To the editor:
We recently reported that intravenous immunoglobulin (IVIg) interferes with the binding of ovalbumin immune complexes (OVA-IC) to phagocytic FcγRs, leading to a decreased internalization inside antigen-presenting cells (APCs) and resulting in a reduced amount of antigen presented by MHC II molecules to CD4 helper T cells.1 Consequently, the antigen-specific helper T-cell response is dampened in the presence of IVIg. In the context of autoimmune diseases, these observations suggest that IVIg treatment could decrease the presentation of self-antigens to CD4 T cells and prevent the subsequent autoantibody production.
Although CD8 cytotoxic T cells play a substantial role in organ destruction in several autoimmune diseases,2-4 the effect of IVIg on this cell compartment has not been studied so far. We hypothesized that CD8 T-cell activation could be impaired in the presence of IVIg, by a mechanism similar to that described for CD4 T-cell activation. We thus used OT-II (CD4) I-Ab–restricted OVA-specific primary T cells5 to first confirm that the previously reported inhibitory effect of IVIg was not limited to I-Ad–restricted OVA-specific CD4 T cells (DO-11.10).1 We also used OT-I (CD8) H2Kb-restricted OVA-specific T cells6 to determine the effect of IVIg on the ability of APCs to activate CD8 T cells by cross-presentation of OVA-IC. OT-I and OT-II cells were purified from the spleen and lymph nodes of C57BL/6-Tg(TcraTcrb)1100Mjb/J and B6.Cg-Tg(TcraTcrb)425Cbn/J mice, respectively. Bone marrow–derived dendritic cells (BMDCs) from C57BL/6 mice were prepared as previously described1 and used as APCs to activate OT-I and OT-II cells in the presence of OVA-IC, with or without IVIg. T-cell activation was determined by flow cytometry using CD69 expression as a marker of cell activation.7
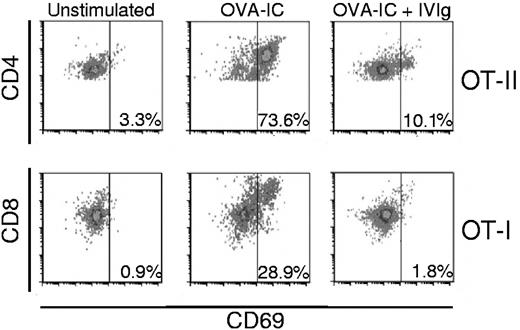
Our results first show an increased proportion of OT-II cells expressing CD69 after OVA-IC presentation, from a background level of 3% up to 74% (Figure 1 top left and middle panels). When IVIg was present during OVA-IC presentation, the percentage of cells expressing CD69 only reached 10% (Figure 1 top right panel), indicating that OT-II cell activation was significantly impaired in the presence of IVIg. The inhibitory effect of IVIg on OT-II cell activation is thus similar to that previously observed with DO-11.10 cells, regardless of their different MHC restriction profile. Our results also reveal the efficient activation of OT-I cells by OVA-IC cross-presentation, as shown by the increase from a background level of 1% to a proportion of 29% of C69-expressing cells (Figure 1 bottom left and middle panels). However, the presence of IVIg completely prevented OT-I cell activation, as illustrated by the absence of induction of CD69 on these cells (bottom right panel).
Prevention of OVA-IC–mediated OT-II (CD4) and OT-I (CD8) activation by IVIg. Equal numbers of bone marrow–derived dendritic cells (BMDCs) and OT-II (top panels) or OT-I (bottom panels) cells were incubated for 3 days in the presence of 2.5 μg/mL of OVA-IC prepared as described previously,1 with or without 10 mg/mL of IVIg (Gamunex). The background activation was established in the absence of OVA-IC (left panels). After incubation, the cells were recovered, washed and labeled with either anti–CD4-FITC or anti–CD8-PE and anti–CD69-APC (eBioscience). T-cell activation was determined by measuring the expression of CD69 on CD4- (top panels) or CD8- (bottom panels) gated cells by flow cytometry (Accuri C6 flow cytometer). The data were analyzed using the FCS Express 4.0 software (De Novo) and are representative of 4 independent experiments done with different lots of BMDCs.
Prevention of OVA-IC–mediated OT-II (CD4) and OT-I (CD8) activation by IVIg. Equal numbers of bone marrow–derived dendritic cells (BMDCs) and OT-II (top panels) or OT-I (bottom panels) cells were incubated for 3 days in the presence of 2.5 μg/mL of OVA-IC prepared as described previously,1 with or without 10 mg/mL of IVIg (Gamunex). The background activation was established in the absence of OVA-IC (left panels). After incubation, the cells were recovered, washed and labeled with either anti–CD4-FITC or anti–CD8-PE and anti–CD69-APC (eBioscience). T-cell activation was determined by measuring the expression of CD69 on CD4- (top panels) or CD8- (bottom panels) gated cells by flow cytometry (Accuri C6 flow cytometer). The data were analyzed using the FCS Express 4.0 software (De Novo) and are representative of 4 independent experiments done with different lots of BMDCs.
The effect of IVIg on CD8 T-cell activation by cross-presentation of immune complexes was predictable, because CD8 T-cell activation requires signals provided by antigenic epitopes presented on MHC I molecules.8,9 We herein provide the experimental demonstration of this inhibitory effect. Whether the interference of IVIg with immune complex uptake by APCs is solely responsible for this inhibition remains to be determined. Kaveri et al previously showed that IVIg contains antibodies specific to a highly conserved portion of human HLA class I antigens and that these antibodies were able to inhibit class I–restricted T cell–mediated cytotoxicity.10 However, we did not observe MHC I blockade by IVIg in our assays, suggesting that HLA class I–specific antibodies are not involved in the inhibitory effects reported here.
In conclusion, we demonstrated that antigen-specific CD8 T-cell activation after cross-presentation of immune complexes by BMDCs is strongly reduced in the presence of therapeutic doses of IVIg. This observation extends our previous observations showing that antigen-specific CD4 T-cell activation is inhibited by IVIg both in vitro and in vivo. Altogether, these results suggest that not only CD4 but also CD8 T-cell activation should be considered as therapeutic targets in the development of potent substitutes to IVIg.
Authorship
Acknowledgments: The authors thank Dominic Chabot for excellent technical assistance.
P.T. receives an Industrial Innovation PhD Scholarship from Fonds de Recherche du Québec–Nature et Technologies (FQRNT)/National Sciences and Engineering Research Council of Canada (NSERC).
Contribution: P.T. designed and performed the experiments, analyzed the results, and wrote the paper; and R.B. designed the research, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renée Bazin, PhD, Department of Research and Development, Héma-Québec, 1070 Avenue des Sciences-de-la-Vie, Québec, QC, Canada, G1V 5C3; e-mail: renee.bazin@hema-quebec.qc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal