In this issue of Blood, Lubenow and colleagues provide the first strong evidence that there is much more besides heparin in triggering the adverse drug reaction, HIT.1
To date, research has focused on identifying whether differences in heparin composition influence the risk of immunization against platelet factor 4/heparin (PF4/heparin) complexes, the target of the immune response that characterizes heparin-induced thrombocytopenia (HIT). And, indeed, longer-chain and more sulfated heparins are more likely to trigger immunization and clinically evident HIT.2
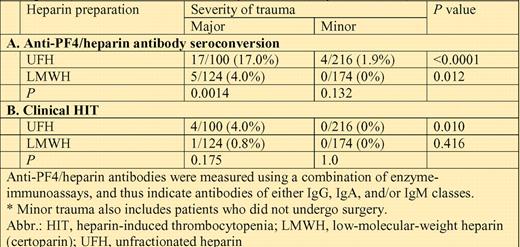
Anti-PF4/heparin immunization frequency and HIT frequency in relation to type of heparin (UFH vs LMWH) and severity of trauma (major vs minor*).
Anti-PF4/heparin immunization frequency and HIT frequency in relation to type of heparin (UFH vs LMWH) and severity of trauma (major vs minor*).
As with any drug reaction, however, only a minority of patients who receive the offending drug are affected. Usually, this is attributed to poorly defined “idiosyncratic” (patient-specific) factors. But in the case of HIT, the perception has grown that there must be additional nondrug, but also nonidiosyncratic, factors that influence immunization risk. The evidence behind this concept has been mostly indirect. For example, surgical patients appear to be more likely to develop HIT than medical patients.3 This is very different from an “idiosyncratic” reason where Mr X has an inherently higher risk of HIT than Mr Y. Rather, both Mr X and Mr Y would have a higher risk of HIT if they received heparin in the context of surgery, rather than if they received heparin as medical patients.
Now, Lubenow et al provide direct evidence that nondrug factors do indeed strongly influence anti-PF4/heparin immunization and HIT risk. They performed a randomized controlled trial comparing 2 types of heparin—unfractionated heparin (UFH) and a low-molecular-weight heparin (LMWH) preparation (certoparin)—administered for thromboprophylaxis after trauma. Patients were classified as “major” and “minor” trauma and were systematically evaluated for immunization (various tests for anti-PF4/heparin antibodies) and for HIT (thrombocytopenia and/or thrombosis bearing a temporal relationship to formation of platelet-activating antibodies). In their study, major trauma referred primarily to fractures of the pelvis, femur, tibia, fibula, or humerus; minor trauma to almost everything else (usually, distal upper-extremity, shoulder, and distal lower-extremity fractures).
The table summarizes the frequency of anti-PF4/heparin antibody immunization, as per a combination of immunoassays, categorized by severity of trauma (major, minor) and for type of heparin (UFH, LMWH). The corresponding data for clinical HIT are also shown.
The data clearly indicate that both heparin type and trauma severity are strong and independent predictors of the anti-PF4/heparin immune response. Consequently, the highest risk of immunization (17%) and of clinical HIT (4%) was seen in patients who received UFH thromboprophylaxis after major trauma. At the other end of the spectrum, not even a single immunization event occurred in 174 patients who received LMWH thromboprophylaxis after minor trauma.
A quick survey of the data in the table suggests that the effect of trauma severity was at least as great as that due to heparin type. Most notably, the frequency of anti-PF4/heparin immunization was approximately twice as high in patients who received LMWH after major trauma (5 of 124, 4.0%) compared with those who received UFH after minor trauma (4 of 216, 1.9%). Indeed, when the Greifswald group applied logistic regression analysis, adjusting for confounders such as age, sex, and type of heparin, the odds ratio for developing any immune response in major trauma compared with minor trauma was 7.98 (95% CI, 2.06-31.00; P = .003). This mirrors the approximately 10-fold greater frequency of HIT that has been shown with UFH compared with LMWH.2,3
The authors speculate that increased release from platelets of PF4 in the context of major surgery, or perhaps a greater degree of inflammation—a plausible potentiator of the anti-PF4/heparin immune response—might be responsible. Perhaps a “double HIT”—simultaneous antigen exposure plus a proinflammatory “danger signal”—is needed for triggering a strong immune response. To date, this concept of proinflammatory potentiators has been largely suggested in case reports. For example, acquired hemophilia has been observed anecdotally to occur in the context of infection, surgery, and malignancy.4-6 More powerfully, this study by Lubenow et al demonstrates through compelling clinical trial data that a nondrug factor—severity of trauma—is of major importance in potentiating the heparin-induced immune response.
Conflict-of-interest disclosure: T.E.W. has received honoraria for lectures on behalf of manufacturers of low-molecular-weight heparin (Pfizer Canada, Sanofi-Aventis). ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal