In this issue of Blood, Aubin and colleagues show that IVIg interacted with FcγRs on APCs, resulting in reduced antigen presentation and inhibition of antigen-specific T-cell response.1 This finding suggests a key role for APCs in IVIg action.
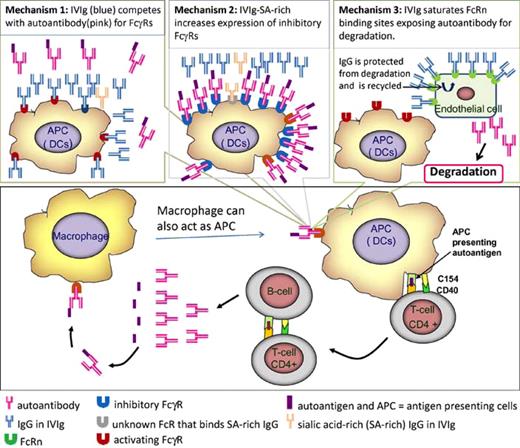
Recent evidence suggests that an essential step in the immunopathology of autoimmune disease (AD) involves antigen-presenting cells (APCs) presenting antigen to antigen-specific CD4+ T helper cells,2 which, in turn, induce antigen-specific B cells to produce autoantibodies (see figure). Costimulatory molecules including CD40 and CD154 play an important role in these cellular interactions, which perpetuate the disease. Autoantibodies bind autoantigens to form immune complexes (ICs) that are taken up by APCs via Fcγ receptors (FcγRs) for antigen processing and presentation, thus maintaining the pathogenic loop.3,4
A model for the immunopathology of autoimmune disease. Some aspects such as the role of cytotoxic T cells and regulatory T cells are not included. Proposed mechanisms whereby IVIg interferes with immune complex–APC interaction are shown in the top 3 panels.
A model for the immunopathology of autoimmune disease. Some aspects such as the role of cytotoxic T cells and regulatory T cells are not included. Proposed mechanisms whereby IVIg interferes with immune complex–APC interaction are shown in the top 3 panels.
Intravenous immunoglobulin (IVIg) has been used to treat AD for more than 2 decades. The precise mechanism(s) of action is still unclear.5 In this issue of Blood, Aubin and colleagues1 provide evidence suggesting that IVIg acts at the level of APC even though they found that IVIg inhibited antigen-specific T-cell and B-cell response. Mice immunized with ovalbumin (OVA) in the presence of IVIg generated reduced numbers of antigen-specific T cells compared with mice immunized with OVA in the absence of IVIg. IVIg treatment during OVA immunization significantly reduced OVA-specific antibody production. These suppressive activities of IVIg were not the result of decreased APC surface expression of MHCII and CD80/CD86 costimulatory molecules, as previously postulated, but were the consequence of IVIg interfering with IC binding to activating FcγRs expressed on APC.
Three mechanisms have been proposed for the immune suppressive action of IVIg6 (see figure) in which pathogenic IgG/IC and FcγRs on APC are believed have a role.
Mechanism 1: IVIg competes with IC for activating FcγRs. In this mechanism, high-dose IVIg competes with IC for activating FcγRs on APC surface.6 Data of Aubin et al1 would favor this mechanism. First, these investigators showed that 2.4G2, FcγRIII–specific monoclonal antibody blocked OVA-IC binding to bone marrow dendritic cells (BM-DCs), used as APC in this study. Second, they found that intact IV IgG inhibited antigen-specific T-cell response but its F(ab′) fragments did not. Third, BM-DCs from γ chain–deficient mice (lacking FcγRs) failed to activate CD4+ T cells in the presence of IC. Altogether, their results suggest that IVIg via its Fc domain competes with IC for binding to activating FcγRs expressed on APCs, consequently reduces APC antigen presentation, and inhibits CD4+ T-cell activation and other downstream immune responses. One reservation in interpreting these data is that monomeric IgG in IVIg binds activating FcγRs (FcγRIII and VI) with low affinity,5 and it would seem surprising that IVIg would be able to compete with IC for binding to these Fc receptors.
Mechanism 2: IVIg induces expression of inhibitory FcγRs. Kaneko and colleagues7 showed recently that 1% to 2% of IgG in IVIg has sialic acid at the Asn297-linked glycan, and IVIg enriched in Fc-sialylated IgGs has increased immune inhibitory activity. These investigators proposed a mechanism of action for IVIg in which Fc-sialylated IgGs bind to a unique receptor (still to be identified) on macrophages/APCs and up-regulate expression of inhibitory FcγRs such as FcγRIIB. Up-regulation of inhibitory FcγRs dilutes out the effects of activating FcγRs. They also argued that as Fc-sialylated IgGs are present only in small quantities in IVIg, this explains the high dose of IVIg required for immune inhibitory effect. In contrast, Aubin et al1 observe that IVIg inhibited antigen presentation to the same degree with BM-DC (APC) from FcγRIIB−/− and FcγRIIB+/+ (wild-type) mice, suggesting that the immune inhibitory effect of IVIg is FcγRIIB-independent.
Mechanism 3: IVIg saturates FcRn binding; FcRn binds pathogenic or nonpathogenic IgGs and protects them from catabolism. The third mechanism postulates that high doses of IVIg saturate the available FcRn binding sites and expose pathogenic autoantibodies, not bound to FcRn, to catabolic removal, thus reducing the amount of circulating autoantibodies. Consistent with this mechanism, Li et al showed that IVIg-treated wild-type mice, but not neonate FcRn-deficient mice, were protected from developing bullous skin disease when the animals were infused with antibodies from patients with pemphigus vulgaris.8 Mechanism 3, however, is not yet widely accepted because there is some evidence against FcRn having a role in IVIg action.5
An important drawback in the study of Aubin et al1 is that they did not use an autoimmune disorder animal model and their findings may not necessarily represent the effects of IVIg in AD. Further studies are required, particularly studies using an autoimmune experimental system. Nevertheless, the findings in this study are helpful in providing insights into the mechanism(s) of IVIg action. If subsequently confirmed by further studies, the knowledge gained may inform development of effective novel therapies for AD,4 such as developing antibodies, peptides, or small molecules that block IC binding to activating FcγRs on APCs.
Conflict-of-interest disclosure: B.H.C. has consulted for Commonwealth Serum Laboratory. J.J.H.C. declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal