Abstract
Reperfusion after brain ischemia causes thrombus formation and microcirculatory disturbances, which are dependent on the platelet glycoprotein Ib–von Willebrand factor (VWF) axis. Because ADAMTS13 cleaves VWF and limits platelet-dependent thrombus growth, ADAMTS13 may ameliorate ischemic brain damage in acute stroke. We investigated the effects of ADAMTS13 on ischemia-reperfusion injury using a 30-minute middle cerebral artery occlusion model in Adamts13−/− and wild-type mice. After reperfusion for 0.5 hours, the regional cerebral blood flow in the ischemic cortex was decreased markedly in Adamts13−/− mice compared with wild-type mice (P < .05), which also resulted in a larger infarct volume after 24 hours for Adamts13−/− compared with wild-type mice (P < .01). Thus, Adamts13 gene deletion aggravated ischemic brain damage, suggesting that ADAMTS13 may protect the brain from ischemia by regulating VWF-platelet interactions after reperfusion. These results indicate that ADAMTS13 may be a useful therapeutic agent for stroke.
Introduction
von Willebrand factor (VWF) is a large multimeric protein that plays a key role in thrombus formation by tethering platelets at sites of vascular injury.1 Smaller VWF multimers are less active, and the potent thrombogenic activity of ultra-large VWF (ULVWF) secreted from endothelium is regulated in vivo through cleavage by ADAMTS13.2,3 The importance of this mechanism for normal hemostasis is supported by evidence that patients with deficiency of ADAMTS13 function, diagnosed with thrombotic thrombocytopenic purpura, have ULVWF in circulating blood and VWF-dependent microvascular thrombosis.2 Recently, we demonstrated that ADAMTS13 cleaves VWF on the surface of platelet thrombi in a shear force–dependent manner, which limits thrombus growth in vitro.4 These data suggest that ADAMTS13 is a key molecule that maintains a physiologic balance between hemostasis and thrombosis through regulation of VWF function in vivo.
ADAMTS13 function is crucial for preventing thrombosis in the cerebral microvasculature, as indicated by the occurrence of neurologic deficits in thrombotic thrombocytopenic purpura, but the role of ADMTS13 in the pathogenesis of reperfusion injury after arterial thrombosis has not been established. To address this issue, we investigated the role of ADAMTS13 in a transient middle cerebral arterial occlusion (MCAO) model of ischemia-reperfusion injury in the mouse brain5 using Adamts−/− mice.6
Because brain ischemia-reperfusion injury is dependent on the platelet glycoprotein Ib–VWF axis7 and platelet thrombosis adversely affects the postischemic cerebral microcirculation8-11 leading to secondary brain damage,10 ADAMTS13 may reduce platelet thrombus growth and thereby ameliorate ischemic brain injury by improving the postischemic no-reflow phenomenon.12 Here we demonstrate that Adamts13 gene deletion aggravates postischemic cerebral blood reflow, resulting in larger infarct volume. This result suggests that ADAMTS13 may indeed suppress excessive platelet thrombus growth in vivo.
Methods
The effect of Adamts13 gene deletion on brain ischemia was studied using male Adamts13−/− (−/−) mice and wild-type (+/+) mice generated by our study group.6 We used male mice only to obtain consistent results because female mice are known to be more resistant to stroke. The experimenters were blinded to the genotype of each animal until all studies had been finished. This study was approved by the institutional ethics committee of Fukuoka University.
Transient MCAO
Focal cerebral ischemia (MCAO by intraluminal thread) was induced in Adamts13−/− and wild-type mice as previously described.5,13,14 Preliminary experiments using 1-hour MCAO gave excessively high mortality of 4 of 5 for one group and 1 of 4 for another. Therefore, we reduced the time of MCAO to 30 minutes. Body temperature was maintained at 36.5°C to 37.0°C during surgery. The success of the left MCAO was confirmed according to the following criteria: (1) regional cerebral blood flow (rCBF) in the left cerebral cortex at the thread insertion less than 20% of pre-MCAO rCBF; and (2) consistent presence of significant ischemic neurologic symptoms of the left cerebral hemisphere, including right forepaw paralysis and right circling behavior during 30-minute MCAO.
rCBF
The rCBF was measured by LASER Doppler flowmetry (LDF; ALF21, Advance Co) as previously described.5 The LDF probe was placed in the left cerebral cortex stereotaxically. The rCBF was monitored in all animals during the period between 30 minutes before MCAO and immediately after reperfusion.
Cerebral infarct volume and histology 24 hours after MCAO
The brains were sectioned coronally (four 2-mm-thick slices) according to a mouse brain matrix 24 hours after MCAO or sham operation. The infarct area was measured using an image-analysis system (National Institutes of Health Image software, Version 1.63) in each slice stained with 2,3,5 triphenyltetrazolium chloride, and the infarct volume was calculated.5,13 Paraffin-embedded brains were stained with phosphotungstic acid hematoxylin (PTAH) to demonstrate fibrin in thrombi or incubated with anti-VWF antibody (sc-8068; Santa Cruz Biotechnology), followed by a standard avidin-biotin-peroxidase complex technique to demonstrate VWF in thrombi.
Neurologic assessment
Neurologic deficit was assessed at 24 hours after MCAO using a neurologic score as previously described,13 and the survival rates were also measured at 24 hours after MCAO.
Statistical analysis
Data are mean plus or minus SEM. For multiple pairwise comparisons, 2-way analysis of variance followed by Scheffé test was performed. When only 2 groups were compared, Student t test was used. Probability values less than .05 were considered to be of statistical significance.
Results and discussion
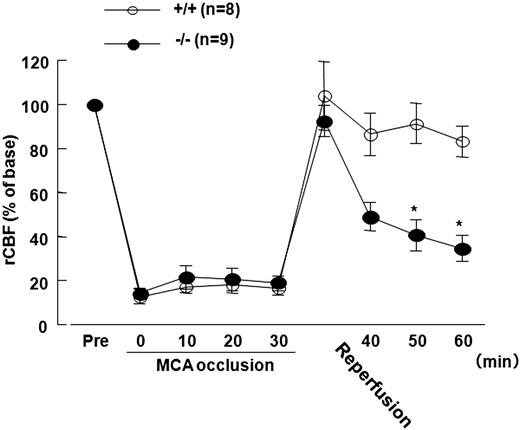
The rCBF decreased to less than 20% of the baseline value during 30-minute MCAO and returned to baseline immediately after reperfusion in both Adamts13−/− and wild-type mice. However, during the subsequent 30 minutes, the rCBF for both groups decreased, suggesting that ischemia-reperfusion had induced thrombosis. The rCBF for Adamts13−/− mice progressively decreased compared with wild-type mice (significantly decreased at 20 and 30 minutes after reperfusion, P < .05, Scheffé test, Figure 1).
Effect of Adamts13 gene deletion on rCBF in mice of 30-minute MCAO model. Male Adamts13−/− (−/−) mice and wild-type (+/+) mice in an SV129-genetic background were used to study the effect of ADAMTS13 deficiency on brain ischemia: −/− (n = 25) and +/+ (n = 25) mice (8-10 weeks of age, 20-23 g of body weight). The focal cerebral ischemia (30-minute MCAO by intraluminal thread) was induced in the −/− (n = 20) and +/+ (n = 20) mice as previously described (sham surgery in −/−, n = 5; and +/+, n = 5). This study was approved by the institutional ethics committee at Nara Medical University. The rCBF was measured by LDF (ALF21; Advance Co). The rCBF was recorded over time (immediately before and after MCAO; 10, 20, and 30 minutes after MCAO; immediately after reperfusion; and 10, 20, and 30 minutes after reperfusion). The rCBFs during occlusion and reperfusion were expressed as a percentage of the baseline LDF value. The rCBF decreased to less than 20% of the baseline value during 30 minutes of MCAO and returned to the baseline immediately after reperfusion in both −/− and WT mice. The rCBF in −/− mice, however, progressively decreased more markedly compared with that in +/+ after reperfusion (percentage rCBF: −/−, n = 9, vs +/+, n = 8, at 20 and 30 minutes after reperfusion; 40.8 ± 7.1 vs 91.4 ± 9.1, and 34.6 ± 5.8 vs 83.2 ± 6.8, respectively). *P < .05 vs WT, Scheffé test after 2-way repeated-measures analysis of variance (F(8,134) = 6.668, P < .01). Values are mean ± SEM.
Effect of Adamts13 gene deletion on rCBF in mice of 30-minute MCAO model. Male Adamts13−/− (−/−) mice and wild-type (+/+) mice in an SV129-genetic background were used to study the effect of ADAMTS13 deficiency on brain ischemia: −/− (n = 25) and +/+ (n = 25) mice (8-10 weeks of age, 20-23 g of body weight). The focal cerebral ischemia (30-minute MCAO by intraluminal thread) was induced in the −/− (n = 20) and +/+ (n = 20) mice as previously described (sham surgery in −/−, n = 5; and +/+, n = 5). This study was approved by the institutional ethics committee at Nara Medical University. The rCBF was measured by LDF (ALF21; Advance Co). The rCBF was recorded over time (immediately before and after MCAO; 10, 20, and 30 minutes after MCAO; immediately after reperfusion; and 10, 20, and 30 minutes after reperfusion). The rCBFs during occlusion and reperfusion were expressed as a percentage of the baseline LDF value. The rCBF decreased to less than 20% of the baseline value during 30 minutes of MCAO and returned to the baseline immediately after reperfusion in both −/− and WT mice. The rCBF in −/− mice, however, progressively decreased more markedly compared with that in +/+ after reperfusion (percentage rCBF: −/−, n = 9, vs +/+, n = 8, at 20 and 30 minutes after reperfusion; 40.8 ± 7.1 vs 91.4 ± 9.1, and 34.6 ± 5.8 vs 83.2 ± 6.8, respectively). *P < .05 vs WT, Scheffé test after 2-way repeated-measures analysis of variance (F(8,134) = 6.668, P < .01). Values are mean ± SEM.
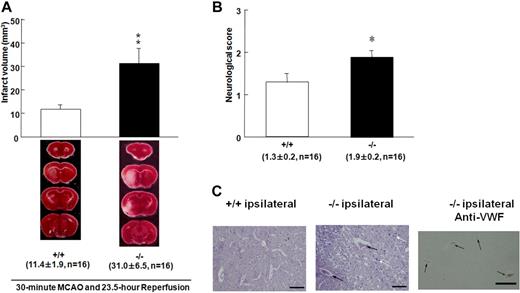
The survival rates of the Adamts13−/− and wild-type mice did not differ (17 of 20 vs 16 of 20, respectively). However, Adamts13−/− mice had larger brain infarctions compared with wild-type mice 24 hours after MCAO (P < .01; Figure 2A), which is reflected by a difference in neurologic score assessing left hemisphere function (Figure 2B). Histologic and immunohistochemical examinations revealed that more thrombi containing fibrin and VWF were observed in Adamts13−/− mice (Figure 2C), which may contribute to the lowered rCBF and increased infarct volume in Adamts13−/− mice. These results indicate that Adamts13 gene deletion aggravates ischemic brain damage.
Effect of Adamts13 gene deletion on brain infarct in mice after 30-minute MCAO. (A) These are coronal sections through the brain in both groups stained with 2,3,5 triphenyltetrazolium chloride. Red areas represent vital brain tissue, and white areas represent cerebral infarction. Adamts13−/− (−/−) mice had significantly larger volume of brain infarct compared with wild-type (+/+) mice after 23.5-hour reperfusion after 30-minute MCAO (−/−, n = 16, vs +/+, n = 16, **P < .01, Student t test). Values are mean ± SEM (mm3). The average infarct volume was 31.0 ± 6.5 mm3 for ADAMTS13 −/− mice and 11.4 ± 1.9 mm3 for +/+ mice. The survival rates of the −/− and +/+ mice did not differ (17 of 20 vs 16 of 20, respectively). No ischemic change was observed in the brain of −/− and +/+ mice after sham operation. (B) Neurologic score was measured 24 hours after MCAO. Neurologic scores were measured from the point according to the neurologic findings as follows: 0 indicates normal motor function; 1, flexion of torso and of contralateral forelimb on lifting of the animal by the tail; 2, circling to the ipsilateral side but normal posture at rest; 3, circling to the ipsilateral side; 4, rolling to the ipsilateral side; and 5, leaning to the ipsilateral side at rest (no spontaneous motor activity). *P < .05. (C) Representative PTAH-stained sections of infarct area for wild-type (+/+) and Adamts13−/− (−/−) mice. There were more thrombi and inflammatory cells in the lesions of Adamts13−/− mice compared with +/+ mice (black arrows represent thrombus; white arrow, inflammatory cells infiltration). The area comparable with PTAH staining for −/− mice was immunostained using anti-VWF antibody. VWF is detected in thrombi as brown staining (−/− ipsilateral anti-VWF). Images were generated using an Olympus BH-2 microscope with an Olympus DP20-5 digital camera (original magnification ×200). Bar represents 40 μm.
Effect of Adamts13 gene deletion on brain infarct in mice after 30-minute MCAO. (A) These are coronal sections through the brain in both groups stained with 2,3,5 triphenyltetrazolium chloride. Red areas represent vital brain tissue, and white areas represent cerebral infarction. Adamts13−/− (−/−) mice had significantly larger volume of brain infarct compared with wild-type (+/+) mice after 23.5-hour reperfusion after 30-minute MCAO (−/−, n = 16, vs +/+, n = 16, **P < .01, Student t test). Values are mean ± SEM (mm3). The average infarct volume was 31.0 ± 6.5 mm3 for ADAMTS13 −/− mice and 11.4 ± 1.9 mm3 for +/+ mice. The survival rates of the −/− and +/+ mice did not differ (17 of 20 vs 16 of 20, respectively). No ischemic change was observed in the brain of −/− and +/+ mice after sham operation. (B) Neurologic score was measured 24 hours after MCAO. Neurologic scores were measured from the point according to the neurologic findings as follows: 0 indicates normal motor function; 1, flexion of torso and of contralateral forelimb on lifting of the animal by the tail; 2, circling to the ipsilateral side but normal posture at rest; 3, circling to the ipsilateral side; 4, rolling to the ipsilateral side; and 5, leaning to the ipsilateral side at rest (no spontaneous motor activity). *P < .05. (C) Representative PTAH-stained sections of infarct area for wild-type (+/+) and Adamts13−/− (−/−) mice. There were more thrombi and inflammatory cells in the lesions of Adamts13−/− mice compared with +/+ mice (black arrows represent thrombus; white arrow, inflammatory cells infiltration). The area comparable with PTAH staining for −/− mice was immunostained using anti-VWF antibody. VWF is detected in thrombi as brown staining (−/− ipsilateral anti-VWF). Images were generated using an Olympus BH-2 microscope with an Olympus DP20-5 digital camera (original magnification ×200). Bar represents 40 μm.
Our results indicate that ADAMTS13 is crucial in vivo to protect the brain from ischemia-reperfusion injury by ameliorating postischemic hypoperfusion. The possible neuroprotective effect of ADAMTS13 may result from the cleavage of ULVWF secreted from endothelium activated by ischemia3,15 and cleavage of VWF multimers on the surface of thrombi formed on the ischemic endothelial cells.4 Without adequate ADAMTS13, progressive platelet thrombus growth may narrow the microvascular lumen, increasing fluid shear stress locally. Without negative feedback regulation by proteolysis of VWF, ischemia-reperfusion injury may cause a vicious cycle in which the VWF-platelet thrombosis and fluid shear stress enhance each other and contribute to the progressive deterioration of cerebral blood reflow, as observed in the Adamts13−/− mice. ADAMTS13 may, therefore, prevent microvascular occlusion under high shear stress and augment the cerebral blood flow after ischemia-reperfusion in vivo.
In addition to VWF-platelet thrombus formation,10,11,16,17 microvascular plugging by activated leukocytes18-20 can lead to no-reflow phenomena in brain ischemia.11 Importantly, platelet-ULVWF strings support leukocyte tethering on the endothelium under high fluid-shear stress.21,22 A recent study suggested that deficiency of ADAMTS13 can increase leukocyte adhesion on the vessels and extravasation.23 Thus, ADAMTS13 deficiency may enhance the leukocyte activation in the ischemic cerebral vasculature after reperfusion and thereby aggravate ischemic brain damage. Indeed, Adamts13−/− mice accumulated more inflammatory cells in the brain tissue than wild-type mice after MCAO (Figure 2C), suggesting that ADAMTS13 may reduce inflammation as well as thrombosis associated with ischemia-reperfusion injury.
In conclusion, ADAMTS13 deficiency causes progressive decline of postischemic rCBF, with a resultant exacerbation of ischemic brain injury, suggesting an important role of ADAMTS13 in neuroprotection. The regulation of VWF-platelet interactions by supplementation with ADAMTS13 may ameliorate ischemia-reperfusion injury of the brain. Because ADAMTS13 tends to dissolve excessive VWF-platelet thrombi with increasing efficiency as the flow path narrows, treatment of acute ischemic stroke with ADAMTS13 might have a relatively low risk of hemorrhagic complications.
This study was presented in part at the 50th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2008.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof.
After submission of our paper, the complementary paper by Zhao et al24 appeared in Blood, which also demonstrated the important role of ADAMTS13 for brain reperfusion injury.
Acknowledgments
The authors thank Dr Kouko Tatsumi and Dr Takahiko Kasai at the Laboratory of the Department of Anatomy and Neuroscience, and Diagnostic Pathology, respectively, in Nara Medical University, for histologic assistance.
Authorship
Contribution: M. Fujioka and K.H. performed most of the animal experiments and prepared the manuscript; A.K., K.I., S.H., T.N., and C.M. helped to perform the animal experiments; H.F. and M.S. worked on the experimental design and data analysis; K.M., M. Fujiwara, K.O., and K.N. provided direction throughout the work, made the overall experimental design, and edited the manuscript; and F.B., K.K., and T.M. produced the ADAMTS13 gene-deleted mice.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenji Nishio, Department of Emergency and Critical Care Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara 634-8522, Japan; e-mail: knishio@naramed-u.ac.jp.
References
Author notes
M. Fujioka and K.H. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal