Abstract
Coagulation factor V (FV), present in plasma and platelets, is indispensable to thrombin formation, yet patients with undetectable plasma FV seldom experience major bleeding. We used thrombin generation assays to explore the role of platelet FV in 4 patients with severe congenital FV deficiency (3 with plasma FV clotting activity [FV:C] < 1%). When triggered with tissue factor (TF) concentrations up to 50pM, platelet-poor plasma (PPP) from the patients with undetectable plasma FV showed no thrombin generation, whereas platelet-rich plasma (PRP) formed thrombin already at 1 to 5pM of TF. Thrombin generation in PRP from the FV-deficient patients was enhanced to near-normal levels by platelet activators (collagen or Ca2+-ionophore) and could be completely suppressed by specific FV inhibitors, suggesting FV dependence. Accordingly, platelet FV antigen and activity were measurable in all FV-deficient patients and platelet FVa could be visualized by Western blotting. Normalization of the tissue factor pathway inhibitor (TFPI) level, which is physiologically low in FV-deficient plasma, almost completely abolished thrombin generation in PRP from the FV-deficient patients. In conclusion, patients with undetectable plasma FV may contain functional FV in their platelets. In combination with low TFPI level, residual platelet FV allows sufficient thrombin generation to rescue these patients from fatal bleeding.
Introduction
Coagulation factor V (FV) is a 330-kDa glycoprotein that is present in plasma (80%) and platelets (20%).1 Plasma FV (20-25nM) is synthesized in the liver and is released in the bloodstream as a single-chain inactive procofactor. After limited proteolysis by thrombin or activated factor X (FXa), FV is converted to its activated form (FVa), consisting of a heavy chain (105 kDa) and a light chain (71/74 kDa) that are noncovalently linked via a single Ca2+ ion.2 FVa acts as a nonenzymatic cofactor of FXa in the conversion of prothrombin to thrombin and increases the rate of prothrombin activation more than 1000-fold.3,4 FVa activity is down-regulated by activated protein C (APC), which inactivates FVa by cleaving the heavy chain at Arg306, Arg506, and Arg679.5,6
Although megakaryocytes are capable of FV synthesis,7,8 platelet FV originates from the plasma pool.9-11 Bone marrow megakaryocytes internalize plasma FV via a specific receptor-mediated process12 and store it in secretory α-granules.13 After endocytosis, FV gains several unique modifications that make platelet FV structurally and functionally different from plasma FV. In particular, platelet FV is stored in a partially activated form that, after exposure on the platelet surface, is further activated by FXa or thrombin and is resistant to APC-catalyzed inactivation.14-16
Congenital FV deficiency (Owren parahemophilia),17,18 caused by loss-of-function mutations in the F5 gene,19,20 is a rare (1:106 [1 person per million]) bleeding disorder inherited as an autosomal recessive trait. Homozygous and compound heterozygous persons have FV levels lower than 10% and present with a lifelong hemorrhagic diathesis whose severity is poorly correlated with the plasma FV level.21 Although a few cases of neonatal intracranial hemorrhage have been reported,22-24 many patients with undetectable FV experience only mild to moderate bleeding21 and do not require routine prophylaxis. The reason for the relatively mild clinical presentation of many FV-deficient patients is presently unknown.
Given the uniform lethality of FV-null mice,25 it has been argued that FV-deficient persons who survive to postnatal life always have some residual FV expression.19,25,26 Since the FV requirement for minimal thrombin generation is well below 1%,26-29 traces of FV would already be sufficient to guarantee thrombin formation and to rescue FV-deficient persons from fatal bleeding. However, in vitro experiments have failed to detect any thrombin generation in plasma from patients with undetectable FV,29,30 although FV-deficient patients have low plasma levels of the anticoagulant protein tissue factor pathway inhibitor (TFPI),29 which considerably reduces the FV requirement for thrombin generation.
Some 30 years ago, Miletich et al31 showed that, in patients with severe FV deficiency, the FXa-binding capacity of platelets (which is a measure of platelet FV) is a better predictor of bleeding tendency than the plasma FV level. Despite this important observation, platelet FV is not routinely evaluated in FV-deficient patients and only 3 other studies report platelet FV levels in patients with severe FV deficiency.32-34 No platelet FV antigen or activity could be demonstrated in 2 patients with undetectable plasma FV.32,33 In another FV-deficient patient, platelet FV could be visualized by Western blotting, but its activity was not determined.34 To get more insight into the role of platelet FV in FV deficiency, we have measured thrombin generation and platelet FV levels in 4 patients with severe congenital FV deficiency.
Methods
Patients
Four unrelated female patients with severe congenital FV deficiency were enrolled. All 4 were referred to Padua Academic Hospital from district hospitals in northeastern Italy and their demographic and clinical characteristics have been previously described.29 Briefly, patient PD I (age 64 years, FV clotting activity [FV:C] < 1%) was diagnosed at the age of 5 years because of recurrent epistaxis and gum bleeding. Immediately after the menarche at the age of 8 years, she presented with abundant menses. During the postpartum period of her only pregnancy, she developed 3 severe hemorrhages, which were treated by transfusion with fresh-frozen plasma, platelets, and red blood cell concentrates. However, she never experienced major spontaneous bleeding. Patient PD II (age 44 years, FV:C < 1%) suffered from recurrent epistaxis in childhood, which led to the diagnosis of severe FV deficiency at the age of 8 years. At the age of 15 years, she presented with excessive bleeding after a tooth extraction. She has always had abundant menses and in her thirties she was admitted to hospital twice for severe metrorrhagia. On both occasions, administration of fresh-frozen plasma was effective in controlling the hemorrhage. Her parents are first-degree cousins. Patient PD III (age 35 years, FV:C < 1%) experienced only very mild episodes of epistaxis and gum bleeding and was diagnosed at the age of 8 years. Her occasional abundant menses have worsened lately because of the presence of uterine fibromatosis. To reduce menstrual bleeding, she was prescribed oral contraceptives, but the treatment had to be stopped after a few cycles because of her intolerance to the drug. Fresh-frozen plasma and/or antifibrinolytic agents were given during all risk situations and bleeding complications never ensued. Patient PD VII (age 62 years, FV:C 6%) has experienced only mild menorrhagia. She was diagnosed at the age of 23 years, when she presented with a postpartum hemorrhage requiring transfusion with fresh-frozen plasma. She also experienced one uncomplicated spontaneous abortion. None of the patients received substitutive therapy in the 3 months preceding blood sampling. All patients were homozygous or compound heterozygous for missense mutations in the F5 gene (Table 1).35-37
F5 gene mutations in FV-deficient patients
| . | F5 gene mutations . | |
|---|---|---|
| Nucleotide change . | Amino acid change . | |
| PD I | 1744G>C (homozygous) | 524 Asp/His |
| PD II | 1744G>C (homozygous) | 524 Asp/His |
| PD III | 853T>C; 4957G>C | 227 Trp/Arg; 1595 Tyr/Asp |
| PD VII | 6509G>A (homozygous) | 2112 Gly/Asp |
| . | F5 gene mutations . | |
|---|---|---|
| Nucleotide change . | Amino acid change . | |
| PD I | 1744G>C (homozygous) | 524 Asp/His |
| PD II | 1744G>C (homozygous) | 524 Asp/His |
| PD III | 853T>C; 4957G>C | 227 Trp/Arg; 1595 Tyr/Asp |
| PD VII | 6509G>A (homozygous) | 2112 Gly/Asp |
The study was approved by the Ethics Committee of Padua Academic Hospital and conducted in accordance with the Declaration of Helsinki. All patients gave informed consent to participate. Eight volunteers with normal FV levels recruited among hospital employees served as healthy controls.
Blood collection and plasma preparation
Blood was collected from each FV-deficient patient on 2 separate occasions, with an interval of approximately 6 months. On each occasion, 40 mL of blood was drawn in 129mM sodium citrate (1:10 vol/vol) for thrombin generation experiments, and 20 mL of blood was drawn in 80mM sodium citrate, 52mM citric acid, 183mM glucose (1:7 vol/vol) for platelet isolation (see “Platelet isolation and preparation”). For each patient, blood from 2 healthy controls was also collected on the same day and handled in the same way.
Citrated blood was centrifuged at 250g for 15 minutes to obtain platelet-rich plasma (PRP). Part of the PRP was further centrifuged at 14 000g for 5 minutes to yield platelet-poor plasma (PPP).
The FV-deficient plasma used in the experiment shown in Figure 1 was purchased from George King Biochemical Inc.
Thrombin generation assays
Thrombin generation in PPP and PRP was determined using the calibrated automated thrombogram method.38 Coagulation was triggered by recalcification (16mM added CaCl2) in the presence of recombinant tissue factor (Innovin; Dade-Behring). The tissue factor (TF) concentration in the Innovin stock solution (prepared according to the manufacturer's instructions) was measured with the Imubind TF-ELISA (American Diagnostica) and found to be 331 ng/mL (7.36nM). For the measurement in PPP, synthetic phospholipids composed of phosphatidylcholine, phosphatidylserine, and sphingomyelin (Phospholipid-TGT; Rossix) were added to a final concentration of 20μM. PRP was adjusted to 1.5 × 108 platelets/mL. In some experiments, PRP was preincubated with collagen (10 μg/mL; Dynamyte Medical) or Ca2+-ionophore A23187 (20μM; Sigma-Aldrich) for 10 minutes at 37°C to activate the platelets before triggering coagulation. To prevent thrombin formation via the intrinsic pathway, all thrombin generation experiments were performed in the presence of 32 μg/mL of corn trypsin inhibitor (Haematologic Technologies Inc). Thrombin generation curves were determined in duplicate and were calibrated against the fluorescence signal obtained in the same plasma with 100nM thrombin calibrator (Thrombinoscope BV). Fluorescence was read in a Fluoroskan Ascent reader (Thermo Labsystems) and thrombin generation curves were analyzed for lag time, peak height, and endogenous thrombin potential (ETP) using the Thrombinoscope software (Thrombinoscope BV).
In some thrombin generation experiments, an anti-FV polyclonal antibody (SAFV-IG; Affinity Biologicals Inc), plasma-purified human APC (Innovative Research), in-house plasma-purified FV, or recombinant human full-length TFPI (kind gift of Prof W. Buurman, Maastricht University) was added to the plasma.
Platelet isolation and preparation
Washed platelet suspensions were prepared as described previously11 and divided in 2 aliquots: one, with a concentration of 0.7 × 109 platelets/mL, was frozen for the preparation of platelet lysates; the other was diluted to 0.5 × 109 platelets/mL in platelet buffer (PB; 10mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 136mM NaCl, 2.7mM KCl, 2mM MgCl2, pH 7.5, containing 2 mg/mL bovine serum albumin and 2 mg/mL glucose) and used for the preparation of activated platelet suspensions. Platelet lysates were used for the measurement of FV and TFPI antigen levels, whereas activated platelet suspensions represented the starting material for platelet FVa activity determination and platelet FVa immunoprecipitation.
For the preparation of platelet lysates, 0.5% (vol/vol) Triton X-100 (Fluka) was added to the frozen platelet suspension in the presence of the following protease inhibitors: 5mM ethylenediaminetetraacetic acid (Merck), 2mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (Fluka), 5mM N-ethylmaleimide (Merck), 10 μg/mL leupeptin (Sigma-Aldrich), 10mM benzamidine (Sigma-Aldrich), and 25 μg/mL soybean trypsin inhibitor (Sigma-Aldrich). After 2-hour lysis on ice, platelet lysates were analyzed immediately for FV and TFPI antigen levels.
For the preparation of activated platelet suspensions, Arg-Gly-Asp-Ser-peptide (H-1155; BACHEM AG) was added to the washed platelet suspensions at a final concentration of 0.3 mg/mL to inhibit platelet aggregation, and platelets (0.5 × 109/mL in PB, pH 7.5) were activated with 34nM thrombin and 20μM Ca2+-ionophore (A23187) in the presence of 5mM CaCl2 for 30 minutes at 37°C. Added thrombin was subsequently neutralized with hirudin (Kordia Life Sciences). Activated platelet suspensions were frozen at −80°C for later determination of FV activity.
Measurement of FV antigen levels in plasma and platelets
Plasma and platelet FV antigen levels were quantified with an enzyme-linked immunosorbent assay (ELISA). Microtiter plates were coated overnight at 4°C with 2.2 μg/well of polyclonal anti-FV antibody (The Binding Site Ltd) in carbonate buffer. After rinsing 5 times with washing buffer (0.05M tris(hydroxymethyl)aminomethane/0.15M NaCl/5mM ethylenediaminetetraacetic acid, pH 7.5, 0.1% Tween 20), 100 μL 1:200-diluted plasma or platelet lysate (2.5 × 107 or 5 × 107 platelets/mL in washing buffer) was added to the wells and incubated at room temperature (RT) for 2 hours. After washing, bound FV was detected by incubation with an horseradish peroxidase (HRP)–conjugated polyclonal anti-FV antibody (The Binding Site Ltd; 0.5 μg/well) for 1.5 hours, and plates were developed with tetramethylbenzidine/peroxide.
Measurement of FV activity levels in plasma and platelets
FV activity levels of plasma and washed platelets were determined with prothrombinase-based assays. Plasma FV was determined as previously described.29 For platelet FV determination, activated platelet suspensions were thawed at 37°C and diluted with Hepes-buffered saline (HBS; 25mM Hepes, 175mM NaCl, pH 7.7) containing 0.5 mg/mL ovalbumin and 2.7mM CaCl2 to obtain a final concentration of 12 × 106 platelets/mL in the assay. Platelet FVa was quantified with 5nM FXa, 1μM prothrombin, 40μM di-oleoyl phosphatidylserine/di-oleoyl phosphatidylcholine (10/90 mol/mol) phospholipid vesicles, and 2.5mM CaCl2. Pooled normal plasma and an activated platelet pool prepared from platelets from 20 healthy persons were used as a reference for plasma and platelet FV measurements, respectively.
Immunoprecipitation of FVa from plasma and platelets
Activated platelet suspensions (individual patient samples and a pool of activated platelets from 20 controls) were solubilized by the addition of 0.5% (final concentration) Triton X-100 in the presence of protease inhibitors (2mM 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, 5mM N-ethylmaleimide, 10 μg/mL leupeptin, 10mM benzamidine, 25 μg/mL soybean trypsin inhibitor) and 5mM CaCl2 for 30 minutes at RT.
FV-deficient or normal pooled plasma was defibrinated with 1 U/mL ancrod (National Institute for Biological Standards and Control) for 10 minutes at 37°C. Plasma FV was activated with 34nM thrombin and 16mM added CaCl2 in the presence of a polyclonal sheep anti–protein C antibody (0.16 mg/mL; Innovative Research) for 10 minutes at 37°C, after which the same cocktail of protease inhibitors as used for the platelet preparation was added.
Platelet lysates and plasma were precleared of endogenous immunoglobulin G with protein A–Sepharose beads (rProtein A Sepharose Fast Flow; GE Healthcare) that were subsequently removed by centrifugation. Precleared platelet lysate (200 μL of 1.5 × 108 platelets/mL) or precleared plasma (200 μl) was added to protein A-Sepharose beads (15 μL drained volume) bearing monoclonal anti-FV heavy chain antibodies (3B1; a kind gift from Prof B. N. Bouma, Utrecht University Hospital) and FVa was immunoprecipitated for 30 minutes at RT under rotation. The beads were collected by centrifugation, washed 3 times with HBS containing 5mM CaCl2 and 10mM benzamidine, and resuspended in HBS containing 1:2 volume sample buffer (40mM tris(hydroxymethyl)aminomethane, pH 6.7, 3.33% sodium dodecyl sulfate (SDS), 6.25% mercaptoethanol, 50% glycerol, 0.01% bromophenol blue). Plasma or platelet samples from FV-deficient patients were resuspended in 3.3 μL (to achieve a 6-fold concentration of the FVa), whereas control samples were resuspended in 200 μL (no concentration). After 5-minute incubation at 96°C, samples were subjected to SDS–polyacrylamide gel electrophoresis on 6% polyacrylamide gels according to Laemmli under reducing conditions. Proteins were subsequently transferred to polyvinylidene fluoride membranes by semidry blotting. Membranes were blocked with 5% (wt/vol) skim milk (Merck) in HBS/0.5% Tween 20 for 1 hour at RT and the primary antibody (monoclonal anti-FV heavy chain AHV-5146 [Haematologic Technologies Inc], 5 μg/mL in blocking buffer) was incubated overnight at 4°C under shaking. Membranes were washed with HBS/0.3% Tween 20 and incubated with a secondary antibody (HRP-conjugated rabbit anti–mouse immunoglobulins [DAKO], 1:2000 in blocking buffer) for 1 hour at RT. HRP activity was detected using chemiluminescence (Supersignal West Pico Chemiluminescent Substrate; Pierce) and the LAS-3000 image analyzer (Fujifilm).
TFPI ELISA
TFPI levels were determined by a full-length TFPI ELISA in plasma as well as in platelet lysates containing 108 platelets/mL. The ELISA was calibrated against normal pooled plasma or a pool of lysed platelets from 20 healthy persons.
Statistical analysis
Data are expressed as mean plus or minus SD. Correlations are expressed as Pearson coefficients (r). Statistical analysis was performed using SPSS 15.0 (SPSS).
Results
Contributions of plasma and platelet FV to thrombin generation
To explore the relative contributions of plasma and platelet FV to thrombin generation, FV-deficient plasma with undetectable FV was supplemented with normal platelets and with increasing concentrations (0%–100%) of purified plasma FV. After preactivating the platelets with collagen, coagulation was triggered with 5pM of TF. Thrombin generation was already optimal in the absence of plasma FV and did not increase further when the concentration of plasma FV was increased up to 100% (Figure 1). This experiment shows that in vitro thrombin generation in the presence of normal platelets does not require plasma FV, in line with the in vivo observation that platelet FV could maintain hemostasis in a patient with an acquired antibody that fully neutralized plasma FV.39
Contribution of plasma and platelet FV to thrombin generation. FV-deficient plasma was reconstituted with 1.5 × 108 normal platelets/mL and increasing amounts of purified plasma FV (0%, 5%, 25%, and 100% = 23nM). Platelets were activated with collagen and thrombin generation was triggered with 5pM of TF.
Contribution of plasma and platelet FV to thrombin generation. FV-deficient plasma was reconstituted with 1.5 × 108 normal platelets/mL and increasing amounts of purified plasma FV (0%, 5%, 25%, and 100% = 23nM). Platelets were activated with collagen and thrombin generation was triggered with 5pM of TF.
Thrombin generation in PPP and PRP from FV-deficient patients
To investigate the role of platelet FV in congenital FV deficiency, 4 patients with severe congenital FV deficiency were enrolled. All were homozygous or compound heterozygous for missense mutations in the F5 gene (Table 1).35-37 Plasma FV clotting activity (FV:C) was undetectable in all patients, except PD VII (FV:C 6%).
Thrombin generation was determined in PPP and PRP from these patients after triggering coagulation with 1, 5, 10, or 50pM of TF. Thrombin generation curves obtained in plasma from the 4 patients and 1 representative control are shown in Figure 2. Thrombin generation parameters obtained at 5pM of TF are presented in Table 2.
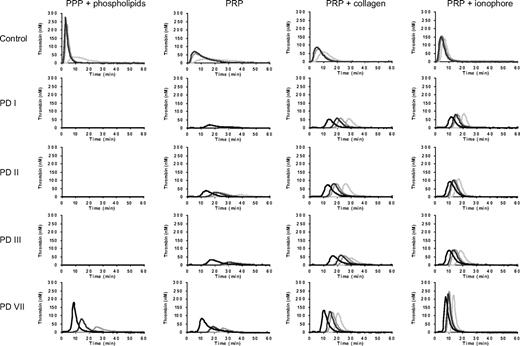
Thrombin generation in PPP and PRP from patients with severe congenital FV deficiency. PPP and PRP from patients PD I, PD II, PD III, and PD VII were triggered with 1 (light gray), 5 (middle gray), 10 (dark gray), or 50 (black) pM of TF, and thrombin generation was determined as described in “Thrombin generation assays.” Thrombin generation in PRP was measured without the addition of any platelet agonist and after preactivation of platelets with collagen (10 μg/mL) or Ca2+-ionophore (20μM). A representative control is shown for comparison.
Thrombin generation in PPP and PRP from patients with severe congenital FV deficiency. PPP and PRP from patients PD I, PD II, PD III, and PD VII were triggered with 1 (light gray), 5 (middle gray), 10 (dark gray), or 50 (black) pM of TF, and thrombin generation was determined as described in “Thrombin generation assays.” Thrombin generation in PRP was measured without the addition of any platelet agonist and after preactivation of platelets with collagen (10 μg/mL) or Ca2+-ionophore (20μM). A representative control is shown for comparison.
Thrombin generation parameters in PRP triggered with 5 pM of TF
| . | No platelet agonist . | Collagen, 10 μg/mL . | Ionophore, 20μM . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag time min . | Peak height nM . | ETP nM · min . | Lag time min . | Peak height nM . | ETP nM · min . | Lag time min . | Peak height nM . | ETP nM · min . | |
| PD I | 27.2 | 6 | 85 | 19.2 | 60 | 384 | 13.0 | 87 | 469 |
| PD II | 17.7 | 26 | 290 | 15.3 | 82 | 624 | 10.7 | 107 | 632 |
| PD III | 28.3 | 12 | 152 | 21.7 | 56 | 438 | 11.0 | 94 | 610 |
| PD VII | 21.5 | 31 | 283 | 13.7 | 127 | 661 | 8.3 | 271 | 808 |
| Controls, n = 8 | 3.0 ± 0.5 | 48 ± 49 | 671 ± 155 | 3.0 ± 0.6 | 86 ± 55 | 681 ± 69 | 2.3 ± 0.2 | 191 ± 44 | 725 ± 94 |
| . | No platelet agonist . | Collagen, 10 μg/mL . | Ionophore, 20μM . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag time min . | Peak height nM . | ETP nM · min . | Lag time min . | Peak height nM . | ETP nM · min . | Lag time min . | Peak height nM . | ETP nM · min . | |
| PD I | 27.2 | 6 | 85 | 19.2 | 60 | 384 | 13.0 | 87 | 469 |
| PD II | 17.7 | 26 | 290 | 15.3 | 82 | 624 | 10.7 | 107 | 632 |
| PD III | 28.3 | 12 | 152 | 21.7 | 56 | 438 | 11.0 | 94 | 610 |
| PD VII | 21.5 | 31 | 283 | 13.7 | 127 | 661 | 8.3 | 271 | 808 |
| Controls, n = 8 | 3.0 ± 0.5 | 48 ± 49 | 671 ± 155 | 3.0 ± 0.6 | 86 ± 55 | 681 ± 69 | 2.3 ± 0.2 | 191 ± 44 | 725 ± 94 |
Thrombin generation parameters were obtained from the thrombin generation curves shown in Figure 2.
ETP indicates endogenous thrombin potential.
In control PPP and PRP, thrombin generation was already appreciable at 1pM of TF and increased at increasing TF concentrations. The lag time of thrombin generation decreased at increasing TF concentrations, and at 50pM of TF it became too short to be measurable. At each TF concentration, thrombin generation was lower in PRP than in PPP, most probably because activated platelets provide a less favorable phospholipid surface for coagulation reactions than the synthetic phospholipid vesicles added to PPP.
No thrombin generation was observed in PPP from patients PD I, PD II, and PD III at any of the TF concentrations tested. PPP from patient PD VII showed thrombin generation at TF concentrations of 5pM or higher, although this was lower and started later in time than in control plasma. In contrast to PPP, PRP from all 4 FV-deficient patients showed thrombin generation, suggesting the presence of some residual FV in the patients' platelets. Thrombin generation was already measurable at 1pM of TF in PRP from patients PD II and PD VII and at 5pM of TF in PRP from patients PD I and PD III.
When PRP was preincubated with collagen to activate platelets before initiating coagulation, thrombin generation was higher and in the FV-deficient patients it also started earlier than in the absence of platelet agonists. In fact, thrombin generation was already observed at 1pM of TF in PRP from all 4 FV-deficient patients. Increasing TF concentrations progressively decreased the lag time, but had no effect on the peak height or ETP.
Preincubation of PRP with Ca2+-ionophore yielded higher and earlier thrombin formation than preincubation with collagen. Also under these conditions, the lag time decreased at increasing TF concentrations, whereas the amount of thrombin formed (peak height and ETP) hardly changed.
Remarkably, in the presence of collagen or Ca2+-ionophore, thrombin generation in PRP from the FV-deficient patients was almost as high as or even higher (PD VII) than in control PRP.
FV dependence of thrombin generation in PRP from the FV-deficient patients
To investigate whether thrombin generation observed in PRP from the FV-deficient patients was FV dependent, thrombin generation was determined in the patients' PRP in the presence of specific inhibitors of FV(a) (ie, an anti-FV antibody or APC).
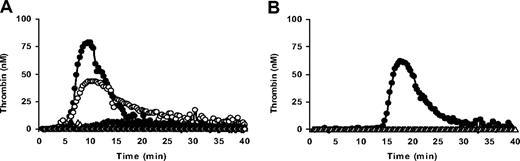
When coagulation was triggered with 50pM of TF in the presence of Ca2+-ionophore, the addition of an anti-FV antibody completely inhibited thrombin generation in FV-deficient PRP (Figure 3A), whereas it had no effect on thrombin generation in control PRP (data not shown).
FV dependence of thrombin generation in PRP from a FV-deficient patient. (A) Effect of an anti-FV antibody on thrombin generation in FV-deficient PRP. PRP from patient PD III was triggered with 50pM of TF in the presence of Ca2+-ionophore and thrombin generation was measured in the absence of an anti-FV antibody (●) and in the presence of 8 μg/mL (○), 28 μg/mL (▴), 60 μg/mL (▵), and 108 μg/mL (◇) of anti-FV antibody. (B) Effect of APC on thrombin generation in FV-deficient PRP. PRP from patient PD III was triggered with 5pM of TF in the presence of calcium ionophore and thrombin generation was measured in the absence of APC (●) and in the presence of 50nM of APC (▵).
FV dependence of thrombin generation in PRP from a FV-deficient patient. (A) Effect of an anti-FV antibody on thrombin generation in FV-deficient PRP. PRP from patient PD III was triggered with 50pM of TF in the presence of Ca2+-ionophore and thrombin generation was measured in the absence of an anti-FV antibody (●) and in the presence of 8 μg/mL (○), 28 μg/mL (▴), 60 μg/mL (▵), and 108 μg/mL (◇) of anti-FV antibody. (B) Effect of APC on thrombin generation in FV-deficient PRP. PRP from patient PD III was triggered with 5pM of TF in the presence of calcium ionophore and thrombin generation was measured in the absence of APC (●) and in the presence of 50nM of APC (▵).
Since APC concentrations up to 200nM had no effect on thrombin generation in PRP triggered with 50pM of TF in the presence of Ca2+-ionophore, the APC sensitivity of thrombin generation in PRP was tested at 5pM of TF in the presence of Ca2+-ionophore. Under these conditions, thrombin generation in FV-deficient PRP was completely inhibited by 50nM APC (Figure 3B). Thrombin generation in control PRP could also be fully inhibited by APC, but a considerably higher APC concentration (200nM) was required (data not shown).
FV antigen and activity levels in plasma and platelets
The fact that thrombin generation was observed in PRP, but not in PPP, from the FV-deficient patients suggested that their platelets contained FV. To verify this, FV antigen and activity levels were measured in washed platelets from the FV-deficient patients and 8 healthy controls, and compared with plasma FV levels (Table 3).
Plasma and platelet FV antigen and activity levels in FV-deficient patients
| . | Plasma FV . | Platelet FV . | ||
|---|---|---|---|---|
| Antigen, % . | Activity, %* . | Antigen, % . | Activity, %* . | |
| PD I | 1.8 | < 0.5 | 4.7 | 1.7 |
| PD II | 5.7 | < 0.5 | 1.7 | 3.7 |
| PD III | 4.2 | < 0.5 | 0.4 | 6.4 |
| PD VII | 9.6 | 4.4 | 4.6 | 11.9 |
| Controls, n = 8 | 98.7 ± 20.9 | 106.5 ± 26.8 | 93.9 ± 32.5 | 93.6 ± 38.6 |
| . | Plasma FV . | Platelet FV . | ||
|---|---|---|---|---|
| Antigen, % . | Activity, %* . | Antigen, % . | Activity, %* . | |
| PD I | 1.8 | < 0.5 | 4.7 | 1.7 |
| PD II | 5.7 | < 0.5 | 1.7 | 3.7 |
| PD III | 4.2 | < 0.5 | 0.4 | 6.4 |
| PD VII | 9.6 | 4.4 | 4.6 | 11.9 |
| Controls, n = 8 | 98.7 ± 20.9 | 106.5 ± 26.8 | 93.9 ± 32.5 | 93.6 ± 38.6 |
FV activity was determined with a prothrombinase-based assay (see “Measurement of FV activity levels in plasma and platelets”).
Although FV antigen was detectable in plasma from all 4 FV-deficient patients, plasma FV activity was below detection limit in all FV-deficient patients except PD VII (4.4%). In platelets, not only FV antigen but also FV activity was measurable in all 4 FV-deficient patients, confirming the presence of functional platelet FV even in the patients with undetectable plasma FV activity. Although platelet FV antigen and activity levels did not show a correlation in the FV-deficient patients, they were well correlated in the healthy controls (r = 0.94, P = .002). Moreover, despite the very small sample size, platelet FV activity levels in FV-deficient patients correlated (r = 0.91, P = .09) with the peak height of thrombin generation determined in the respective PRPs in the presence of Ca2+-ionophore, a condition where all platelet FV(a) is exposed.
Immunoprecipitation of plasma and platelet FVa
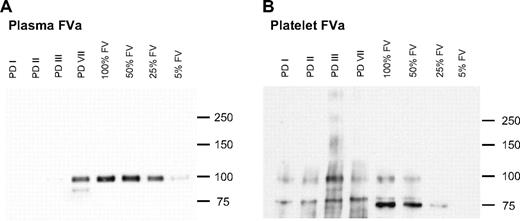
To visualize any residual FV present in plasma and/or platelets from the FV-deficient patients, FV was concentrated from plasma and platelets by immunoprecipitation and detected by Western blotting (Figure 4). Since plasma FV is structurally different from platelet FV, whereas the corresponding activated forms are identical,40 activated plasma and platelets (containing FVa) were used as a starting material for the immunoprecipitation. As shown in Figure 4A, no FVa was detectable in PPP from the FV-deficient patients, except for PD VII. In contrast, all patients showed FVa on the Western blot of activated platelet lysates (Figure 4B). Since an anti–heavy chain antibody was used for the detection of FVa, only the heavy chain is visible on the Western blot. This appears as a single band of 105 kDa in plasma FVa, and as multiple bands in platelet FVa. The additional bands of 75/80 kDa observed in platelet FVa represent degradation products of the FVa heavy chain that are probably generated during platelet activation and/or immunoprecipitation.41,42
Immunoprecipitation of FVa from plasma and platelets. FVa was immunoprecipitated from 200 μL of plasma (A) or 200 μL of 1.5 × 108 activated platelets/mL (B), concentrated 6 times (FV-deficient patients PD I, PD II, PD III, PD VII) or not concentrated (control), and subjected to SDS–polyacrylamide gel electrophoresis and Western blotting. Control plasma and platelet FVa preparations were run at different dilutions (100%, 50%, 25%, or 5%). FVa was detected with a monoclonal anti-FV heavy chain antibody and chemiluminescence. The FVa heavy chain has a molecular weight of 105 kDa; smaller fragments (only visible in platelet FVa) are degradation products of the FVa heavy chain.
Immunoprecipitation of FVa from plasma and platelets. FVa was immunoprecipitated from 200 μL of plasma (A) or 200 μL of 1.5 × 108 activated platelets/mL (B), concentrated 6 times (FV-deficient patients PD I, PD II, PD III, PD VII) or not concentrated (control), and subjected to SDS–polyacrylamide gel electrophoresis and Western blotting. Control plasma and platelet FVa preparations were run at different dilutions (100%, 50%, 25%, or 5%). FVa was detected with a monoclonal anti-FV heavy chain antibody and chemiluminescence. The FVa heavy chain has a molecular weight of 105 kDa; smaller fragments (only visible in platelet FVa) are degradation products of the FVa heavy chain.
Platelet TFPI level
We have recently shown that full-length TFPI is markedly reduced in FV-deficient plasma,29 a finding that was confirmed in this study (Table 4). Since platelets also contain full-length TFPI,43,44 we measured platelet TFPI antigen in the FV-deficient patients (Table 4). Platelet TFPI levels showed a large interindividual variation both in controls and in FV-deficient patients. On average, platelet TFPI levels were slightly but not significantly lower in FV-deficient patients (58.3% ± 19.9%) than in healthy controls (77.4% ± 31.7%).
Plasma and platelet TFPI antigen levels
| . | Full-length TFPI level . | |
|---|---|---|
| Plasma, % . | Platelet, % . | |
| PD I | 36.9 | 81.6 |
| PD II | 55.8 | 58.7 |
| PD III | 22.0 | 32.9 |
| PD VII | 51.0 | 60.1 |
| Controls, n = 8 | 96.7 ± 22.0 | 77.4 ± 31.7 |
| . | Full-length TFPI level . | |
|---|---|---|
| Plasma, % . | Platelet, % . | |
| PD I | 36.9 | 81.6 |
| PD II | 55.8 | 58.7 |
| PD III | 22.0 | 32.9 |
| PD VII | 51.0 | 60.1 |
| Controls, n = 8 | 96.7 ± 22.0 | 77.4 ± 31.7 |
Effect of TFPI on thrombin generation in PRP
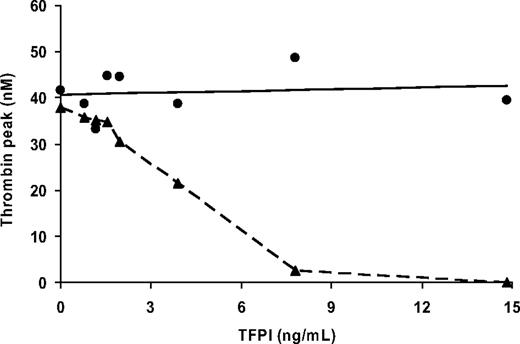
To verify whether the low plasma levels of full-length TFPI also improve thrombin generation in FV-deficient PRP (as they do in PPP),29 increasing concentrations of TFPI (0.8 to 14.8 ng/mL) were added to PRP from patient PD II and to control PRP, and thrombin generation was initiated with 50pM of TF in the presence of collagen. In control plasma, addition of TFPI increased the lag time of thrombin generation from 4.2 minutes to 8.0 minutes, but did not affect peak height (Figure 5). In contrast, in FV-deficient PRP, increasing the TFPI concentration not only prolonged the lag time (from 14.3 minutes to 29.5 minutes), but it also profoundly reduced the amount of thrombin formed (Figure 5). The addition of 7.8 ng/mL of TFPI, a concentration that normalizes the plasma TFPI level in FV-deficient PRP, almost completely abolished thrombin generation in FV-deficient PRP, which confirms the beneficial effect of low plasma levels of TFPI for thrombin generation in FV-deficient patients.
TFPI titration of thrombin generation in PRP. Control PRP (●) and FV-deficient PRP (patient PD II, ▴) were supplemented with increasing amounts of recombinant full-length TFPI and coagulation was initiated with 50pM of TF in the presence of collagen (10 μg/mL). The peak height of thrombin generation is plotted as a function of the concentration of added TFPI.
TFPI titration of thrombin generation in PRP. Control PRP (●) and FV-deficient PRP (patient PD II, ▴) were supplemented with increasing amounts of recombinant full-length TFPI and coagulation was initiated with 50pM of TF in the presence of collagen (10 μg/mL). The peak height of thrombin generation is plotted as a function of the concentration of added TFPI.
Discussion
Despite the essential role of FV in prothrombin activation, many persons with severe congenital FV deficiency experience only mild to moderate bleeding.21 The present study provides an explanation for this paradox by showing that patients with undetectable plasma FV may contain enough functional FV in their platelets to guarantee thrombin generation and protect them against major bleeding.
Four patients with severe congenital FV deficiency and relatively mild bleeding symptoms were investigated. Although no thrombin generation was observed in PPP from the 3 patients with undetectable plasma FV even when plasma was triggered with 50pM of TF, the corresponding PRP showed appreciable thrombin generation already at 5pM of TF. Thrombin generation in PRP from the FV-deficient patients was already observed in the absence of platelet agonists and was greatly stimulated by preactivating the platelets with collagen or Ca2+-ionophore. The progressive increase in the amount of thrombin formed going from no platelet agonist to collagen and Ca2+-ionophore probably reflects the extent of FV secretion and the parallel exposure of procoagulant phospholipids (phosphatidylserine) on the outer platelet membrane surface.45,46 Remarkably, the total amount of thrombin formed in PRP from FV-deficient patients stimulated with collagen or Ca2+-ionophore was similar to that of controls, although the lag time of thrombin generation was considerably prolonged. Moreover, when PRP from the FV-deficient patients was activated with collagen or Ca2+-ionophore, the amount of thrombin formed was independent of the TF concentration, suggesting that a fixed prothrombinase activity was generated at all TF concentrations. A possible explanation for this observation is that the amount of platelet FV(a) that is released is the limiting factor for prothrombinase activity in FV-deficient PRP, although more factor X (FX) is activated at increasing TF concentrations.
To prove that thrombin generation in PRP from the FV-deficient patients was dependent on platelet FV and not caused by an unknown platelet protein capable of stimulating prothrombin activation, we showed that thrombin generation in PRP from FV-deficient patients could be completely abolished by 2 specific inhibitors of FV, an anti-FV antibody and APC. Thrombin generation in control plasma was much less sensitive to the FV antibody and to APC, which is likely caused by the excessive amount of FVa in combination with the reduced sensitivity of platelet FVa for APC.14-16
The presence of FV in platelets from the FV-deficient patients was confirmed in several ways. First, platelet preparations from all 4 patients showed FV activity in a FVa activity assay, that is, they were able to stimulate FXa-catalyzed prothrombin activation in a model system. Second, platelet FVa from the FV-deficient patients could be visualized on Western blot. In addition to the 105-kDa band, corresponding to the full-length heavy chain, platelet FVa showed several smaller fragments on the Western blot. These fragments represent degradation products of the FVa heavy chain that are probably generated during platelet activation47 and/or FVa immunoprecipitation, as many platelet proteases, including calpain41 and lysosomal enzymes,42 can cleave FV(a). Finally, FV antigen was also detectable in the patients' platelets by an ELISA. Although FV antigen was also measurable in the patients' plasma, this is most likely nonfunctional as the patients' PPP showed no FV activity in the prothrombinase-based and thrombin generation assays (except PD VII). The rather poor correlation between the amount of platelet FV antigen measured by ELISA and that visible on Western blot (eg, for PD III) might be due to interference of the F5 mutations with the recognition of FV by the different antibodies used in the ELISA, immunoprecipitation, and Western blot.
The presence of functional FV in platelets from patients with congenital FV deficiency can be explained only if the underlying F5 gene mutations are compatible with some residual FV expression. Remarkably, all patients in the study carried F5 missense mutations as the underlying cause of FV deficiency. Although all amino acid substitutions involved charge changes at highly conserved residues, it should be noted that missense mutations do not per se prevent protein synthesis, but rather impair the folding, secretion, and/or stability of the mutant protein.19 Moreover, being point mutations, their effects can be abolished by rare somatic reversion events or by ribosome slippage during mRNA translation.26 Therefore, they can hardly be considered truly “null” mutations. Although it has been argued that all patients with severe FV deficiency actually retain some minimal FV expression,19,25,26 as suggested by the uniform lethality of FV knockout mice25 and by the absence of gross deletions from the F5 mutational spectrum,19 further studies are needed to verify the presence of functional platelet FV in patients with F5 molecular defects other than missense mutations. As a matter of fact, no functional FV could be demonstrated in plasma or platelets from 2 young patients with severe FV deficiency caused by short out-of-frame deletions/insertions32,33 and from 2 other patients with unknown F5 mutations.31 Patients with undetectable platelet FV tend to have more severe clinical manifestations31,33 than the patients in our study and their PRP would be expected to generate less or no thrombin.
The mechanism underlying the preferential localization of FV in platelets rather than in plasma from patients with severe FV deficiency remains unclear. One possibility is that megakaryocytes take up all FV available in plasma, simultaneously depleting the plasma FV pool. This would imply a high-affinity receptor for FV endocytosis. Alternatively, minimal FV synthesis might occur in megakaryocytes as well as in the liver, but plasma FV might be more rapidly cleared than the FV stored in platelets. This hypothesis is supported by the observation that platelet concentrates confer FV-deficient patients a longer-lasting protection from bleeding than fresh-frozen plasma, suggesting that platelet FV has a longer half-life than plasma FV.48
Since the FV requirement for minimal hemostasis is less than 1%, the small amount of FV found in platelets may well be sufficient to explain the relatively mild clinical phenotype of our patients. In fact, the specific characteristics of platelet FV (rapid activation by FXa and resistance to APC-catalyzed inactivation)14,40 and its targeted release at the site of injury, resulting in high local concentrations,39 make platelet FV a very efficient procoagulant, able to maintain hemostasis even in the absence of plasma FV. This concept is illustrated by the in vitro observation that, in the presence of normal activated platelets (and thus of platelet FVa), plasma FV does not contribute to thrombin generation (Figure 1). Moreover, in a patient with a circulating FV inhibitor and undetectable plasma FV, platelet FV proved sufficient to ensure adequate hemostasis even during surgery.39 The pivotal role of platelet FV is further supported by the bleeding diathesis associated with FV New York,49 a selective deficiency of platelet FV with normal levels of plasma FV.
As we have recently reported,29 FV deficiency is associated with markedly reduced plasma levels of full-length TFPI, a condition that reduces the FV requirement for minimal thrombin generation. In the present study, we show that normalization of the plasma TFPI level almost completely abolishes thrombin generation in PRP from FV-deficient patients. This effect of TFPI is likely due to inhibition of the initiation phase of coagulation, since physiologic concentrations of TFPI do not inhibit prothrombin activation.50 The low TFPI level contributes to enhance thrombin generation in patients with severe FV deficiency and may explain the near-normal thrombin generation observed in collagen- and Ca2+-ionophore–stimulated PRP from these patients (Figure 2, Table 2).
In conclusion, this study demonstrates that patients with congenital FV deficiency and undetectable plasma FV may contain functional FV in their platelets. In combination with the low TFPI level, residual platelet FV supports enough thrombin generation to rescue patients with undetectable plasma FV from fatal hemorrhage. These findings further suggest that differences in residual platelet FV level, possibly determined by the nature of the underlying molecular defect, may be responsible for the variation in clinical phenotype observed among patients with equally undetectable plasma FV levels. Therefore, platelet FV should be routinely evaluated for a more accurate estimate of bleeding tendency in these patients.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the FV-deficient patients for their enthusiastic participation in this study. We thank Dr E. Zanon from the Hemophilia Center of Padua University Hospital and Dr G. Barillari from the Institute of Immuno-Hematology and Transfusion Medicine of Udine General and University Hospital (Italy) for their help in contacting patients and collecting blood samples. Dr V. Tchaikovski is gratefully acknowledged for his expert assistance and helpful suggestions for Western blot analysis.
This study was supported by a VIDI grant (no. 917-76-312; E.C.) from the Dutch Organisation for Scientific Research (NWO) and by a Ministero dell'Università della Ricerca Scientifica e Tecnologica (ex 60%) grant (no. 60A07-2430/05; P.S.).
Authorship
Contribution: C.D. performed research, analyzed data, and wrote the paper; P.S. recruited the patients, collected clinical histories, and critically reviewed the manuscript; L.S. recruited the patients and collected clinical histories; C.R., P.D., and S.G. performed research; J.R. provided major intellectual input and critically reviewed the manuscript; E.C. designed and performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elisabetta Castoldi, Department of Biochemistry, Maastricht University, PO Box 616, 6200 MD Maastricht, The Netherlands; e-mail: e.castoldi@bioch.unimaas.nl.