Abstract
Regulation of growth factor and cytokine signaling is essential for maintaining physiologic numbers of circulating hematopoietic cells. Thrombopoietin (Tpo), acting through its receptor c-Mpl, is required for hematopoietic stem cell maintenance and megakaryopoiesis. Therefore, the negative regulation of Tpo signaling is critical in many aspects of hematopoiesis. In this study, we determine the mechanisms of c-Mpl degradation in the negative regulation of Tpo signaling. We found that, after Tpo stimulation, c-Mpl is degraded by both the lysosomal and proteasomal pathways and c-Mpl is rapidly ubiquitinated. Using site-directed mutagenesis, we were able to determine that c-Mpl is ubiquitinated on both of its intracellular lysine (K) residues (K553 and K573). By mutating these residues to arginine, ubiquitination and degradation were significantly reduced and caused hyperproliferation in cell lines expressing these mutated receptors. Using short interfering RNA and dominant negative overexpression, we also found that c-Cbl, which is activated by Tpo, acts as an E3 ubiquitin ligase in the ubiquitination of c-Mpl. Our findings identify a previously unknown negative regulatory pathway for Tpo signaling that may significantly impact our understanding of the mechanisms affecting the growth and differentiation of hematopoietic stem cells and megakaryocytes.
Introduction
Hematopoiesis is tightly regulated by several cytokines and growth factors to ensure that numbers of circulating blood cells remain constant under normal conditions. In many hematologic disorders, cytokine and growth factor signaling is dysfunctional, resulting in the overproduction or underproduction of 1 or more blood cell lineages. Thrombopoietin (Tpo) is a hematopoietic cytokine that, via its receptor c-Mpl, supports hematopoietic stem cell maintenance and proliferation and is the primary regulator of megakaryopoiesis.1,2 Absence of Tpo signaling results in thrombocytopenia, reduced numbers of transplantable stem cells, and eventually aplastic anemia in humans.3-5 Conversely, excessive Tpo signaling, usually due to mutations in c-Mpl or its secondary signaling proteins, results in hyperproliferation of numerous cell lineages, causing myeloproliferative syndromes.6-8 Therefore, the control of Tpo-mediated signaling is critical in maintaining physiologic numbers of circulating blood cells.
Protein phosphatases, suppressors of cytokine signaling (SOCS) proteins, and inhibitory intracellular mediators are all mechanisms that contribute to the negative regulation of cytokine signaling.9-12 However, the process of receptor internalization and degradation is one of the quickest and most effective ways in which activated receptors are negatively regulated. We recently demonstrated a mechanism for Tpo-stimulated c-Mpl internalization, through the interaction of adaptor protein 2 with YRRL motifs located at Y521 and Y591 in the c-Mpl intracellular domain; elimination of these sites significantly reduced degradation of the receptor.13
Ubiquitination is a posttranslational modification involving the covalent attachment of the small (∼ 8 kDa) protein ubiquitin to lysine residues of target proteins. Ubiquitination relies on the activities of 3 groups of enzymes; ubiquitin-activating enzymes (E1), ubiquitin carrier proteins (E2), and ubiquitin protein ligases (E3).14 The attachment of single ubiquitin molecules to target proteins (monoubiquitination) has previously been shown to mediate protein trafficking and intracellular signaling.15 However, activated ubiquitin is also able to form direct interactions with other ubiquitin molecules via 1 of the 7 lysine residues (usually lysine 48). Once 4 or more ubiquitins are conjugated in a polyubiquitin chain, the protein is targeted to the proteasome and degraded. The process of ubiquitination and proteasomal degradation of transmembrane growth factor receptors is a common regulatory mechanism and has been identified in several different systems (reviewed in Marmor and Yarden16 ). Studies of the ubiquitination and degradation of the epidermal growth factor receptor and platelet derived growth factor receptor, colony-stimulating factor-1, ErbB-2, and Met have identified c-Cbl as one of the E3 ligases responsible for receptor ubiquitination in response to growth factor stimulation.17-21 In these examples, stimulation of the receptor leads to recruitment of Cbl, either directly through phosphorylated tyrosine residues in the receptor or indirectly via adaptor proteins.22,23 Cbl is then phosphorylated, stimulating its E3 ligase activity.
In the current studies, we further explored the molecular mechanisms of c-Mpl degradation, specifically testing the role of ubiquitination as a negative regulator of Tpo signaling in c-Mpl–expressing cell lines, murine megakaryocytes, and human platelets. We then identified which lysines in the intracellular domain of c-Mpl are ubiquitinated and determined their effects on c-Mpl degradation, Tpo-dependent cell growth, and levels of receptor phosphorylation in response to Tpo. Finally, using short interfering RNA (siRNA) and dominant negative overexpression, we determined that c-Cbl significantly contributes to c-Mpl ubiquitination and degradation through its E3 ubiquitin ligase activity. Our findings identify a previously unknown negative regulatory mechanism for Tpo signaling that may significantly impact our understanding of the mechanisms affecting the growth and differentiation of hematopoietic stem cells and megakaryocytes.
Methods
Chemicals and reagents
The proteasome inhibitor MG-132 and Janus kinase 2 (JAK2)–specific inhibitor JAKI were purchased from Calbiochem. The lysosome inhibitor NH4Cl, cycloheximide, and mouse monoclonal anti-Na+/K+-ATPase α1 antibody were purchased from Sigma-Aldrich. N-terminal–specific polyclonal rabbit anti–human c-Mpl antibody was provided by Amgen Pharmaceuticals, and C-terminal–specific polyclonal rabbit anti–human c-Mpl antibody was purchased from Millipore. The mouse monoclonal antipolyubiquitin antibody was purchased from Biomol. Rabbit anti–c-Cbl, rabbit anti-phospho–signal transducer and activator of transcription 5 (STAT5) and rabbit anti-STAT5 were purchased from Cell Signaling Technologies. Monoclonal mouse antiactin was purchased from Sigma-Aldrich. Secondary antibodies, goat anti–rabbit horseradish peroxidase and rabbit anti–mouse horseradish peroxidase were purchased from Santa Cruz Biotechnology and fluorescently labeled goat anti–rabbit 488 was purchased from Invitrogen. Membrane-impermeable sulfo-N-hydroxysuccinimido-biotin and neutravidin-coupled agarose beads were purchased from Pierce. Recombinant human (rh) Tpo was a gift from Don Foster (Zymogenetics, Seattle, WA).
Cell lines, cell culture, and platelet isolation
The interleukin-3 (IL-3)–dependent prolymphoid cell line BaF3 expressing human c-Mpl (BaF-Mpl) was maintained in RPMI1640 medium (Invitrogen) containing 10% fetal bovine serum (FBS) supplemented with IL-3 (2 μL/mL of conditioned medium from IL-3–producing baby hamster kidney cells). The retroviral packaging cell line Platinum-E was maintained in Dulbecco modified Eagle medium supplemented with 10% FBS. To generate BaF-Mpl clones, subconfluent Platinum-E cells were transfected with pMX-puro-c-Mpl constructs using Lipofectamine 2000 transfection reagent (Invitrogen) for 48 hours, prior to viral supernatant collection. BaF3 cells lines were then incubated in viral supernatant supplemented with IL-3 for 48 hours before selection with puromycin (2 μg/mL; Invitrogen). Clonal populations were generated by limiting dilution. Total c-Mpl protein expression was determined by Western blot and cell surface expression was confirmed using flow cytometry for membrane-localized c-Mpl as previously described.13 Platelets were isolated after collection of venous blood from healthy volunteers and resuspended in Walsh buffer (137mM NaCl, 20mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5.6mM dextrose, 1 mg/mL bovine serum albumin, 1mM MgCl2, 2.7mM KCl, 3.3mM NaH2PO4, pH 7.4) prior to treatment.
Receptor constructs
Wild-type human c-Mpl (NM_005373.1) was cloned into the retroviral expression vector pMX-puro using EcoRI and XhoI cloning sites. The c-Mpl intracellular domain point mutations (c-Mpl K553R, c-Mpl K573R, and c-Mpl K553+ 573R) were introduced using the QuikChange mutagenesis kit (Stratagene) with the following oligonucleotides: K553R forward primer: 5′-CCTGAGCCCGCCCAGGGCCACAGTC-3′, K553R reverse primer: 5′-GACTGTGGCCCTGGGCGGGCTCAGG-3′, K573R forward primer: 5′-CTTGAAATCCTCCCCAGGTCCTCAGAGAGGACTCC-3′; and K573R reverse primer: 5′-GGAGTCCTCTCTGAGGACCTGGGGAGGATTTCAAG-3′. Mutations were confirmed by sequencing of genomic DNA isolated from individual clones. Wild-type mouse c-Cbl (NM_007619.2) cDNA was amplified from the cDNA of BaF3-Mpl and was cloned into the mammalian expression vector pcDNA 3.1 (Invitrogen) using NotI and XhoI cloning sites. The c-Cbl point mutation in the RING-finger domain (c-Cbl C379A) was introduced using the QuikChange mutagenesis kit (Stratagene) with the following oligonucleotides: C379A forward primer: 5′-GCTATCAACAAGGCGGAGGTGCCACTGCTAACCCTGTGGCC-3′, C379A reverse primer: 5′-GGCCACAGGGTTAGCAGTGGCACCTCCGCCTTGTTGATAGC-3′.
Immunoblotting and immunoprecipitation
After chemical treatment or Tpo stimulation, BaF-Mpl cell lines were lysed in Nonidet P40 (NP-40) lysis buffer (50mM tris(hydroxymethyl)aminomethane-HCl, pH 7.4, 1% NP-40, 150mM NaCl, 1mM ethylenediaminetetraacetic acid, 10mM β-glycerolphosphate, 1mM Na3VO4, 10mM NaF) containing 1% protease inhibitors (Sigma-Aldrich). Denatured proteins were fractionated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. Protein expression was detected by incubating with specific antibodies and visualized by chemiluminescent detection reagent (ECL-plus; GE Healthcare). Western blots were quantified by densitometry using ImageJ analysis software (National Institutes of Health, http://rsbweb.nih.gov/ij). For immunoprecipitations, cells were lysed in NP-40 lysis buffer. Supernatants were precleared by preincubating for 1 hour at 4°C with protein A beads (Millipore) before antibody incubation overnight. Immunoprecipitates were washed before resuspension in Laemmli buffer (125mM tris(hydroxymethyl)aminomethane-HCl, 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue), and boiled for 5 minutes at 100°C. Samples were all subjected to SDS–polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes.
c-Mpl turnover assay
BaF-Mpl and BaF-MplK553+573R cells growing in log phase in IL-3 were washed 3 times in phosphate-buffered saline before membrane proteins were labeled with sulfo-N-hydroxysuccinimido-biotin for 1 hour at 4°C as previously described.13 The biotinylation reaction was quenched by the addition of 100mM glycine and unbound biotinylation reagent was removed by repeated washing. Cells were then returned to media containing 10% FBS without cytokines for 0 to 6 hours before cell lysis. Biotinylated protein was pulled down using neutravidin-labeled agarose beads and precipitates were analyzed by Western blot for c-Mpl. Loading of membrane proteins was controlled by reprobing for the ubiquitous membrane localized Na+/K+ ATPase α1.
MTT proliferation assays
BaF-Mpl clones in log-phase growth were washed 3 times with phosphate-buffered saline to remove IL-3, resuspended in RPMI1640 supplemented with 2% FBS, and plated into 96-well plates at a concentration of 1000 cells/well. rhTpo was then added at concentrations ranging from 1 pg/mL to 100 ng/mL. Cells were incubated for 48 hours before treatment with 2 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (Sigma) and incubated at 37°C for 5 hours. Cells were lysed and formazan crystals dissolved in 100 μL of MTT lysis solution (dH2O, 20% SDS, 40% N,N-dimethyl formamide, 2% acetic acid, 0.2% HCl) for 6 hours at 37°C. Absorbance was then read on a colorimetric plate reader at 570 nm. Each data point is expressed as a percentage of proliferation stimulated by a maximal dose of murine IL-3 (4 μL/mL murine IL-3 supernatant). Each experiment was performed in triplicate with 2 different clones for each wild-type or mutant c-Mpl construct.
RNA interference
siRNA to murine c-Cbl was purchased from Thermo Scientific. A combination of 4 specific siRNAs was used (siGenome SMARTpool M-040165-00-0005): (1) GAUCUGACCUGCAAUGAUU, (2) GGAGACACUUUCCGGAUUA, (3) GGCGAAACCUGACCAAAUU, and (4) GAAGAGGACACAGAAUAUA. siRNA was transfected into cells using an Amaxa nucleofector (Lonza) as described previously.13 Protein knockdown was analyzed by Western blot 48 hours after transfection.
Results
Tpo stimulation mediates proteasomal and lysosomal degradation of c-Mpl
First, we determined whether c-Mpl was degraded via the proteasomal and/or lysosomal pathways after Tpo stimulation. After incubation for 12 hours in starvation medium (RPMI, 2% FBS without cytokines), BaF-Mpl cells were treated with the proteasome inhibitor MG-132, or the lysosome inhibitor NH4Cl, in combination with cycloheximide (Chx) to prevent de novo protein synthesis, and rhTpo for the times indicated in Figure 1.
Tpo-stimulated c-Mpl is degraded by both the proteasome and the lysosome. (A) BaF-Mpl cells were treated with Chx for 0 to 240 minutes to inhibit synthesis of new protein in conjunction with or without rhTpo, MG-132, and NH4Cl. c-Mpl degradation was determined by the presence or absence of the mature 85-kDa form of c-Mpl. (B) Similar experiments were also performed on bone marrow–derived murine megakaryocytes and human platelets. The data shown are representative of 3 independent experiments.
Tpo-stimulated c-Mpl is degraded by both the proteasome and the lysosome. (A) BaF-Mpl cells were treated with Chx for 0 to 240 minutes to inhibit synthesis of new protein in conjunction with or without rhTpo, MG-132, and NH4Cl. c-Mpl degradation was determined by the presence or absence of the mature 85-kDa form of c-Mpl. (B) Similar experiments were also performed on bone marrow–derived murine megakaryocytes and human platelets. The data shown are representative of 3 independent experiments.
The mature cell surface c-Mpl receptor displays a molecular weight of 85 kDa due to extensive glycosylation, compared with that localized to the secretory pathway, which is an 80-kDa form.13,24 In the absence of rhTpo, BaF-Mpl cells slowly degraded the surface receptor form of c-Mpl protein (Figure 1A). In contrast, cells treated with rhTpo exhibited extensive degradation of mature c-Mpl protein within 60 minutes, which was greatly inhibited by pretreatment with MG-132. Although the expression of c-Mpl did not vary between control BaF-Mpl cells and those pretreated with the lysosomal inhibitor NH4Cl for the first 60 minutes after the addition of Tpo to the cultures, significantly less receptor degradation was observed between 60 and 120 minutes. To confirm these findings in a more physiologically relevant setting, we purified and studied murine megakaryocytes from C57/Bl6 mice (Figure 1B left panel). In contrast to BaF-Mpl cells, murine megakaryocytes display proportionately far more of the mature 85-kDa protein than the immature intracellular 80-kDa form, which was almost absent. Megakaryocytic c-Mpl protein was significantly reduced after 60 minutes of Tpo stimulation, which was blocked by adding both MG-132 and NH4Cl. Interestingly, c-Mpl was not degraded after Tpo stimulation of isolated human platelets (Figure 1B right panel).
Tpo stimulation causes ubiquitination of c-Mpl
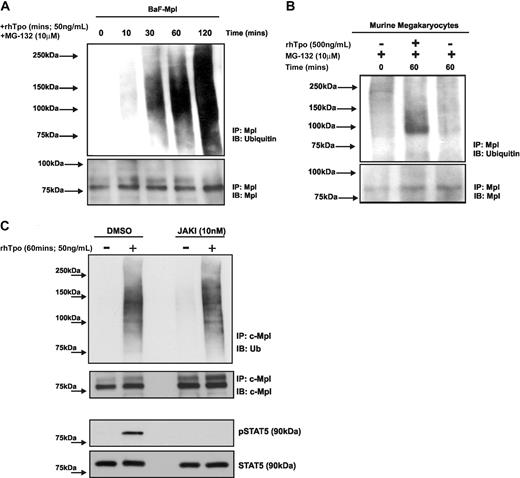
As proteasomal degradation of proteins requires polyubiquitination, we next examined the effects of Tpo stimulation on c-Mpl ubiquitination. BaF-Mpl cells were treated with MG-132 for 135 minutes and Tpo was added 10 to 120 minutes before the end of incubation with MG-132. Control cells were incubated with MG-132 alone for 135 minutes. Whole-cell lysates were immunoprecipitated with an anti-Mpl antibody and analyzed by Western blotting using an antiubiquitin antibody. Ubiquitinated c-Mpl was detected 10 to 30 minutes after Tpo stimulation as a high-molecular-weight smear (Figure 2A). Using murine megakaryocytes we also found increased c-Mpl ubiquitination 60 minutes after Tpo stimulation (Figure 2B). Next we determined the role of Janus kinase 2 (JAK2) in Tpo-stimulated c-Mpl ubiquitination by pretreating BaF-Mpl cells with the JAK2 inhibitor JAKI for 30 minutes before Tpo stimulation. We found no significant difference in Tpo-stimulated c-Mpl ubiquitination in the presence of JAKI (Figure 2C). The effectiveness of JAKI was determined by performing Western blots for phospho-STAT5, which is directly phosphorylated by active JAK2. We found that the inhibitor was effective in fully blocking STAT5 phosphorylation.
Tpo stimulates ubiquitination of c-Mpl. (A) BaF-Mpl cells were treated with or without MG-132 and rhTpo for 0 to 120 minutes and c-Mpl ubiquitination was analyzed by c-Mpl immunoprecipitation (IP) and immunoblot (IB) analysis using an anti-mono/polyubiquitin antibody. Ubiquitination is seen as a high-molecular-weight smear. The data shown are representative of 3 independent experiments. The equivalence of protein loading was analyzed by reprobing the IB with an anti–c-Mpl antibody. (B) Bone marrow–derived megakaryocytes were pretreated with MG-132 for 0 or 60 minutes, with or without rhTpo. Megakaryocyte c-Mpl ubiquitination was analyzed as described in panel A and the blot shown is representative of 2 independent experiments. (C) BaF-Mpl cells were pretreated with vehicle control (DMSO) or the JAK2 inhibitor JAKI for 30 minutes before Tpo stimulation (50 ng/mL, 60 minutes) and c-Mpl ubiquitination was analyzed. The effectiveness of JAKI to inhibit JAK2 activity was determined by analyzing levels of phosphor (p)–STAT5. Data are representative of 2 independent experiments.
Tpo stimulates ubiquitination of c-Mpl. (A) BaF-Mpl cells were treated with or without MG-132 and rhTpo for 0 to 120 minutes and c-Mpl ubiquitination was analyzed by c-Mpl immunoprecipitation (IP) and immunoblot (IB) analysis using an anti-mono/polyubiquitin antibody. Ubiquitination is seen as a high-molecular-weight smear. The data shown are representative of 3 independent experiments. The equivalence of protein loading was analyzed by reprobing the IB with an anti–c-Mpl antibody. (B) Bone marrow–derived megakaryocytes were pretreated with MG-132 for 0 or 60 minutes, with or without rhTpo. Megakaryocyte c-Mpl ubiquitination was analyzed as described in panel A and the blot shown is representative of 2 independent experiments. (C) BaF-Mpl cells were pretreated with vehicle control (DMSO) or the JAK2 inhibitor JAKI for 30 minutes before Tpo stimulation (50 ng/mL, 60 minutes) and c-Mpl ubiquitination was analyzed. The effectiveness of JAKI to inhibit JAK2 activity was determined by analyzing levels of phosphor (p)–STAT5. Data are representative of 2 independent experiments.
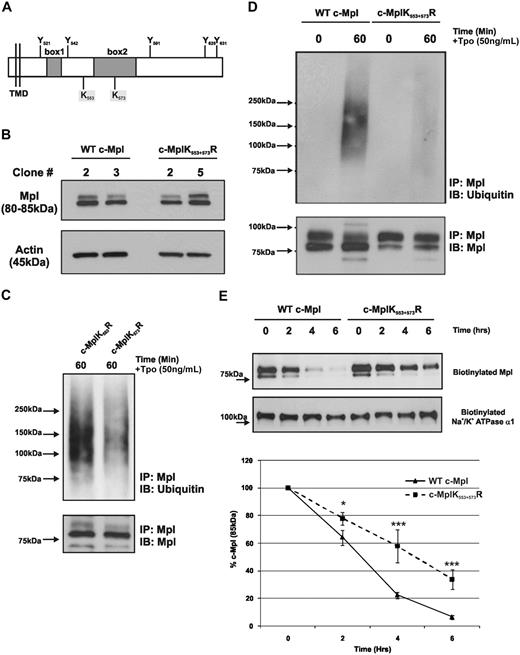
To further investigate the effects of monoubiquitination and polyubiquitination on the function of c-Mpl, we mutated the 2 intracellular lysine (K) residues K553 and K573 in the wild-type receptor to arginine (R; Figure 3A). We next generated 2 wild-type c-Mpl and 2 mutant c-MplK553+573R clones and confirmed equal total expression of c-Mpl protein by Western blotting (Figure 3B) as well as equal cell surface expression by flow cytometry (data not shown). Receptors bearing only 1 mutation (c-MplK553R and K573R) exhibited normal ubiquitination, indicating that both lysines are sites of ubiquitination (Figure 3C). By mutating both intracellular lysine residues, we prevented Tpo-stimulated ubiquitination of c-Mpl (Figure 3D). All of the 3 mutated cell lines (K553R, K573R, and K553+573R) exhibited normal Tpo-stimulated c-Mpl internalization compared with control cells (data not shown). Next, we determined whether ubiquitination of c-Mpl is involved in the turnover of mature, cell surface–localized receptor in the absence of Tpo, using cells expressing wild-type (WT)–Mpl and MplK553+573R. To detect the level of cell surface c-Mpl, we developed a sensitive membrane protein biotinylation assay, in which all surface proteins are first biotinylated, and after various time intervals, the cells were lysed, the remaining surface proteins were specifically captured with avidin beads, and c-Mpl was recognized by Western blotting. We found a significant decrease in the normal cell surface turnover of c-MplK553+573R compared with WT c-Mpl (Figure 3E). For example, after 4 hours, 58% (± 12%) of c-MplK553+573R remained on the cell surface, compared with just 22% (± 2%) of WT c-Mpl. A similar pattern was observed at 6 hours (c-MplK553+573R 34% (± 7%) versus WT c-Mpl 6% (± 1%).
c-Mpl is ubiquitinated at K553 and K573. (A) Schematic representation of the human c-Mpl intracellular domain, showing the position of the 2 intracellular lysine (K) residues in relation to tyrosine (Y) and box 1 and box 2. (B) Western blot analysis of total c-Mpl expression in 2 WT c-Mpl clones and 2 c-MplK553+573R clones. (C) Cells expressing c-Mpl with a single K to R mutation (c-Mpl K553R and c-Mpl K573R) display normal levels of c-Mpl ubiquitination in response to Tpo stimulation. (D) c-Mpl ubiquitination after 60-minute Tpo stimulation in BaF cells expressing WT c-Mpl or c-MplK553+573R. The data shown are representative of 3 independent experiments. (E) Biotinylation of mature, membrane-localized c-Mpl in BaF cells expressing WT c-Mpl and c-MplK553+573R to determine normal turnover over a time period of 6 hours. The Western blot shown is representative of 3 independent experiments. The graph displays a quantitation of the turnover of mature, 85-kDa c-Mpl in the 2 cell lines as determined by densitometry, compared with time 0 (100%). Protein loading is standardized by comparison to surface Na+/K+ ATPase α1 expression. The data points represent the mean ± SE of 3 independent experiments (*P < .05, ***P < .001; Student t test).
c-Mpl is ubiquitinated at K553 and K573. (A) Schematic representation of the human c-Mpl intracellular domain, showing the position of the 2 intracellular lysine (K) residues in relation to tyrosine (Y) and box 1 and box 2. (B) Western blot analysis of total c-Mpl expression in 2 WT c-Mpl clones and 2 c-MplK553+573R clones. (C) Cells expressing c-Mpl with a single K to R mutation (c-Mpl K553R and c-Mpl K573R) display normal levels of c-Mpl ubiquitination in response to Tpo stimulation. (D) c-Mpl ubiquitination after 60-minute Tpo stimulation in BaF cells expressing WT c-Mpl or c-MplK553+573R. The data shown are representative of 3 independent experiments. (E) Biotinylation of mature, membrane-localized c-Mpl in BaF cells expressing WT c-Mpl and c-MplK553+573R to determine normal turnover over a time period of 6 hours. The Western blot shown is representative of 3 independent experiments. The graph displays a quantitation of the turnover of mature, 85-kDa c-Mpl in the 2 cell lines as determined by densitometry, compared with time 0 (100%). Protein loading is standardized by comparison to surface Na+/K+ ATPase α1 expression. The data points represent the mean ± SE of 3 independent experiments (*P < .05, ***P < .001; Student t test).
BaF-MplK553+573R cells are hyperproliferative in response to Tpo
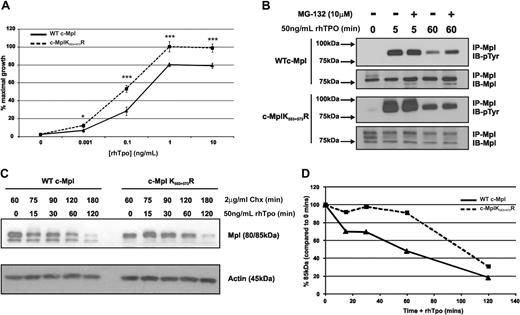
Next, we used the BaF-Mpl and BaF-MplK553+573R cells to determine the biologic significance of c-Mpl ubiquitination on Tpo-stimulated proliferation and signaling. Treatment with rhTpo caused the cells to proliferate in a concentration-dependent manner in all clones. Overall, BaF-MplK553+573R cells displayed increased Tpo-stimulated proliferation compared with BaF-Mpl cells. At submaximal concentrations of rhTpo (0.1 ng/mL), BaF-MplK553+573R reached 53% (± 1.3%) of IL-3–induced maximal cell proliferation compared with 28% (± 1.5%) in BaF-Mpl. At maximal concentrations of rhTpo (1 ng/mL), BaF-MplK553+573R cells proliferated to 98% (± 3.5%) of IL-3–induced maximal concentration, compared with 80% (± 2.8%) in BaF-Mpl cells (Figure 4A). To determine whether Tpo-induced signaling of c-MplK553+573R cells differed from BaF-Mpl cells, we examined several major signaling pathways normally stimulated by Tpo, including JAK2, STAT5, extracellular signal-related kinase 1/2, and phosphoinositide-3 kinase/AKT. Levels of activation at increasing concentrations of Tpo and signaling kinetics over a period of 180 minutes were determined, and no significant difference in these signaling pathways was observed (data not shown). However, c-Mpl phosphorylation was prolonged in BaF-Mpl cells pretreated with MG-132 60 minutes after Tpo stimulation (Figure 4B). In addition, compared with BaF-Mpl cells, BaF-MplK553+573R cells exhibited greatly increased (5 minutes) and prolonged (60 minutes) c-Mpl phosphorylation after Tpo stimulation. In contrast, MG-132 had no effect on prolonging phosphorylation in BaF-MplK553+573R cells. We next investigated the effects of c-MplK553+573R on c-Mpl degradation. BaF-Mpl and BaF-MplK553+573R cells were pretreated with Chx for 60 minutes, prior to stimulation with rhTpo for 0 to 120 minutes before c-Mpl protein was analyzed by Western blotting (Figure 4C-D). After 60 minutes, 49% of the wild-type c-Mpl protein remained, whereas 89% of the c-MplK553+573R was still present in Chx- and Tpo-treated cells. Degradation of c-MplK553+573R continued after 60 minutes, with only 32% of c-Mpl still detectable after 120 minutes.
c-MplK553+573R exhibits altered Tpo-induced proliferation and degradation. (A) MTT proliferation assay using BaF cells expressing WT c-Mpl or c-Mpl553+573R. Cells were treated with rhTpo at increasing concentrations for up to 48 hours. The data represent the mean (± SE) of 3 individual experiments using 2 stably expressing clones for each group (*P < .05, ***P < .001). (B) Western blot analysis of Tpo-induced c-Mpl phosphorylation with and without MG-132. The data are representative of 3 individual experiments. (C) Western blot analysis of Tpo-induced c-Mpl degradation of WT c-Mpl and c-MplK553+573R. Cells were pretreated with Chx for 60 minutes before Tpo stimulation for up to 120 minutes. (D) Graphic representation of c-Mpl degradation, comparing mature (85-kDa) c-Mpl at time 0 using densitometry.
c-MplK553+573R exhibits altered Tpo-induced proliferation and degradation. (A) MTT proliferation assay using BaF cells expressing WT c-Mpl or c-Mpl553+573R. Cells were treated with rhTpo at increasing concentrations for up to 48 hours. The data represent the mean (± SE) of 3 individual experiments using 2 stably expressing clones for each group (*P < .05, ***P < .001). (B) Western blot analysis of Tpo-induced c-Mpl phosphorylation with and without MG-132. The data are representative of 3 individual experiments. (C) Western blot analysis of Tpo-induced c-Mpl degradation of WT c-Mpl and c-MplK553+573R. Cells were pretreated with Chx for 60 minutes before Tpo stimulation for up to 120 minutes. (D) Graphic representation of c-Mpl degradation, comparing mature (85-kDa) c-Mpl at time 0 using densitometry.
C-Cbl acts as an E3 ligase in the ubiquitination of c-Mpl
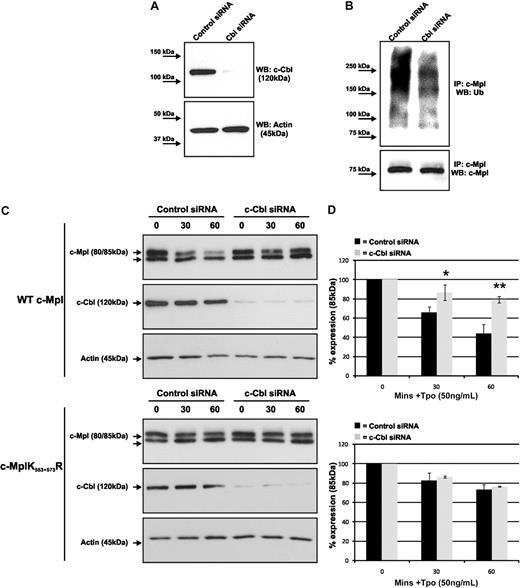
E3 ubiquitin ligases are required to covalently attach ubiquitin to lysine residues of target proteins. C-Cbl has previously been shown to act as an E3 ligase and is expressed in megakaryocytes and other hematopoietic cells. C-Cbl can also be activated by Tpo.25,26 To examine whether c-Cbl is involved in the degradation of c-Mpl, we first used specific siRNA to attenuate c-Cbl protein expression in BaF-Mpl cells (Figure 5A). Reduced c-Cbl protein expression resulted in a marked reduction of c-Mpl ubiquitination after Tpo stimulation compared with nontargeted siRNA-treated control BaF-Mpl cells (Figure 5B). We then determined the effects of c-Cbl siRNA on Tpo-stimulated c-Mpl degradation by preincubating nontargeted and c-Cbl siRNA-treated cells with Chx for 30 minutes before stimulating with Tpo for up to 60 minutes. After 60 minutes, 82% of mature (85-kDa) c-Mpl remained in the c-Cbl siRNA-treated cells, compared with 49% in the sample treated with a nontarget siRNA (Figure 5C-D top panels). c-Cbl siRNA experiments were also performed on BaF-MplK553+573R cells, which cannot be ubiquitinated (Figure 3D). In these cells, c-Cbl siRNA had no significant effect on Tpo-stimulated degradation (Figure 5C-D bottom panels). To confirm that c-Cbl is an E3 ligase for c-Mpl and not part of an indirect ubiquitination pathway, we determined the effects of c-CblC379A on c-Mpl turnover, a RING-finger domain E3 function inactivating mutant of c-Cbl. Stable WT-c-Cbl or c-CblC379A–overexpressing BaF-Mpl cells (Figure 6A) were first analyzed for Tpo-stimulated c-Mpl ubiquitination after 60 minutes after MG-132 pretreatment for 30 minutes. We found that ubiquitination was markedly reduced in cells that overexpressed c-CblC379A (Figure 6B) compared with wild-type c-Cbl. To examine whether overexpression of c-CblC379A would mimic the hyperproliferative phenotype found in BaF-MplK553+573R cells (Figure 4A), we performed an MTT proliferation assay with BaF-Mpl cells overexpressing the 2 forms of c-Cbl. We found a significant increase in cell growth at 1 and 10 ng/mL of rhTpo in BaF-Mpl/c-CblC379A compared with cells overexpressing wild-type c-Cbl (Figure 6C), consistent with our findings in the BaF-MplK553+573R cells.
c-Cbl siRNA reduces Tpo-induced c-Mpl ubiquitination and degradation. (A) c-Cbl siRNA significantly reduced expression of c-Cbl in BaF-Mpl cells 48 hours after transfection. (B) c-Mpl ubiquitination was reduced in cells treated with c-Cbl compared with those treated with nontargeting siRNA. The data shown are representative of 4 individual experiments. (C) Tpo-stimulated c-Mpl degradation was analyzed by Western blot using lysates from BaF-WT-Mpl and BaF-MplK553+573R cells treated with c-Cbl–specific and nontargeting siRNAs and pretreated with Chx for 60 minutes before Tpo stimulation for up to 60 minutes. (D) Graphic representation of degradation, comparing the mature (85-kDa) form of c-Mpl in cells treated with c-Cbl–specific siRNA compared with nontargeting siRNA. The data points represent the mean ± SE of 3 independent experiments (*P < .05, **P < .01; Student t test).
c-Cbl siRNA reduces Tpo-induced c-Mpl ubiquitination and degradation. (A) c-Cbl siRNA significantly reduced expression of c-Cbl in BaF-Mpl cells 48 hours after transfection. (B) c-Mpl ubiquitination was reduced in cells treated with c-Cbl compared with those treated with nontargeting siRNA. The data shown are representative of 4 individual experiments. (C) Tpo-stimulated c-Mpl degradation was analyzed by Western blot using lysates from BaF-WT-Mpl and BaF-MplK553+573R cells treated with c-Cbl–specific and nontargeting siRNAs and pretreated with Chx for 60 minutes before Tpo stimulation for up to 60 minutes. (D) Graphic representation of degradation, comparing the mature (85-kDa) form of c-Mpl in cells treated with c-Cbl–specific siRNA compared with nontargeting siRNA. The data points represent the mean ± SE of 3 independent experiments (*P < .05, **P < .01; Student t test).
c-Cbl acts as a ubiquitin E3 ligase in the Tpo-stimulated ubiquitination of c-Mpl. (A) BaF-Mpl cells overexpressing WT-Cbl or the E3 ligase dead CblC379A. (B) WT-Cbl– and CblC379A-expressing cells were pretreated with MG-132, then with or without rhTpo for 60 minutes, and c-Mpl ubiquitination was analyzed by IP and Western blot. The data shown are representative of 3 independent experiments. (C) These cells were analyzed for proliferation using an MTT assay at increasing concentrations of rhTpo. The data shown represent mean (± SE) of 3 independent experiments (*P < .05, ***P < .001).
c-Cbl acts as a ubiquitin E3 ligase in the Tpo-stimulated ubiquitination of c-Mpl. (A) BaF-Mpl cells overexpressing WT-Cbl or the E3 ligase dead CblC379A. (B) WT-Cbl– and CblC379A-expressing cells were pretreated with MG-132, then with or without rhTpo for 60 minutes, and c-Mpl ubiquitination was analyzed by IP and Western blot. The data shown are representative of 3 independent experiments. (C) These cells were analyzed for proliferation using an MTT assay at increasing concentrations of rhTpo. The data shown represent mean (± SE) of 3 independent experiments (*P < .05, ***P < .001).
Discussion
After ligand-induced activation of growth factor receptors, several negative feedback mechanisms modulate the strength and duration of intracellular signaling events to attain the appropriate cellular response.27-29 Lack or gain of function can lead to uncontrolled cell growth and malignancies.30 Although negative regulatory mechanisms have been extensively studied for several different membrane receptors, the mechanisms of regulation of activated c-Mpl after Tpo stimulation, in particular those of receptor degradation, are largely unknown. In this work, we demonstrate that c-Mpl is degraded by both proteasomal and lysosomal pathways after Tpo stimulation and that inhibition of receptor ubiquitination (and consequently proteasomal degradation) results in a hyperproliferative phenotype in hematopoietic cell lines.
Previous work on the erythropoietin receptor (EpoR), with which c-Mpl shares significant homology, has similarly shown roles for both proteasomal and lysosomal degradation of the receptor after stimulation with Epo.31 Indeed, kinetics of both degradation and ubiquitination of the receptors after activation also seem similar. However, Walrafen et al found that c-Cbl–specific siRNA and overexpression of mutated c-Cbl had no effect on EpoR ubiquitination.31 In this study, we demonstrated that c-Cbl, which can act as an E3 ligase, is important for Tpo-mediated ubiquitination of c-Mpl. Taken together, these 2 findings suggest that different molecular pathways mediate the ubiquitination of the 2 receptors. Although we were unable to demonstrate direct interactions between c-Mpl and c-Cbl by coimmunoprecipitation (data not shown), this may be due to the transient nature of the interaction between c-Cbl and its targets, or may indicate that an adaptor protein is required to associate c-Cbl and the c-Mpl receptor. The phosphatase SHP2 has been identified as an adaptor protein for the signaling subunit of the IL-6 receptor, gp130, and c-Cbl, enabling the ubiquitination of the receptor by c-Cbl.32 We have found that SHP2 can be recruited to activated c-Mpl (I.S.H., unpublished data), making a similar mechanism for the recruitment of c-Cbl to c-Mpl plausible. Interestingly, we also found that Tpo-stimulated c-Mpl ubiquitination appears to be a JAK2-independent pathway. JAK2-independent c-Mpl ubiquitination also supports our finding that ubiquitination and degradation of c-Mpl occur in the absence of cytokines. The results presented in Figure 3E suggest that surface-localized c-Mpl has a level of background turnover in which the majority of mature receptor is replaced roughly every 6 hours. Presumably, stimulation with Tpo greatly increases the kinetics of this turnover, by stimulating the same or different internalization and degradation pathways. It should be noted that reduced c-Cbl function, either by siRNA or overexpression of c-CblC379A, reduced rather than entirely prevented c-Mpl ubiquitination. Therefore it is highly likely that other ubiquitin E3 ligases, of which there are hundreds, also contribute to c-Mpl ubiquitination. We are currently investigating the signaling pathways responsible for unstimulated and Tpo-stimulated c-Mpl turnover, c-Mpl and c-Cbl interactions, and identification of other E3 ligases potentially involved in c-Mpl ubiquitination.
Because monoubiquitination of some membrane receptors has previously been described as critical for their internalization (reviewed in Hitchcock et al13 ), we addressed this question using a BaF-MplK553+573R cell model, which cannot be ubiquitinated in the intracellular domain. Using this cell line, we found that internalization of c-Mpl in response to Tpo was normal, demonstrating that alternative mechanisms are required for c-Mpl internalization. We recently described the importance of the intracellular motif Y591RRL for the recruitment of adaptor protein, which allows the rapid internalization of c-Mpl from the cell surface in a clathrin-dependent manner.33-37 Other receptors, such as those for epidermal growth factor, growth hormone, leptin, and transferrin, require ubiquitination for clathrin-dependent internalization.38,39 These receptors contain conserved intracellular tyrosine, serine, and lysine residues that are necessary for their ubiquitin-dependent internalization.6 In the case of c-Mpl, the replacement of both intracellular lysine residues with arginine did not alter the internalization kinetics. Consequently, we conclude that the ubiquitination of c-Mpl is not required for its internalization.
We also identified a role for c-Mpl ubiquitination as a negative feedback regulator of Tpo signaling. BaF-MplK553+573R cells, which cannot undergo ubiquitination in the intracellular domain, are hyperproliferative in response to Tpo. Although we found no significant difference in the major signaling pathways between c-MplK553+573R and WT-c-Mpl (data not shown), we did find increased and prolonged receptor phosphorylation. The prolonged presence of phosphorylated docking sites for other signaling proteins, particularly at intracellular Y625 and Y631, known binding sites for STATs and adaptor proteins growth factor receptor-bound protein 2, Son of sevenless, and SHC (reviewed in Rathinam et al40 ), may explain the observed hyperproliferative phenotype. Confirming our observations with the mutated c-Mpl, we found a similar, but not as potent, hyperproliferative phenotype after overexpression of c-Cbl with a disabled RING-finger domain. These findings are concurrent with the study by Rathinam et al, in which they described a hyperproliferative phenotype in hematopoietic stem cells that lack endogenous c-Cbl and instead express c-Cbl with an inactive RING-finger domain. The researchers described increased levels of STAT5 phosphorylation after Tpo stimulation and subsequently augmented c-Myc expression.41,42 Not only do our data support these findings, we hypothesize that one of the roles of c-Cbl is to act directly on c-Mpl as an ubiquitin E3 ligase, therefore mediating signaling after stimulation with Tpo. We are currently investigating the role of c-Cbl in the ubiquitination of c-Mpl in hematopoietic stem cells and primary mature megakaryocytes.
Interestingly, novel mutations affecting the E3 ligase function of c-Cbl have recently been described in patients with acute myeloid leukemia, suggesting a significant role for c-Cbl as a hematopoietic oncogene. In this regard, it would be extremely interesting to determine whether identical or different mutations of c-Cbl are present in the myeloproliferative disorders that are reliant on Tpo signaling, such as essential thrombocythemia and primary myelofibrosis.
Our studies provide novel insights into the regulatory mechanisms of Tpo signaling, determine the intracellular lysine residues of c-Mpl that are ubiquitinated after Tpo stimulation, and identify a role for c-Cbl as an E3 ubiquitin ligase for c-Mpl. Our findings provide novel insights into the regulation of Tpo signaling and degradation of c-Mpl. Similar mechanisms may also be of importance in regulating the signaling of numerous hematopoietic growth factors, critically affecting the proliferation and differentiation of hematopoietic lineages.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This research was supported by National Institutes of Health grants R01DK049855 and P01 HL078784-04.
National Institutes of Health
Authorship
Contribution: All authors have substantially contributed to the content of the paper and have agreed to the submission in its current format; V.S. performed research and analyzed data; A.E.G. and K.K. designed experiments, interpreted data, and edited the manuscript; and S.J.S. and I.S.H. designed and performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ian S. Hitchcock, Department of Medicine, University of California San Diego, 9500 Gilman Dr, MD 0726, La Jolla, CA 92093; e-mail: ihitchcock@ucsd.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal