune diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tony Tremblay, Patrick Trépanier, and Isabelle Paré for technical assistance and Sophie Dubuc for statistical analysis of the data.
This work was supported by the Bayer/Talecris/CBS/HQ Partnership Fund (R.B., R.L.).
Authorship
Contribution: E.A. designed and performed experiments, analyzed results, and wrote the paper; and R.L. and R.B. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renée Bazin, Department of Research and Development, Héma-Québec, 1070 Ave des Sciences-de-la-Vie, Québec City, QC, Canada G1V 5C3; e-mail: renee.bazin@hema-quebec.qc.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal