Abstract
We hypothesized that progression of monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma (MM) reflects the escape of transformed plasma cells from T-cell recognition because of impaired antigen processing-presenting machinery (APM). We studied plasma cells and CD8+ T cells from bone marrow of 20 MGUS patients, 20 MM patients, and 10 control patients. Immunofluorescence and flow cytometry revealed significantly different patterns of APM component expression in plasma cells from the 3 groups. Compared with control patients, MM samples had lower expression of proteasome subunits and peptide transporters and greater expression of chaperones, considering both percentages of stained cells and molecular equivalents of soluble fluorochrome. MGUS samples had intermediate percentages of stained cells but molecular equivalents of soluble fluorochrome similar to control patients. Real-time polymerase chain reaction documented that APM changes occurred at the transcriptional level. Cytotoxicity assays demonstrated that MGUS CD8+ T cells lysed autologous transformed plasma cells more than MM CD8+ T cells did. MGUS progression correlated directly with calnexin, calreticulin, and tapasin and indirectly with δ, LMP2, and LMP10 expression levels; MM disease status did not correlate with APM levels. APM changes may allow transformed plasma cells to elude immunesurveillance in the MGUS-MM pathogenetic sequence.
Introduction
Monoclonal gammopathy of undetermined significance (MGUS) is a condition characterized by the clonal expansion of transformed plasma cells. Although MGUS may remain stable for several years, in some cases it progresses to multiple myeloma (MM), an inexorably lethal malignancy.1 Therefore, MGUS is considered a clinically premalignant condition. The mechanisms by which MGUS progresses into full-blown MM are incompletely understood. It has been recently recognized that both patients with MGUS and those with MM have functional CD8+ T cells specific for transformed plasma cells in the bone marrow (BM),2,3 thereby establishing that there is no tolerance to plasma cell tumors. Nonetheless, in MM, CD8+ T cells are unable to control the expansion of the malignant plasma cells. These observations have rekindled interest in the immunesurveillance mechanisms of tumor growth.4
One issue in particular under intense discussion is why CD8+ T cells fail to control the clonal expansion of transformed plasma cells in MM despite the fact that the malignant cells do not substantially differ from their premalignant MGUS precursors in terms of cytogenetic abnormalities5,6 and gene expression profiles, at least regarding the 5483 genes evaluated on HuGeneFL GeneChip microarrays.7 An answer to this conundrum has been sought, thus far, in the functional properties of CD8+ T cells from MM and MGUS patients, with somehow-conflicting results. Some reports showed that MM CD8+ T cells need prolonged in vitro stimulation to exert effector functions, whereas MGUS CD8+ T cells have a strong ex vivo reactivity to autologous preneoplastic plasma cells (unknown antigens) and to shared myeloma-associated antigens2,3,8 ; these findings indicate that MM CD8+ T cells are functionally impaired, as suggested previously.9 On the contrary, another study10 found that MM CD8+ T cells had prompt reactivity and intact efficiency for a human leukocyte antigen (HLA)–A2–restricted tumor-associated antigen peptide. An alternative explanation to why MM CD8+ T cells fail to control tumor progression could be that transformed plasma cells are impaired in the normal presentation of tumor antigens necessary for T-cell recognition. To the best of our knowledge, the ability of transformed plasma cells to process and present antigens via the HLA class I pathway has not been investigated.
The processing and presentation of HLA class I antigen-derived peptides is accomplished through a complex series of intracellular events involving multiple molecular species.11,12 These species include the constitutive proteasome subunits δ (β1), MB1 (X or β5), and ζ (β2), as well as the interferon-γ (IFN-γ)–inducible proteasome (immunoproteasome) β-type subunits LMP2, LMP7, and LMP10. Also involved are the peptide transporters TAP1 and TAP2 and the endoplasmic reticulum chaperones calnexin, calreticulin, ERp57, and tapasin. Acting in concert, these proteins are responsible for the generation of antigenic peptides and for their translocation to the endoplasmic reticulum and loading onto β2-microglobulin (β2m)–associated HLA class I heavy chains.13 Defects in the function or expression of antigen processing-presenting machinery (APM) components could affect the formation of HLA class I–β2m-peptide trimolecular complexes and their recognition by CD8+ cells expressing cognate T-cell receptors.
The present study was therefore conducted to test the hypothesis that progression of MGUS to MM involves the escape of transformed plasma cells from CD8+ T-cell recognition and destruction because of alterations in the plasma cells' APM. We measured the expression levels of the diverse APM components in premalignant and malignant plasma cells in comparison with normal cells. We determined whether altered expression levels were associated with impaired recognition by BM cytotoxic CD8+ T cells and whether they were regulated by interleukin-6 (IL-6), the central growth factor for transformed plasma cells,14,15 or by IFN-γ, the primary inducer of immunoproteasomes.16 Finally, we assessed the relation between these changes and both disease progression in MGUS patients and response to therapy in MM patients.
Methods
Study subjects and biologic samples
Paired peripheral blood (PB) and BM samples were obtained from 40 patients with newly diagnosed monoclonal gammopathies. Patients were classified as having MGUS (n = 20) or symptomatic MM (n = 20) according to the International Myeloma Working Group criteria.17 PB and BM samples also were obtained from 10 healthy control patients who were healthy family members or unrelated BM donors of patients undergoing allogeneic transplantation. Clinical records of MGUS patients were searched for information regarding serum levels of M component at the time of sampling and after 24 months of observation. Clinical records of MM patients were consulted for information regarding the type of chemotherapy administered after sampling and the patients' responses to treatment (remission vs stable or progressive disease).
The study protocol was approved by the University of Bari Medical School Ethics Committee and conformed to the good clinical practice guidelines of the Italian Ministry of Health and the ethical guidelines of the Declaration of Helsinki, as revised and amended in 2004. Written informed consent was obtained from each subject in accordance with the Declaration of Helsinki.
Primary cell isolation
BM and PB mononuclear cells (BMMCs and PBMCs) were isolated by Ficoll-Hypaque (Pharmacia Biotech) density gradient centrifugation. Normal plasma cells (CD38+) were isolated from healthy control patients' BMMC by automated magnetic cell sorting with anti-CD38 microbeads (Miltenyi Biotec). Premalignant and malignant (CD138+) plasma cells were isolated from MGUS and MM patients' BMMCs, respectively, by automated magnetic cell sorting with anti-CD138 microbeads (Miltenyi Biotec); both the microbead-selected cell fraction (transformed plasma cells) and the nonbinding (nonmalignant) cell fraction were cryopreserved in aliquots and retained for further use. CD8+ T cells were isolated from patients' nonmalignant BM-cell fraction by automated magnetic cell sorting with anti-CD8 microbeads (Miltenyi Biotec). Mean recovery was 26 680 CD8+ T cells per subject (SD = 8510).
Protein expression analysis
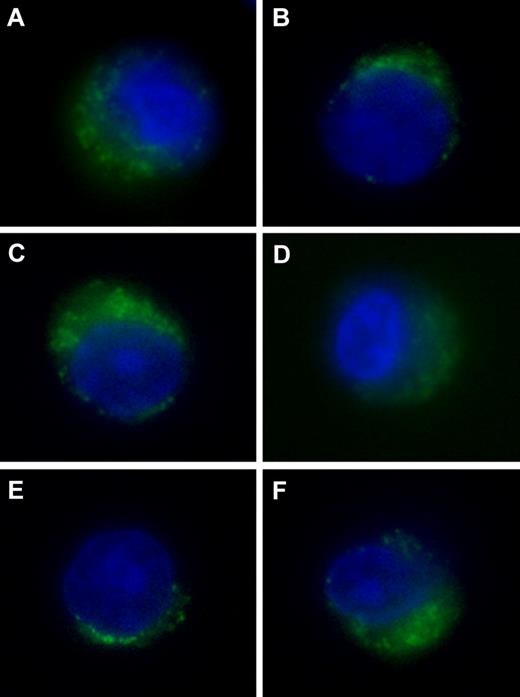
Flow cytometry and epifluorescence microscopy were performed on cells stained with a set of in-house and commercial antibodies. The following antibodies were produced and characterized as described previously18-22 : the δ-specific monoclonal antibody (mAb) SY-5; the MB1-specific mAbSJJ-3; the ζ-specific mAb NB1; the LMP2-specific mAb SY-1; the LMP7-specific mAb HB2; the LMP10-specific mAb TO-7; the TAP1-specific mAb NOB1; the TAP2-specific mAb NOB2; the calnexin-specific mAb TO-5; the calreticulin-specific mAb TO-11; the ERp57-specific mAb TO-2; the tapasin-specific mAb TO-3; and the β2-m–specific mAb NAMB-1. They were labeled with fluorescein isothiocyanate (FITC) by the use of the Pierce FITC Antibody Labeling Kit. FITC-conjugated mouse IgG1 anti–human HLA-ABC mAb, R-phycoerythrin (PE)–conjugated mouse IgG1 anti–human CD138 mAb, and peridinin-chlorophyll-protein complex–conjugated mouse IgG1 anti–human CD38 mAb were purchased from BD Pharmingen. The mouse IgG1 anti–human NY-ESO-1 (E978) mAb and the goat FITC-conjugated anti–mouse IgG1 Ab were purchased from Santa Cruz Biotechnology. For flow cytometry, BMMC from patients and controls were immunostained as described in supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). Expression levels of APM components were expressed as the percentage of positively staining cells and also were quantified in units of molecular equivalents of soluble fluorochrome (MESF).23 For epifluorescence microscopy, premalignant and malignant plasma cells from patients were fixed, permeabilized, and stained intracellularly as described for BMMCs. Cells were then incubated with 0.1 mg/mL DAPI (4,6 diamidino-2-phenylindole; Sigma-Aldrich), washed, and examined under a Nikon TE2000 inverted microscope with an epifluorescence source (Figure 1).
Expression of components of the HLA class I APM in transformed plasma cells. Patients with MGUS are shown on the left; MM on the right. CD138+ cells were immunomagnetically purified from BMMCs and stained with DAPI (blue fluorescence) and with FITC-conjugated mAb (green fluorescence) to proteasome subunits δ (A-B) and LMP2 (C-D) or to the chaperone calnexin (E-F) and examined by epifluorescence microscopy.
Expression of components of the HLA class I APM in transformed plasma cells. Patients with MGUS are shown on the left; MM on the right. CD138+ cells were immunomagnetically purified from BMMCs and stained with DAPI (blue fluorescence) and with FITC-conjugated mAb (green fluorescence) to proteasome subunits δ (A-B) and LMP2 (C-D) or to the chaperone calnexin (E-F) and examined by epifluorescence microscopy.
Gene expression analysis
mRNA levels of APM components were determined by the use of standard techniques and commercial kits (supplemental Methods).
Preparation of antigen-presenting cells
Antigen-presenting cells consisted of dendritic cells (DCs) exposed to plasma cell lysate, antibody-coated apoptotic plasma cells, or synthetic peptide. To prepare plasma cell lysates, premalignant and malignant plasma cells were resuspended in phosphate-buffered saline and lysed by 5 freeze-thaw cycles (on methanol-dry ice and at room temperature); total cell disruption was confirmed with the use of trypan blue staining. Lysed cells were sonicated for 10 minutes and centrifuged at 15 000g (30 minutes, 4°C). The top lipid layer was discarded, and the supernatant liquid was recovered, filtered, and quantified for protein concentration by spectrophotometry. To prepare antibody-coated apoptotic plasma cells, premalignant and malignant plasma cells were opsonized by incubation with anti–syndecan-1 mAb (1 μg/mL; Serotec) for 30 minutes at 4°C and then made apoptotic by γ-irradiation (50 Gy). HLA-A*0201–restricted NY-ESO-1157-165 (SLLMWITQV) and HIV-1 gag p1776-84 (SLYNTVATL) synthetic peptides were purchased from Proimmune.
DCs were generated by adherent culture of patients' PBMCs in the presence of granulocyte-macrophage colony-stimulating factor and IL-4 (both from PeproTech) as already described.24 After 5 days, DCs were transferred to 96-well U-bottom plates and pulsed with autologous plasma cell lysates (100 μg/mL), autologous opsonized apoptotic plasma cells (1:1 ratio), NY-ESO-1157-165 (10μM), or control HIV-1 gag p1776-84 peptide (10μM); some wells received no treatment (unloaded DCs). After 20 hours, a cytokine cocktail consisting of 10 ng/mL tumor necrosis factor-α, IL-6, and IL-1β (all from R&D Systems) and 1 μg/mL PGE2 (Sigma-Aldrich) was added to all wells. Cells were incubated overnight for maturation.
Generation of T-cell lines specific for premalignant and malignant plasma cell antigens
Patients' CD8+ T cells were cocultured in 96-well round-bottom plates with mature autologous unloaded DCs or with mature autologous DCs previously loaded with plasma cell lysate, antibody-coated apoptotic plasma cells, or synthetic peptide. Each well contained 2 × 105 T cells and from 0.67 × 104 to 2 × 104 DCs (DC/T-cell ratio between 1:10 and 1:30). Cells were grown at 37°C in 200 μL of RPMI 1640 supplemented with 10% heat-inactivated human AB serum, 2mM l-glutamine, 100 U/mL penicillin, and 10 μg/mL streptomycin (all from Sigma-Aldrich; culture medium). The medium was replaced with fresh culture medium containing 10 U/mL rIL-2 (PeproTech) on days 4, 7, 11, 14, and 18; on day 7, the fresh culture medium also contained 10 ng/mL rIL-7 (PeproTech). Cultures were restimulated with the same autologous DC preparations on days 7 and 14. On day 21, cells from several similarly treated wells were pooled and either subjected to automated magnetic cell sorting with anti-CD8 microbeads (to isolate CD8+ T cells) or stained with the NY-ESO-1157-165 HLA-A*0201 PE-conjugated pentamer (Proimmune) according to the manufacturer's instructions and then subjected to automated magnetic cell sorting with anti-PE microbeads (Miltenyi Biotec; to isolate pentamer-binding cells). These cells were used as effectors in cytotoxicity assays.
Preparation of targets for cytotoxicity assays
Target cells consisted of autologous premalignant or malignant plasma cells, autologous Epstein-Barr virus (EBV)–transformed B cells, and HLA-A*0201+ U266 myeloma cells (ATCC). EBV-transformed B cells were generated by infecting patients' PBMC with the supernatant of the EBV producer cell line B95.8 (LGC Promochem), as previously described.24 The resulting EBV-transformed B-cell lines were pulsed overnight with 10 μg/mL cell lysate from autologous premalignant or malignant plasma cells or 10 μg/mL control HIV-1 gag p1776-84 peptide. Pulsed EBV-transformed B cells, premalignant and malignant plasma cells, and U266 cells were labeled for 1 hour with 25 μCi of 51Cr (PerkinElmer) and washed twice with phosphate-buffered saline before being used as targets in cytotoxicity assays.
Cytotoxicity assays
CD8+ T cells specific for premalignant or malignant plasma cell antigens (effectors) were incubated with radiolabeled EBV-transformed B cells, autologous premalignant or malignant plasma cells, or U266 myeloma cells (target cells) in culture medium in round-bottom 96-well plates; each well contained 3 × 103 target cells and a serial dilution of effectors (from 1.5 to 6 × 104 cells). In some wells, the HLA-ABC–specific mAb TP25.99.8.425 was added at 10 μg/mL. The assay was performed in triplicate and repeated twice. After 6 hours at 37°C, the culture plates were centrifuged and the radioactivity released into the supernatant was quantified. Percent specific lysis was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release), where spontaneous release and maximum release reflect target cell lysis in the absence of effector cells and in the presence of 10% Triton X-100 (Sigma-Aldrich), respectively.
Effects of IL-6 and IFN-γ stimulation
Control patients' BMMC and patients' transformed (CD138+) plasma cells were incubated with 10 ng/mL IFN-γ (R&D Systems), 100 ng/mL IL-6 (R&D Systems), or no cytokine in RPMI 1640 supplemented with 10% heat-inactivated human AB serum and 2mM l-glutamine. After 36 hours, the expression levels of all 12 APM components were assessed at the protein level by immunofluorescence and flow cytometry and at the mRNA level by polymerase chain reaction, as described in the preceding paragraphs. Before selected cytotoxicity assays, targets (autologous premalignant and malignant plasma cells) were treated overnight with 100 ng/mL IL-6 before radiolabeling.
Effects of DNA demethylation
Myeloma cell lines were treated with decitabine and analyzed for APM component expression as described in supplemental Methods.
Statistical analysis
Statistical analyses were performed with Prism (GraphPad Software). Nonparametric statistics were used because many data were not normally distributed, and 2-tailed P values were calculated. Kruskal-Wallis analysis of variance was used to compare protein expression levels among 3 groups. The Mann-Whitney test was used to compare mRNA levels, protein levels, and percent specific lysis between MGUS and MM patients. The paired t test was used to compare percent specific lysis in the absence and presence of IL-6. The Wilcoxon signed rank test was used to compare protein expression levels before and after cytokine stimulation. The Spearman rank test was used to assess correlations between protein expression levels and either percent specific lysis or patients' clinical status. For clinical status of MGUS patients, we used, as an indicator of disease progression, the serum level of the M component 24 months after BM sampling, expressed as a percentage of the initial value. For MM patients, we considered each patient's disease status after first-line chemotherapy, assigning a score of 1 to patients in complete or partial remission and a score of 0 to patients with stable or progressive disease.
Results
To understand why MM develops and progresses despite the immune system's ability to generate tumor-specific CD8+ T-cell responses, we looked for alterations in the APM of transformed plasma cells from 20 patients with MGUS and 20 patients with newly diagnosed symptomatic MM compared with 10 healthy control patients.
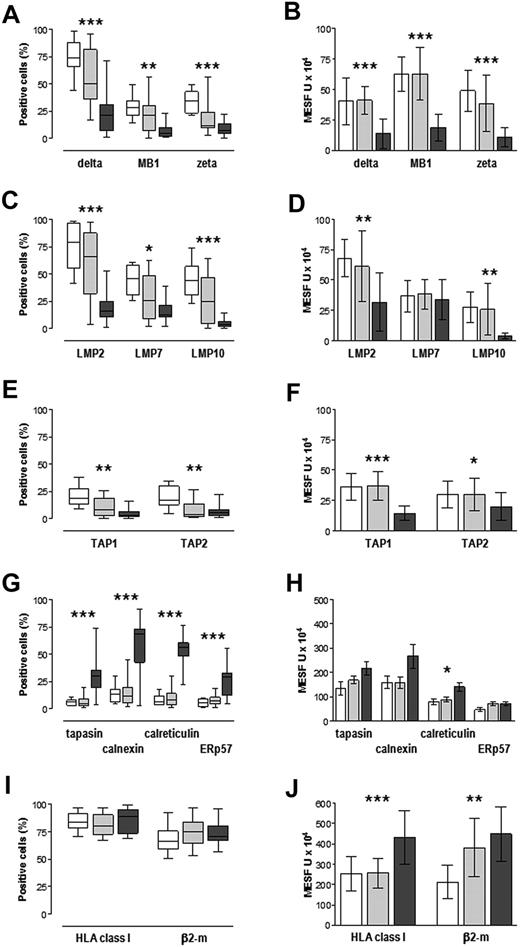
First, we determined the steady-state levels of APM proteins in normal (CD38+) and in premalignant and malignant (CD38+CD138+) plasma cells from BM of control patients and MGUS and MM patients, respectively. Overall, proteins were expressed with considerable variability, within and among the groups, as shown by both the percentage of cells staining positively (Figure 2 left) and the mean expression levels in positive cells, expressed as MESF units (Figure 2 right). Regarding the constitutive proteasome subunits δ, MB1, and ζ, the median percentage positive cells was greater in control patients than in MM samples, with intermediate but wide ranging values in MGUS samples (Figure 2A), whereas the expression levels (MESF units) were similar in control and MGUS samples but lower in MM samples (Figure 2B); these differences were significant (P < .001). Similar patterns were observed for the inducible proteasome subunits LMP2 and LMP10, whereas for LMP7 the expression levels (MESF units) were similar among the 3 groups (Figure 2C-D). The transporters TAP1 and TAP2 were expressed by low percentages of cells (median < 20% in all groups), but still similar patterns emerged and were significant: the control group had the greatest median percentage positive cells whereas the MM group had the lowest (P < .001; Figure 2E); expression levels (MESF units) were similar in control and MGUS samples and lower in MM samples (Figure 2F).
APM component expression in normal, premalignant, and malignant plasma cells. Control patients (□), MGUS patients ( ; n = 20), and MM patients (
; n = 20), and MM patients ( ; n = 20) are shown. BMMCs were immunostained for surface expression of CD38 (controls) or both CD38 and CD138 (MGUS and MM), HLA class I antigens, β2m, and for intracellular expression of individual APM proteins and analyzed by flow cytometry. (A,C,E,G,I) Percentages of CD38+ or CD38+CD138+ cells that stained positively for APM components, expressed as median, interquartile range (box), and range (whiskers). (B,D,F,H,J) Expression levels in positive cells, reported in units of MESF and expressed as median and standard deviation. *P < .05, **P ≤ .005, ***P < .001, Kruskal-Wallis test for variance among the 3 groups.
; n = 20) are shown. BMMCs were immunostained for surface expression of CD38 (controls) or both CD38 and CD138 (MGUS and MM), HLA class I antigens, β2m, and for intracellular expression of individual APM proteins and analyzed by flow cytometry. (A,C,E,G,I) Percentages of CD38+ or CD38+CD138+ cells that stained positively for APM components, expressed as median, interquartile range (box), and range (whiskers). (B,D,F,H,J) Expression levels in positive cells, reported in units of MESF and expressed as median and standard deviation. *P < .05, **P ≤ .005, ***P < .001, Kruskal-Wallis test for variance among the 3 groups.
APM component expression in normal, premalignant, and malignant plasma cells. Control patients (□), MGUS patients ( ; n = 20), and MM patients (
; n = 20), and MM patients ( ; n = 20) are shown. BMMCs were immunostained for surface expression of CD38 (controls) or both CD38 and CD138 (MGUS and MM), HLA class I antigens, β2m, and for intracellular expression of individual APM proteins and analyzed by flow cytometry. (A,C,E,G,I) Percentages of CD38+ or CD38+CD138+ cells that stained positively for APM components, expressed as median, interquartile range (box), and range (whiskers). (B,D,F,H,J) Expression levels in positive cells, reported in units of MESF and expressed as median and standard deviation. *P < .05, **P ≤ .005, ***P < .001, Kruskal-Wallis test for variance among the 3 groups.
; n = 20) are shown. BMMCs were immunostained for surface expression of CD38 (controls) or both CD38 and CD138 (MGUS and MM), HLA class I antigens, β2m, and for intracellular expression of individual APM proteins and analyzed by flow cytometry. (A,C,E,G,I) Percentages of CD38+ or CD38+CD138+ cells that stained positively for APM components, expressed as median, interquartile range (box), and range (whiskers). (B,D,F,H,J) Expression levels in positive cells, reported in units of MESF and expressed as median and standard deviation. *P < .05, **P ≤ .005, ***P < .001, Kruskal-Wallis test for variance among the 3 groups.
Finally, an opposite pattern emerged for the 4 chaperone proteins resident in the endoplasmic reticulum: low percentages of control and MGUS cells scored positively for these proteins (median < 10% for calreticulin, ERp57, and tapasin; < 15% for calnexin), whereas in MM samples the median percentages of cells staining positively were high with wide interpatient variability (Figure 2G); this pattern was significant (P < .001 for all 4 proteins). Expression levels (MESF units) of the chaperone proteins tended to be greater in MM samples, but this was significant only for calreticulin (P = .006; Figure 2H). Altogether, these results document altered patterns of expression of APM proteins in MM samples and also in MGUS samples, at least regarding the percentages of cells positive for proteasome and transporter proteins.
We next examined the cell surface expression of HLA class I antigens and β2m in the same samples. The majority of cells stained positively for HLA class I antigens, with median values of 84%, 80%, and 89% in control, MGUS, and MM samples, respectively. Median values of positive cells expressing β2m were also high (67%, 75%, and 71%, respectively). The variance among groups regarding the percentage cells positive for HLA or β2m was not significant (Figure 2I). A significant pattern emerged, however, when expression levels (MESF units) were assessed (Figure 2J). HLA class I antigens were expressed at greater levels in MM samples than in control patients and MGUS samples (P < .001). Expression of β2m was greater in MM samples than in controls, whereas MGUS samples had intermediate levels (P < .001).
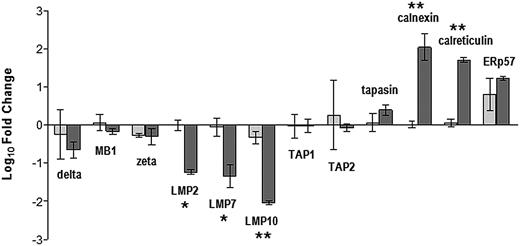
To determine whether the variations in protein expression reflected differences in the transcriptional activity of the relevant gene loci, we quantified mRNA encoding the 12 APM components in premalignant and malignant plasma cells and expressed the data as a fold change relative to the mean level for normal plasma cells from controls (Figure 3). Regarding the constitutive proteasome subunits, mRNA levels in MGUS and MM samples were similar to or slightly less than those of normal cells, without significant differences between the 2 groups. Regarding the inducible proteasome subunits, mRNA levels in MGUS samples were similar to those of control cells, whereas in MM samples they were reduced more than 10-fold (LMP2 and LMP7, P = .026 and P = .009) or more than 100-fold (LMP10, P < .001). mRNA levels of the 2 transporter proteins were similar to control levels. Regarding the chaperone proteins, MGUS samples had normal levels of mRNA encoding calnexin, calreticulin, and tapasin but elevated levels of ERp57; instead, in MM samples mRNA levels were elevated for all 4 chaperones, exceeding normal levels by 10-fold for calnexin, calreticulin, and ERp57 (P < .001 vs MGUS samples).
Relative levels of mRNA encoding individual APM components in immunomagnetically purified premalignant and malignant (CD138+) BM plasma cells determined by real-time reverse-transcription polymerase chain reaction and the 2−ΔΔCt method. indicates MGUS patients; and
indicates MGUS patients; and  , MM patients. APM transcript levels were normalized to those of glyceraldehyde 3-phosphate dehydrogenase in the same samples and then expressed as fold change relative to the average value for normal (CD38+) plasma cells from controls. Values shown are mean and SD for 20 MGUS and 20 MM patients. *P < .05, **P ≤ .005, Mann-Whitney test.
, MM patients. APM transcript levels were normalized to those of glyceraldehyde 3-phosphate dehydrogenase in the same samples and then expressed as fold change relative to the average value for normal (CD38+) plasma cells from controls. Values shown are mean and SD for 20 MGUS and 20 MM patients. *P < .05, **P ≤ .005, Mann-Whitney test.
Relative levels of mRNA encoding individual APM components in immunomagnetically purified premalignant and malignant (CD138+) BM plasma cells determined by real-time reverse-transcription polymerase chain reaction and the 2−ΔΔCt method. indicates MGUS patients; and
indicates MGUS patients; and  , MM patients. APM transcript levels were normalized to those of glyceraldehyde 3-phosphate dehydrogenase in the same samples and then expressed as fold change relative to the average value for normal (CD38+) plasma cells from controls. Values shown are mean and SD for 20 MGUS and 20 MM patients. *P < .05, **P ≤ .005, Mann-Whitney test.
, MM patients. APM transcript levels were normalized to those of glyceraldehyde 3-phosphate dehydrogenase in the same samples and then expressed as fold change relative to the average value for normal (CD38+) plasma cells from controls. Values shown are mean and SD for 20 MGUS and 20 MM patients. *P < .05, **P ≤ .005, Mann-Whitney test.
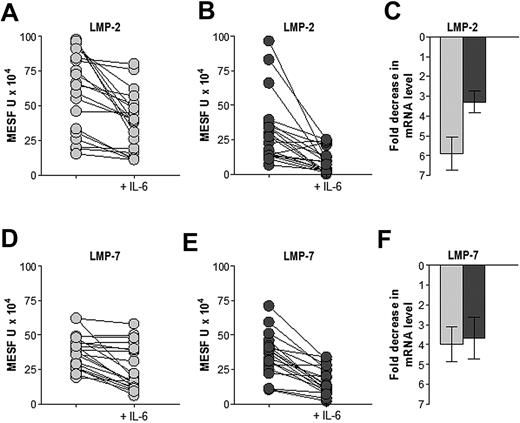
Considering the possibility that the expression of APM components could be regulated by IL-6, the central growth factor for transformed plasma cells, or by IFN-γ, the primary inducer of immunoproteasomes, we examined the effects of cytokine treatment (36 hours) on both protein and mRNA levels (Figure 4). IL-6 treatment reduced LMP2 and LMP7 protein levels (MESF units) in most MM and MGUS samples; although the down-regulation appeared stronger in MM than in MGUS samples, the reductions were significant in both groups (P < .001). IL-6 treatment also substantially reduced mRNA transcripts of both LMP2 and LMP7 (P < .05; Figure 4C,F). IL-6 treatment had no substantial effects on LMP10 or other APM component (data not shown). In contrast, IFN-γ treatment did not lead to substantial changes in any APM component in normal, premalignant, or malignant plasma cells (data not shown).
Effect of IL-6 treatment on the expression of APM components LMP2 and LMP7 in premalignant and malignant BM plasma cells. indicates MGUS patients; and
indicates MGUS patients; and  , MM patients. Samples were incubated without or with 100 ng/mL IL-6 for 36 hours before immunostaining and flow cytometry analysis (A,B,D,E) or mRNA extraction and quantification (C,F). IL-6 treatment significantly reduced levels of LMP2 and LMP7 protein (P < .001, Wilcoxon signed rank test) and down-regulated the corresponding transcripts in both MGUS and MM samples (P < .05, Wilcoxon signed rank test).
, MM patients. Samples were incubated without or with 100 ng/mL IL-6 for 36 hours before immunostaining and flow cytometry analysis (A,B,D,E) or mRNA extraction and quantification (C,F). IL-6 treatment significantly reduced levels of LMP2 and LMP7 protein (P < .001, Wilcoxon signed rank test) and down-regulated the corresponding transcripts in both MGUS and MM samples (P < .05, Wilcoxon signed rank test).
Effect of IL-6 treatment on the expression of APM components LMP2 and LMP7 in premalignant and malignant BM plasma cells. indicates MGUS patients; and
indicates MGUS patients; and  , MM patients. Samples were incubated without or with 100 ng/mL IL-6 for 36 hours before immunostaining and flow cytometry analysis (A,B,D,E) or mRNA extraction and quantification (C,F). IL-6 treatment significantly reduced levels of LMP2 and LMP7 protein (P < .001, Wilcoxon signed rank test) and down-regulated the corresponding transcripts in both MGUS and MM samples (P < .05, Wilcoxon signed rank test).
, MM patients. Samples were incubated without or with 100 ng/mL IL-6 for 36 hours before immunostaining and flow cytometry analysis (A,B,D,E) or mRNA extraction and quantification (C,F). IL-6 treatment significantly reduced levels of LMP2 and LMP7 protein (P < .001, Wilcoxon signed rank test) and down-regulated the corresponding transcripts in both MGUS and MM samples (P < .05, Wilcoxon signed rank test).
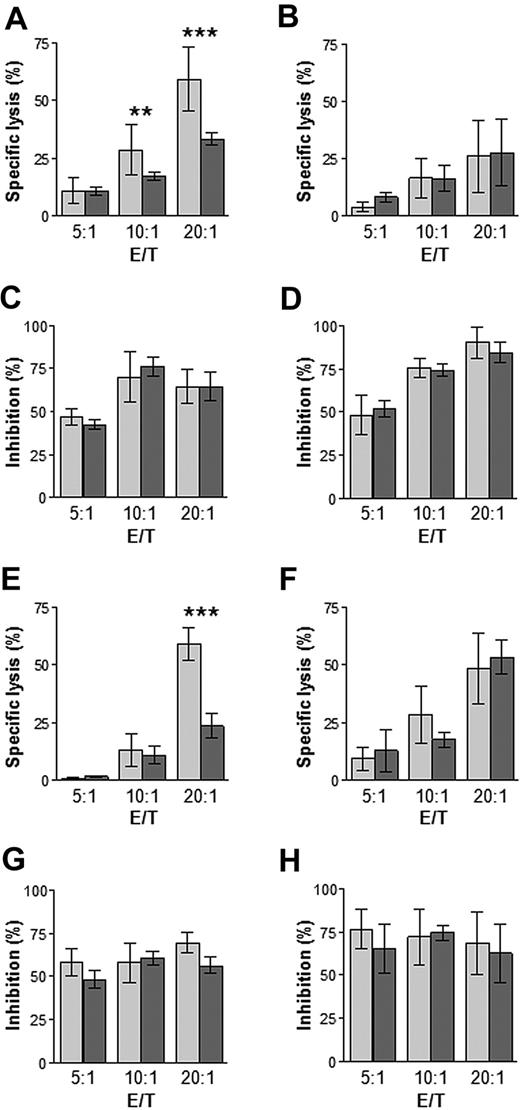
To assess the effects that altered levels of APM components might have on the recognition of premalignant and malignant plasma cells by cytotoxic T lymphocytes, we performed cytotoxicity assays by using, as effectors, CD8+ T cells from the BM of MGUS and MM patients, expanded in vitro in the presence of autologous DCs pulsed with autologous plasma cell lysates; targets were ex vivo autologous plasma cells or, as positive control, autologous EBV-transformed B cells previously pulsed with autologous plasma cell lysates (Figure 5). Specific lysis of autologousplasma cells was observed at all 3 effector/target ratios tested, but CD8+ T cells from MGUS subjects exhibited significantly greater lysis than those from MM patients at the 2 greater ratios (Figure 5A). No significant differences in percent specific lysis were observed between MGUS and MM samples regarding EBV-transformed B-cell targets (Figure 5B). Lysis of both premalignant/malignant plasma cells and EBV-transformed B cells was inhibited by blocking the HLA class I molecules with a monoclonal antibody (Figure 5C-D), indicating the HLA class I–restricted nature of the cytotoxic cells.
Cytotoxicity of CD8+ T cells. CD8+ T cells (effectors) from the BM of MGUS patients ( ; n = 20) and MM patients (
; n = 20) and MM patients ( ; n = 20) were expanded in vitro in the presence of autologous DCs pulsed either with plasma cell lysates (A-D) or opsonized apoptotic plasma cells (E-H). Targets were ex vivo autologous plasma cells (A,C,E,G) or autologous EBV-transformed B cells previously pulsed with autologous plasma cell lysates (B,D,F,H). Shown is percentage-specific lysis at different effector/target ratios (A,B,E,F) and the inhibition of this lysis by a mAb to HLA class I antigen (C,D,G,H). **P ≤ .005, ***P < .001, Mann-Whitney test.
; n = 20) were expanded in vitro in the presence of autologous DCs pulsed either with plasma cell lysates (A-D) or opsonized apoptotic plasma cells (E-H). Targets were ex vivo autologous plasma cells (A,C,E,G) or autologous EBV-transformed B cells previously pulsed with autologous plasma cell lysates (B,D,F,H). Shown is percentage-specific lysis at different effector/target ratios (A,B,E,F) and the inhibition of this lysis by a mAb to HLA class I antigen (C,D,G,H). **P ≤ .005, ***P < .001, Mann-Whitney test.
Cytotoxicity of CD8+ T cells. CD8+ T cells (effectors) from the BM of MGUS patients ( ; n = 20) and MM patients (
; n = 20) and MM patients ( ; n = 20) were expanded in vitro in the presence of autologous DCs pulsed either with plasma cell lysates (A-D) or opsonized apoptotic plasma cells (E-H). Targets were ex vivo autologous plasma cells (A,C,E,G) or autologous EBV-transformed B cells previously pulsed with autologous plasma cell lysates (B,D,F,H). Shown is percentage-specific lysis at different effector/target ratios (A,B,E,F) and the inhibition of this lysis by a mAb to HLA class I antigen (C,D,G,H). **P ≤ .005, ***P < .001, Mann-Whitney test.
; n = 20) were expanded in vitro in the presence of autologous DCs pulsed either with plasma cell lysates (A-D) or opsonized apoptotic plasma cells (E-H). Targets were ex vivo autologous plasma cells (A,C,E,G) or autologous EBV-transformed B cells previously pulsed with autologous plasma cell lysates (B,D,F,H). Shown is percentage-specific lysis at different effector/target ratios (A,B,E,F) and the inhibition of this lysis by a mAb to HLA class I antigen (C,D,G,H). **P ≤ .005, ***P < .001, Mann-Whitney test.
A similar experiment was performed by the use of, as effectors, CD8+ T cells expanded in the presence of autologous DCs loaded with opsonized, apoptotic plasma cells. Specific lysis of plasma cells was observed at the 2 greater effector/target ratios tested and, at the highest ratio, CD8+ T cells from MGUS patients exhibited significantly greater lysis than those from MM patients (Figure 5E). As observed in the preceding experiment, there was no significant difference between MGUS and MM samples when targets wereEBV-transformed B cells (Figure 5F); mAb blocking of HLA class I molecules demonstrated again the HLA class I–restricted nature of the CD8+ T cells (Figure 5G-H). Notably, the percent specific lysis observed in this experiment (Figure 5E) at the highest effector/target ratio correlated directly with the percentage of premalignant plasma cells stained positively for δ, LMP2, and LMP10 in MGUS patients and with the percentage of malignant plasma cells stained positively for δ, MB1, LMP2, LMP10, and TAP1 in MM patients (Table 1).
Correlation between percent-specific lysis of transformed plasma cells in cytotoxicity assays with the use of autologous CD8+ T cells and percentage of transformed plasma cells stained ex vivo by APM component-specific mAb
| Component . | MGUS . | MM . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| δ | 0.911 | < .001 | 0.261 | .043 |
| MB1 | 0.004 | n.s. | 0.450 | .009 |
| LMP2 | 0.307 | .009 | 0.592 | .006 |
| LMP10 | 0.453 | .025 | 0.651 | .002 |
| TAP1 | 0.267 | n.s | 0.567 | .018 |
| Component . | MGUS . | MM . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| δ | 0.911 | < .001 | 0.261 | .043 |
| MB1 | 0.004 | n.s. | 0.450 | .009 |
| LMP2 | 0.307 | .009 | 0.592 | .006 |
| LMP10 | 0.453 | .025 | 0.651 | .002 |
| TAP1 | 0.267 | n.s | 0.567 | .018 |
APM indicates antigen processing-presenting machinery; mAb, monoclonal antibodies; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; and n.s., not significant.
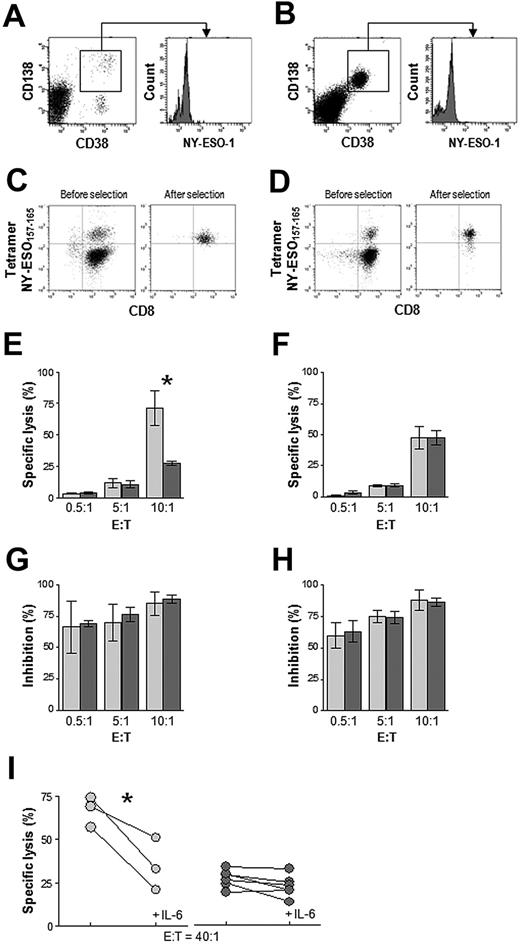
To confirm that the observed differences in target killing between MGUS and MM cytotoxic T lymphocytes were the result of their different capacities to recognize transformed plasma cells, we enriched for BM-derived CD8+ T cells specific for a particular HLA class I–restricted tumor peptide (derived from the cancer testis protein NY-ESO-1) and tested them in cytotoxicity assays against autologous plasma cells expressing the protein antigen and, as control, a myeloma plasma cell line. Therefore, we identified from the study population a subset of HLA-A*0201–positive patients whose BM-derived transformed plasma cells stained positively for NY-ESO-1 (Figure 6A-B). A total of 3 MGUS and 6 MM patients met these criteria. CD8+ T cells from their BM samples were expanded in vitro with autologous DCs pulsed with the HLA-A*0201–restricted NY-ESO-1157-165 peptide, stained with the NY-ESO-1157-165 HLA-A*0201 PE-conjugated pentamer, immunomagnetically enriched (Figure 6C-D), and tested for killing of autologous NY-ESO-1–expressing plasma cells or HLA-A*0201–positive NY-ESO-1–expressing U266 myeloma cells. Specific lysis of autologous plasma cells by tetramer-binding CD8+ T cells from the 3 MGUS patients was high at an E/T ratio of 10:1 and significantly greater (P = .027) than that observed with CD8+ T cells from the 6 MM patients (Figure 6E).
Cytotoxicity of in vitro–generated and immunomagnetically sorted NY-ESO-1–specific CD8+ T cells from BM of HLA-A*0201–positive MGUS patients (n = 3) and MM patients (n = 6) in whom we also detected NY-ESO-1–expressing plasma cells. Shown are representative flow cytometric plots of CD38-, CD138-, and NY-ESO-1–immunostained BMMC from 1 MGUS (A) and 1 MM (B) subject, as well as representative flow cytometry plots before and after selection of NY-ESO-1157-165 pentamer-binding CD8+ T cells (expanded in vitro with NY-ESO-1157-165 peptide) from 1 MGUS (C) and 1 MM (D) subject. Percentage-specific lysis of autologous plasma cells (E) and U266 myeloma cells (F) at different effector:target ratios by MGUS ( ) and MM (
) and MM ( ) NY-ESO-1157-165 pentamer-binding CD8+ T cells (*P < .001, Mann-Whitney test). (G,H) Inhibition of this lysis by a mAb to HLA class I antigen. (I) Percentage-specific lysis of autologous plasma cells by MGUS (
) NY-ESO-1157-165 pentamer-binding CD8+ T cells (*P < .001, Mann-Whitney test). (G,H) Inhibition of this lysis by a mAb to HLA class I antigen. (I) Percentage-specific lysis of autologous plasma cells by MGUS ( ) and MM (
) and MM ( ) NY-ESO-1157-165 pentamer-binding CD8+ T cells in the absence or presence of IL-6. *P < .001, paired t test.
) NY-ESO-1157-165 pentamer-binding CD8+ T cells in the absence or presence of IL-6. *P < .001, paired t test.
Cytotoxicity of in vitro–generated and immunomagnetically sorted NY-ESO-1–specific CD8+ T cells from BM of HLA-A*0201–positive MGUS patients (n = 3) and MM patients (n = 6) in whom we also detected NY-ESO-1–expressing plasma cells. Shown are representative flow cytometric plots of CD38-, CD138-, and NY-ESO-1–immunostained BMMC from 1 MGUS (A) and 1 MM (B) subject, as well as representative flow cytometry plots before and after selection of NY-ESO-1157-165 pentamer-binding CD8+ T cells (expanded in vitro with NY-ESO-1157-165 peptide) from 1 MGUS (C) and 1 MM (D) subject. Percentage-specific lysis of autologous plasma cells (E) and U266 myeloma cells (F) at different effector:target ratios by MGUS ( ) and MM (
) and MM ( ) NY-ESO-1157-165 pentamer-binding CD8+ T cells (*P < .001, Mann-Whitney test). (G,H) Inhibition of this lysis by a mAb to HLA class I antigen. (I) Percentage-specific lysis of autologous plasma cells by MGUS (
) NY-ESO-1157-165 pentamer-binding CD8+ T cells (*P < .001, Mann-Whitney test). (G,H) Inhibition of this lysis by a mAb to HLA class I antigen. (I) Percentage-specific lysis of autologous plasma cells by MGUS ( ) and MM (
) and MM ( ) NY-ESO-1157-165 pentamer-binding CD8+ T cells in the absence or presence of IL-6. *P < .001, paired t test.
) NY-ESO-1157-165 pentamer-binding CD8+ T cells in the absence or presence of IL-6. *P < .001, paired t test.
In contrast, similar rates of specific lysis of U266 myeloma cells were found for CD8+ T cells from MGUS and MM patients (Figure 6F). No specific lysis was observed when these CD8+ T cells were tested against EBV-B cells pulsed with a negative control, the HLA-A*0201–restricted HIV-1 gag p1776-84 peptide (data not shown). mAb blocking of HLA class I molecules confirmed the HLA class I–restricted nature of the CD8+ T cells (Figure 6G-H). Preincubation of autologous plasma cell targets with IL-6 reduced significantly (P = .045) the percent specific lysis in MGUS but not in MM patients (Figure 6I). The impairment in specific lysis by CD8+ T cells from MM patients, compared with MGUS patients, shown by these cytotoxicity experiments, was strictly dependent on the use of autologous plasma cells as targets and is consistent with both the aberrant expression levels of APM components in MM patients' plasma cells (Figures 2–3) and the APM down-regulating effects of IL-6 (Figure 4).
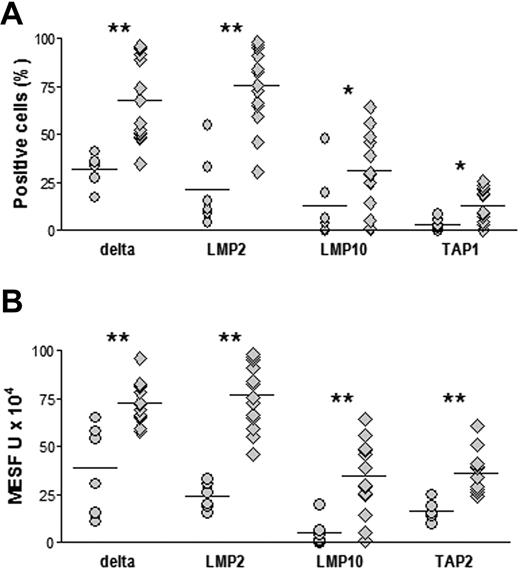
Next, we wished to establish whether alterations in the expression of APM proteins, HLA class I antigens, and β2m in premalignant and malignant plasma cells had clinical implications. We therefore looked for correlations between individual proteins at the time of BM sampling and either changes in the serum level of the M component during the successive 24 months in MGUS patients or in disease status (remission vs stable or progressive disease) after first-line chemotherapy in MM patients. The analysis was performed considering APM protein expression in terms of both percentage positive cells and MESF units. In MGUS patients (Table 2), significant negative correlations were observed for δ, LMP2, and LMP10 in both analyses and for TAP1 in the analysis with the use of MESF units. In addition, significant positive correlations were found for calnexin, calreticulin, and tapasin inboth analyses and for HLA class I and β2m in the analysis with the use of MESF units. Therefore, decreases in APM proteins δ, LMP2, LMP10, and TAP1 and increases in calnexin, calreticulin, tapasin, HLA class I, and β2m are associated with MGUS progression. Interestingly, when we compared the 6 MGUS patients who progressed to MM in the successive 24 months to the 14 patients who did not progress, we found that those who progressed had significantly lower expression levels of δ, LMP2, LMP10, and, depending on the assay, also TAP1 and TAP2 at the time of BM sampling (Figure 7).
Correlation between levels of APM components in transformed plasma cells of MGUS patients at the time of BM sampling and changes in serum M component during the course of 24 months
| Component . | Positive cells, % . | MESF units . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| δ | −0.596 | .001 | −0.342 | .043 |
| LMP2 | −0.321 | .021 | −0.224 | .049 |
| LMP10 | −0.442 | .003 | −0.381 | .045 |
| TAP1 | −0.317 | n.s. | −0.338 | .026 |
| Calnexin | 0.586 | .009 | 0.436 | .024 |
| Calreticulin | 0.539 | .006 | 0.399 | .028 |
| Tapasin | 0.441 | .012 | 0.340 | .039 |
| β2-m | 0.300 | n.s. | 0.589 | .001 |
| HLA class I | 0.233 | n.s. | 0.463 | .006 |
| Component . | Positive cells, % . | MESF units . | ||
|---|---|---|---|---|
| r . | P . | r . | P . | |
| δ | −0.596 | .001 | −0.342 | .043 |
| LMP2 | −0.321 | .021 | −0.224 | .049 |
| LMP10 | −0.442 | .003 | −0.381 | .045 |
| TAP1 | −0.317 | n.s. | −0.338 | .026 |
| Calnexin | 0.586 | .009 | 0.436 | .024 |
| Calreticulin | 0.539 | .006 | 0.399 | .028 |
| Tapasin | 0.441 | .012 | 0.340 | .039 |
| β2-m | 0.300 | n.s. | 0.589 | .001 |
| HLA class I | 0.233 | n.s. | 0.463 | .006 |
Levels of APM components were expressed as both percentage of cells staining positively and MESF units. Only APM components that had at least 1 significant correlation are shown; Spearman rank test.
β2-m indicates β2-microglobulin; BM, bone marrow; HLA, human leukocyte antigen; and MESF, molecular equivalents of soluble fluorochrome. Other abbreviations as in Table 1.
APM component expression in premalignant plasma cells from BM of MGUS patients with different disease progression in the 24 months after BM sampling. MGUS patients who progressed to MM (○, n = 6) and who did not progress (◇, n = 14) are shown. Immunostaining and flow cytometric analysis were performed to obtain percentages of positive cells (A) and MESF (B), as described in Figure 2. *P < .05, **P ≤ .005, Mann-Whitney test.
APM component expression in premalignant plasma cells from BM of MGUS patients with different disease progression in the 24 months after BM sampling. MGUS patients who progressed to MM (○, n = 6) and who did not progress (◇, n = 14) are shown. Immunostaining and flow cytometric analysis were performed to obtain percentages of positive cells (A) and MESF (B), as described in Figure 2. *P < .05, **P ≤ .005, Mann-Whitney test.
These observations in MGUS patients, when considered together with the results on APM protein levels in MM patients (Figure 2), suggest that changes in APM component expression in MGUS patients may be predictive of disease progression to MM. However, in MM patients, no significant correlation was observed between individual protein levels and response to treatment when dichotomized into remission versus stable or progressive disease (data not shown). Of note, no correlation was found even in the 6 patients who achieved disease remission after a chemotherapy regimen containing bortezomib, a proteasome inhibitor.
Finally, to determine whether the reduced expression of proteasome subunits and peptide transporters TAP1 and TAP2 in premalignant and malignant plasma cells was associated with epigenetic changes and, in particular, promoter DNA methylation, we measured the effects of decitabine treatment. Decitabine is a potent DNA methyltransferase inhibitor that can reactivate the expression of methylation-silenced genes,26 including some genes that encode APM proteins in malignant cells.27 Because of limitations in the recovery of plasma cells from BM aspiration, it was impossible to perform the experiment on primary cell samples, so U266 and RPMI 8226 myeloma cell lines were used instead. In these cells, demethylation led to a general increase in the number of cells staining positively for each APM component investigated, although the extent of the increases and their temporal patterns varied (supplemental Figure 1). These results provide further evidence that APM component expression is primarily regulated at the transcriptional level.
Discussion
In this study, we found that malignant transformation of plasma cells in monoclonal gammopathies was accompanied by changes in the expression of HLA class I APM components. These changes were evident ex vivo, occurred at the transcriptional level, were not reversed by IFN-γ, and, in some cases, were enhanced by IL-6. Moreover, malignant plasma cells from MM patients were less readily lysed by autologous, in vitro–expanded cytotoxic CD8+ T cells than were premalignant plasma cells from MGUS patients. This difference in cytotoxicity was detectable at the epitope level and is unlikely to be caused by the intrinsic properties of CD8+ T cells, given that no difference was found when CD8+ T cells were tested with other HLA-matched target cells (autologous EBV-transformed B cells pulsed with transformed plasma cell lysates or U266 myeloma cells expressing NY-ESO-1). For many APM components, changes in expression level in plasma cells correlated with the extent of the cells' specific lysis by CD8+ T cells and with changes in the serum level of the M component in MGUS patients.
This study confirms and extends the pioneering work reported by Dhodapkar et al in 2 successive articles.2,3 It confirms that clinical progression of MGUS to MM is not caused by the deletion of antitumor CD8+ T cells from the tumor bed because these cells can be expanded in vitro from MM patients if properly activated by tumor antigen-presenting DCs.3 It also confirms that, despite no loss in the antigen-specific CD8+ T-cell pool, the antitumor CD8+ T-cell response in the BM of MM patients is less efficient than that of MGUS patients.2 Moreover, this study extends our knowledge of the cellular mechanisms that are deranged in MM: Dhodapkar et al3 found that freshly isolated T cells from the BM of MGUS patients readily recognized tumor antigen-presenting DCs and secreted IFN-γ, whereas these T cells from MM patients did not. We further demonstrated that MM patients' CD8+ T cells, when expanded in vitro, also failed to recognize transformed plasma cells.
There are 2 possible reasons why a reduced cytotoxicity of MM patients' CD8+ T cells was documented in this study but was not observed by Dhodapkar et al,2 Wen et al,28 or Choi et al.10 First, only in this study was the cytotoxic potential of CD8+ T cells from MM patients compared with that of MGUS patients; this permitted us to observe a significantly lower efficiency of specific lysis in MM samples. Second, as targets, we (and Dhodapkar et al2 ) used transformed plasma cells that, as shown here, have abnormal APM expression. It is possible that the observation of reduced cytotoxicity of MM samples in this study was facilitated by the use of this target, rather than in vitro-generated, plasma cell-loaded DCs (recently shown to have different expression patterns of the constitutive and inducible proteasome subunits29 ) or peptide-loaded T2 cells (which are not the “natural” targets of MM CD8+ T cells and which are not known to have APM abnormalities), as used by Wen et al28 and Choi et al,10 respectively.
It remains to be determined whether the present findings have clinical impact in terms of understanding the prognosis of MGUS patients or predicting the response to therapy with proteasome inhibitors in MM patients. This study suggests that changes in expression of APM components are associated with, and therefore may predict, the progression of MGUS to MM. However, we are currently unable to reconcile our findings at the cellular level with work by Jakob et al,30 who observed that proteasome levels in serum were greater in patients with active (advanced) MM than in those with “smoldering” MM and who found that proteasome concentration was an independent prognostic factor. Concerning the response to therapy, this study found no correlation between APM expression levels and disease status (remission vs stable or progressive), even in patients treated with the proteasome inhibitor bortezomib. This result contrasts with the recent observation that the sensitivity of tumor B-cell lines to proteasome inhibitors was associated with the pattern of expression of the proteasome subunits.31
In conclusion, this study supports the hypothesis that aberrations in the expression of HLA class I APM components in transformed plasma cells disrupt the cells' ability to be recognized and killed by plasma cell antigen-specific cytotoxic CD8+ T cells residing in the BM. These T cells may help suppress tumor growth but fail to eradicate it, depending on the proliferative rate and clonogenic potential of the targeted subpopulation and on the nature of the tumor antigen being targeted. Therefore, changes in APM component expression may be considered an immune escape mechanism that contributes to the MGUS-MM progression. Additional studies are required to understand the molecular mechanisms responsible for the observed abnormalities in APM component expression in these premalignant and malignant cells. In particular, epigenetic events, including DNA methylation of gene promoters, merit further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Valerie Matarese provided scientific editing.
This study was supported by research funding from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy; from Fondazione Cassa di Risparmio di Puglia, Bari, Italy; and by PHS grants CA109688, CA110249, and CA113861 awarded by the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: V.R., P.L., and S.F. designed research; M.A.F. and C.B. performed research; S.F. contributed vital new reagents; P.L. and F.P. performed statistical analysis; V.R., P.L., F.P., and F.D. analyzed data; V.R. and F.D. interpreted data; and V.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vito Racanelli, MD, Department of Internal Medicine and Clinical Oncology, University of Bari Medical School, Policlinico, 11 Piazza G. Cesare, 70124 Bari, Italy; e-mail: v.racanelli@dimo.uniba.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal