Abstract
Kaposi sarcoma–associated herpesvirus (KSHV) infection is essential to the development of Kaposi sarcoma (KS). Notch signaling is also known to play a pivotal role in KS cell survival and lytic phase entrance of KSHV. In the current study, we sought to determine whether KSHV regulates Notch components. KSHV-infected lymphatic endothelial cells showed induction of receptors Notch3 and Notch4, Notch ligands Dll4 and Jagged1, and activated Notch receptors in contrast to uninfected lymphatic endothelial cells. In addition, KSHV induced the expression of endothelial precursor cell marker (CD133) and mural cell markers (calponin, desmin, and smooth muscle alpha actin), suggesting dedifferentiation and trans-differentiation. Overexpression of latency proteins (LANA, vFLIP) and lytic phase proteins (RTA, vGPCR, viral interleukin-6) further supported the direct regulatory capacity of KSHV viral proteins to induce Notch receptors (Notch2, Notch3), ligands (Dll1, Dll4, Jagged1), downstream targets (Hey, Hes), and endothelial precursor CD133. Targeting Notch pathway with γ-secretase inhibitor and a decoy protein in the form of soluble Dll4 inhibited growth of KSHV-transformed endothelial cell line. Soluble Dll4 was also highly active in vivo against KS tumor xenograft. It inhibited tumor cell growth, induced tumor cell death, and reduced vessel perfusion. Soluble Dll4 is thus a candidate for clinical investigation.
Introduction
Kaposi sarcoma (KS) is the most common neoplasm in HIV-infected persons. KS lesions are composed of endothelial proliferation, immature and defective vessel formation, and infiltration with immune cells.1 KS-associated herpesvirus (KSHV) infection is linked to KS, primary effusion lymphoma, and Castleman disease.1 KSHV, a herpesvirus, uses 2 different programs to sustain and propagate infection, the most prominent of which is latency, during which only a few viral genes are expressed.1,2 The second program is the lytic phase, during which most of the viral genes are expressed in sequential manner, the viral DNA is amplified, and infectious virions are produced. The lytic phase also induces host cell toxicity.1,2
KS tumors show latent infection of spindle-shaped tumor cells, with rare cells undergoing lytic infection.2 Latency proteins may play a critical role in initiating and propagating KSHV-related tumors, whereas the lytic phase may expand the target cell population by infecting permissive cells. Investigation of latency and lytic cycle proteins has revealed that several proteins can transform target cells. The latency genes, including LANA and vFLIP, can regulate cell survival, transform target cells, and induce proliferation.3 LANA can stabilize activated forms of Notch receptor, a pathway with diverse functions, including transformation and regulation of the vascular system.4 vFLIP activates the nuclear factor-κB (NF-κB) pathway, induces spindle cell phenotype, and induces transformation in vitro and in vivo.5 Lytic phase viral proteins also regulate major signaling pathways, which may lead to transformation, regulate self-renewal, and induce angiogenesis and inflammation.6,7 Among them, replication and transcription activator (RTA) expression is necessary and sufficient to reactivate and complete viral lytic phase.8 RTA regulates several viral protein promoters.8 It also regulates genes via interaction with recombination signal sequence–binding protein J (RBP-J), also called CSL (CBF1 [Cp-binding factor 1]/Su(H)/Lag2),9 a transcription factor that is central to the Notch signaling pathway. Viral G-protein–coupled receptor (vGPCR) is a constitutively active GPCR that has close homology to interleukin-8 (IL-8) receptor.6 vGPCR transforms NIH3T3 cells and induces KS-like vascular lesion in transgenic mouse lines.6
It is thus of interest to determine whether KSHV viral proteins use host cellular genes to induce and sustain KS-related tumors. Notch pathway is of great interest based on its role as a transforming agent and its induction in KS and lymphoma.10 The Notch signaling pathway is evolutionarily conserved and proven to regulate cell fate decision in various cell types during and after development.11 On ligand binding, Notch receptor undergoes cleavage and releases Notch intracellular domain (NICD), which translocates to the nucleus and interacts with and activates RBP-J.11 The activated RBP-J protein induces expression of Notch target genes, including members of Hey and Hes helix-loop-helix family proteins.11 In higher vertebrates, multiple Notch homologs have been identified, including Notch1 through Notch4.12 Multiple ligands for the Notch receptors have also been identified in mammals (Jagged/Serrate 1 and 2 and Delta like [Dll] 1, 3, and 4).12 There is accumulating evidence for the spatial and temporal expression of Notch receptors and ligands, and their functional diversity.12 Notch receptors (Notch1, Notch3, and Notch4) and ligands (Dll4 and Jagged1) are expressed in the blood vessels.13 Specifically, Notch4 and Dll4 are restricted to arterial endothelial cells, whereas Notch3 is expressed only in mural cells/vascular smooth muscle cells.14 Notch1 knockout is lethal with profound vascular defects.15 Jagged1 knockout is embryonic lethal,16 and Dll4 single-copy loss is lethal with vascular defects.17 Notch3 knockout only has defects in the vascular wall of the small vessels predominantly in the arteries.14
Notch signaling regulates cell fate by mediating the differentiation of progenitor cells during development and the self-renewal of stem cells in adults.12 In many settings, a high level of Notch signaling maintains the stem cell phenotype, whereas a low level allows differentiation.18 Notch gene amplification, chromosomal translocation, and mutations in Notch components promote tumor growth and tumor stem cell–like characteristics.12,19 Inhibitors of Notch signaling have tumor inhibitory activity in many tumor models, including KS.10
Activated Notch pathway in KS and response to Notch inhibitors led us to the current studies. Here we show that KSHV induces Notch receptors, Notch ligands, and downstream targets. Notch activation in KS promotes cell survival, and Notch inhibitors have potent antitumor activity.
Methods
Antibodies and other reagents
Antibodies against Notch1, Notch3, Jagged1, cleaved NICD, and CD133 were from Cell Signaling. Notch4 and Dll1 antibodies were from R&D Systems. Dll4 antibody and soluble Dll4 (amino acids 1-526) fused to human IgG1 Fc fragment were generated at VasGene Therapeutics. α-Smooth muscle actin (α-SMA) antibody was from Santa Cruz Biotechnology. β-Actin antibody was from Sigma-Aldrich. CD31 antibody was from BD Biosciences. Ki67 and Notch3 (for immunostaining) antibodies were from Abcam. NG2 antibody, hypoxyprobe-1, and hypoxyprobe antibody were from Chemicon International. Horseradish peroxidase–conjugated secondary antibodies, anti–human Fc polyclonal antibody, and human IgG1 Fc fragment were from Rockland. Rhodamine-labeled ricinus communis agglutinin I (RCA) was from Vector Laboratories. TdT-mediated dUTP nick-end labeling (TUNEL) assay kit was from Promega. Inhibitors Bay 11-7085, U0126, and LY294002 were from EMD Biosciences. γ-Secretase inhibitor 1 (GSI-1) and 4-hydroxytamoxifen (4-OHT) were from Sigma-Aldrich.
Cell culture
Human umbilical artery endothelial cells (HUAECs), human umbilical vein endothelial cells (HUVECs), and human umbilical artery smooth muscle cells (UASMCs) were purchased from Lonza Walkersville and maintained in corresponding medium provided by Lonza using University of Southern California Institutional Review Board–approved tissue procurement protocol. Human primary lymphatic endothelial cells (LECs) were isolated as previously described.20 Purified LECs were immortalized with pBabe retrovirus containing human papillomavirus oncoproteins E6 and E7 and then infected with rKSHV.219 virus,21 followed by selection with puromycin resistance. rKSHV.219 expresses green fluorescent protein (GFP) from the EF-1α promoter and contains the gene for puromycin resistance as a selectable marker. Confocal microscopy and flow cytometry analyses showed that nearly all puromycin-resistant, GFP+ LECs were chronically infected with KSHV. LEC/KSHV cells used in this study were all less than 5 passages. LECs and LEC/KSHV cells were maintained in EGM2-MV medium from Lonza Walkersville. KS-IMM cell line was obtained from Dr A. Albini22 and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Mediatech). Telomerase-immortalized HUVECs stably expressing vFLIP-ERTAM or a control vector were generated as described previously and were obtained from P.M.C.23 Cells were treated with 4-OHT (100nM) for induction of gene expression as described previously.23
Constructs, SiRNA, and transfection
Expression vectors for KSHV genes were obtained from J.J. They include pcDNA3/LANA, pcDNA4/vFLIP, pcDNA3.1/RTA, pcDNA4/vGPCR, and pcDNA3.1/viral interleukin-6 (vIL-6). Transfection of 293T cells (ATCC) grown on 6-well plates was performed with Lipofectamine 2000 following the manufacturer's protocol (Invitrogen). Cells were harvested for RNA isolation 72 hours after transfection. We first screened various amounts of plasmid DNA for transfection to compare the viral protein level in transient expression system and KSHV-infected endothelial cells. Latency protein transient expression was compared with latently infected LECs, whereas lytic protein transient expression was compared with LECs/KSHV undergoing lytic phase. We used 0.1 to 0.4 μg of plasmid DNA, which resulted in expression level comparable with the gene expression in LEC/KSHV. Notch3 SiRNA was synthesized at QIAGEN according to the sequence described by Liu et al.24 Nontargeting control SiRNA was also purchased from QIAGEN (sequence was not disclosed by the vendor). Transfection was performed with HiPerfect (QIAGEN) following the manufacturer's protocol. Cells were subjected to RNA isolation, Western blot, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay approximately 72 hours after transfection.
RT-PCR and quantitative PCR
Total RNA was extracted from cell lysate with RNA extraction kit from QIAGEN. First-strand cDNA was synthesized from 2 μg of total RNA with the kit from Fermentas and then used for polymerase chain reaction (PCR) with specific primers (primer pairs used for this study are available on request) as described previously.25 Comparative quantitative PCR analysis was performed as previously described.26
Western blotting
Cell lysates were prepared as previously described.25 Typically, 20 μg of whole-cell lysates was run on 4% to 20% Tris-glycine gradient gel (Bio-Rad), transferred onto nitrocellulose membrane (Bio-Rad), and probed with primary and horseradish peroxidase conjugated secondary antibodies (Rockland). Femto Maximum Sensitivity chemiluminescent substrate from Thermo Scientific was used to develop signal.
Cell viability assay
Cells were seeded in 24-well plates at a density of 104 cells/well in a total volume of 500 μL. The medium was changed after cells were attached, and triplicate wells were treated. Cell viability was assessed using MTT as described previously.26
Flow cytometry
LECs and LEC/KSHV cells were washed with phosphate-buffered saline (PBS) and then washed with PBS containing 5mM ethylenediaminetetraacetic acid (EDTA). Cells were detached by incubated with PBS containing 5mM EDTA at 37°C for 5 minutes. After washing with PBS, cells were fixed by 4% paraformaldehyde at 37°C for 10 minutes. Cells were then washed by PBS and blocked with 0.5% bovine serum albumin, followed by incubation with rabbit anti-CD133 antibody (20 μg/mL) or control rabbit IgG (20 μg/mL) for 1 hour at room temperature. Cells were next washed twice and incubated with AlexaFluor488-conjugated antirabbit IgG antibody (1:700, Invitrogen). Finally, cells were washed twice and fluorescent cells were counted by flow cytometry.
Coculture and angiogenesis assay
Except LEC/KSHV, which has GFP expression, all cells were stained with 2μM orange or green CellTracker from Invitrogen according to the manufacturer's protocol. Cells were then grown under normal growth condition overnight. The second day, cells were trypsinized, centrifuged, and resuspended in HUAEC growth medium EGM2-MV supplemented with 10 ng/mL platelet-derived growth factor (PDGFbb; R&D Systems). Cells were counted, mixed at 1:1 ratio with various combinations, and then plated onto 12-well plates precoated with Matrigel (BD Biosciences). A total of 8 × 104 cells (1 cell type) were plated in each well. Plate was placed back to 37°C incubator maintaining 5% CO2. Six hours later, pictures were taken with an Olympus AX70 fluorescence microscope and Spot, Version 2.2.2 (Diagnostic Instruments) digital imaging system.
Immunofluorescence
Fresh-frozen tissue embedded in Optimum Cutting Temperature (OCT) medium (Sakura Finetek) was sectioned at 5 μm and fixed in phosphate-buffered 4% paraformaldehyde and washed in PBS. Sections were then incubated with primary antibody overnight at 4°C. After washing in PBS, antibody binding was localized with Alexa Fluor–conjugated appropriate secondary antibodies (Invitrogen). Nuclei were counterstained with 6-diamidino-2-phenylindole dihydrochloride hydrate. Images were obtained with an Olympus AX70 fluorescence microscope and Spot, Version 2.2.2 digital imaging system. Confocal images were taken with a Zeiss confocal microscope (Carl Zeiss).
Murine tumor xenograft models
KS-IMM cells were propagated, collected by trypsin digestion, and resuspended in serum-free medium. Cells (2 × 106) were injected in the flank of 10- to 12-week-old male Balb/C athymic mice. Tumor growth was measured 3 times a week, and tumor volume was estimated as 0.52 × a × b2, where a and b are the largest and smallest lengths of the palpable tumor. Six days after cell implantation, tumor volumes were calculated to ensure uniformity in size, and animals were divided randomly into 2 groups (n = 6 mice per group, repeated twice). Each group was administered 3 times per week intraperitoneal injection of 200 μL of PBS alone or 10 mg/kg sDll4-Fc in PBS. Animals were killed and tumors were harvested after 28 days treatment. Distribution and intensity of hypoxia were assessed by immunostaining of hypoxyprobe HP1-100, which was infused intraperitoneally at a dose of 60 mg/kg 1 hour before tumor harvest. Vessel perfusion was assessed by RCA, which was infused 10 to 15 minutes before tumor harvest following the manufacturer's protocol. All murine studies were conducted under a University of Southern California Animal Care and Use Committee–approval protocol.
Results
KSHV regulates Notch receptors and ligands and activates Notch pathway
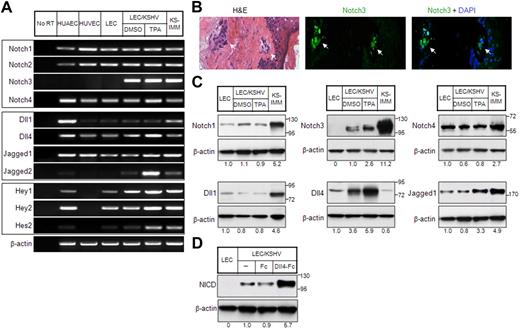
Notch receptors (Notch1, Notch2, and Notch 4) and Notch ligand jagged1 expression in KS tumor cells and KS cell line SLK have been shown in the past.10 Furthermore, GSI-1, which inhibits Notch signaling by preventing NICD release, was shown to reduce the proliferation of KS cell line SLK.10 However, the role of KSHV in the regulation of Notch components has not been investigated previously. To this end, we examined the expression of Notch components, including its downstream targets in LECs latently infected with KSHV (LEC/KSHV) or undergoing lytic phase on phorbol ester tetradecanoyl phorbol acetate (TPA) treatment. TPA treatment had no effect on the expression of Notch pathway genes in LECs (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), suggesting that its effect on LEC/KSHV was specifically related to the KSHV lytic cycle. Notch1 and Notch4 were expressed in LECs and LEC/KSHV, whereas Notch3 was only induced in LEC/KSHV with further induction (2.6-fold) during lytic phase (Figure 1A,C). Notch3 expression was also determined in 5 KS tumor samples, which showed positive signal in KS tumor cells and not the overlying skin. Even the earliest KS lesion showed Notch3 expression (Figure 1B).
Expression of Notch pathway genes in KS cells. (A) Expression of Notch receptors (top panel), Notch ligands (middle panel), and Notch pathway downstream target genes (bottom panel) in endothelial cells and KS cells was analyzed by RT-PCR. HUAECs, HUVECs, and LECs were included as controls. KSHV-infected LECs (LEC/KSHV) were treated with TPA (25 ng/mL) or dimethyl sulfoxide (DMSO) for 48 hours before harvest. KS-IMM was a tumor cell line isolated from KS lesion.22 β-Actin expression indicates that an equal amount of RNA was used to perform PCR. (B) Expression of Notch3 in human KS tumor tissue was analyzed by immunostaining. The KS tumor regions are indicated by arrows. (C) Expression of Notch receptors and Notch ligands in KS cells were analyzed by Western blot. The relative expression level was quantitated by ImageJ (National Institutes of Health), normalized to β-actin, and is shown below each panel. (D) sDll4-Fc or human Fc fragment was clustered with anti-Fc antibody (1:1 ratio) at room temperature for 60 minutes. LEC/KSHV cells were then treated with clustered sDll4-Fc or Fc fragment (1 μg/mL) for 90 minutes, and whole cell lysate was subjected to Western blot with antibody against activated Notch.
Expression of Notch pathway genes in KS cells. (A) Expression of Notch receptors (top panel), Notch ligands (middle panel), and Notch pathway downstream target genes (bottom panel) in endothelial cells and KS cells was analyzed by RT-PCR. HUAECs, HUVECs, and LECs were included as controls. KSHV-infected LECs (LEC/KSHV) were treated with TPA (25 ng/mL) or dimethyl sulfoxide (DMSO) for 48 hours before harvest. KS-IMM was a tumor cell line isolated from KS lesion.22 β-Actin expression indicates that an equal amount of RNA was used to perform PCR. (B) Expression of Notch3 in human KS tumor tissue was analyzed by immunostaining. The KS tumor regions are indicated by arrows. (C) Expression of Notch receptors and Notch ligands in KS cells were analyzed by Western blot. The relative expression level was quantitated by ImageJ (National Institutes of Health), normalized to β-actin, and is shown below each panel. (D) sDll4-Fc or human Fc fragment was clustered with anti-Fc antibody (1:1 ratio) at room temperature for 60 minutes. LEC/KSHV cells were then treated with clustered sDll4-Fc or Fc fragment (1 μg/mL) for 90 minutes, and whole cell lysate was subjected to Western blot with antibody against activated Notch.
Among Notch ligands, Dll1 is normally expressed in endothelial cells, predominantly in arterial endothelial cells.27 It was expressed at a low level in LECs and LEC/KSHV with or without induction of lytic cycle (Figure 1A,C). Dll4 expression in vivo is highly restricted to arterial endothelial cells.13 It was highly induced in LEC/KSHV with further induction in lytic phase (Figure 1A). Jagged1 and Jagged2 were also expressed in latently infected LECs with further induction during lytic phase (Figure 1A,C). The lack of reagents did not allow assessment of Jagged2 protein levels. Downstream targets of Notch pathway, particularly Hey1, Hey2, and Hes2, were expressed during latency with marked induction of Hes2 during lytic phase (Figure 1A). Similar results in Notch induction were observed in another KSHV-infected endothelial cell line, LTC-TIVE28 (data not shown). We next examined the activation state of Notch receptors. Antibody specific for cleaved intracellular domain of Notch NICD showed constitutive activation of Notch receptor in KSHV-infected LECs (Figure 1D). Furthermore, clustered soluble ligand Dll4 was applied to demonstrate that Notch receptors are responsive to their cognate ligand-mediated activation (Figure 1D).
It has been reported previously that, in normal blood vessel, Notch 3 is expressed only in mural cell/vascular smooth muscle cells but not endothelial cells.14 Furthermore, when endothelial cells are cocultured with stromal fibroblast, endothelial cell-specific Jagged1 can induce fibroblast to acquire mural cell markers, including Notch3.14 Current data showing expression of Notch3, calponin, desmin, and smooth muscle cell actin further support the findings that KSHV-infected endothelial cells can also acquire smooth muscle cell phenotype (Figure 2C). These results are consistent with the previous findings that KS cells express vascular smooth muscle cell phenotype and arterial endothelial cell phenotype.25,29 Notch4 and Dll4, on the other hand, are a highly specific receptor-ligand pair for arterial endothelial cells (Figure 1A,C). Taken together, these results indicate that KSHV can induce both mural cell and arterial endothelial cell phenotype.
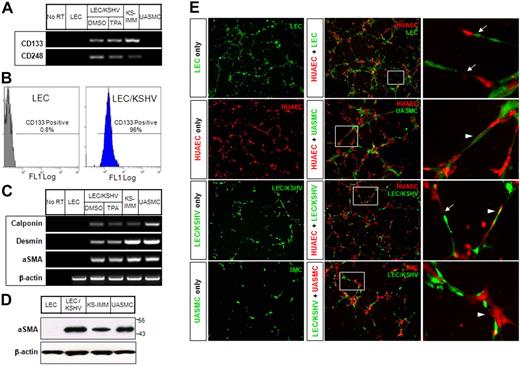
KS cells have both endothelial and mural cell characteristics. (A) Expression of endothelial progenitor markers (CD133 and CD248) in KS cells was analyzed by RT-PCR. (B) LECs and LEC/KSHV cells were sorted for cell surface CD133 expression by flow cytometry. (C) Expression of mural cell markers (calponin, desmin, and α-SMA) in KS cells was analyzed by RT-PCR. Human UASMCs were used as positive control for mural cell markers. (D) Western blots analysis for α-SMA expression in KS cells. (E) Cells were labeled with green or orange CellTracker and cultured on Matrigel for 6 hours, alone or in various combinations. (Right panel) Enlarged boxed area in the middle panel. Arrows point to the tubes formed by integration of 2 types of endothelial cells. Arrowheads point to the endothelial cells lined with mural cells.
KS cells have both endothelial and mural cell characteristics. (A) Expression of endothelial progenitor markers (CD133 and CD248) in KS cells was analyzed by RT-PCR. (B) LECs and LEC/KSHV cells were sorted for cell surface CD133 expression by flow cytometry. (C) Expression of mural cell markers (calponin, desmin, and α-SMA) in KS cells was analyzed by RT-PCR. Human UASMCs were used as positive control for mural cell markers. (D) Western blots analysis for α-SMA expression in KS cells. (E) Cells were labeled with green or orange CellTracker and cultured on Matrigel for 6 hours, alone or in various combinations. (Right panel) Enlarged boxed area in the middle panel. Arrows point to the tubes formed by integration of 2 types of endothelial cells. Arrowheads point to the endothelial cells lined with mural cells.
Endothelial and mural cell precursor characteristics in KS cells
Endothelial- and mural-pericyte–specific expression pattern raised the possibility that KSHV may skew the endothelial cells toward dedifferentiated phenotype, such that LEC/KSHV induce endothelial precursor–like features and transdifferentiate to have pericyte precursor features. To this end, we examined the expression of endothelial precursor marker CD133,30 CD248 (endosialin),31 along with early and late mural cell markers calponin, desmin, and smooth muscle-α actin. Expression of CD133 and CD248 indicates that LEC/KSHV express endothelial precursor cell markers (Figure 2A-B). Mural cell-specific markers were also expressed in LEC/KSHV but not in endothelial cells (Figure 2C-D). KHSV thus has a unique capability to activate precursor cell phenotype and mural cell marker. We next determined whether LEC/KSHV display endothelial or mural cell characteristics in tube formation assays in which endothelial cells organize to make tube-like structures but smooth muscle cells on their own do not (Figure 2E). Coculture of endothelial cells instructed smooth muscle cells to promote tube formation and localize in close proximity to endothelial cells (Figure 2E). Coculture of endothelial cells and LECs or LEC/KSHV resulted in tube-like structures, and cocultures showed cooperation between HUVECs and LECs as well as HUVECs and LEC/KSHV (Figure 2E arrow). Furthermore, LEC/KSHV align tightly adjacent to endothelial cells and behave like mural cells, a feature that was not observed with endothelial cell-LEC coculture (Figure 2E arrowhead). An endothelial cell-like characteristic of LEC/KSHV was also maintained when these cells were cocultured with vascular smooth muscle cells (Figure 2E). Thus, KSHV-infected endothelial cells not only express endothelial- and mural-specific genes but can also function as endothelial and mural cells.
KSHV latency proteins regulate Notch receptors and ligands
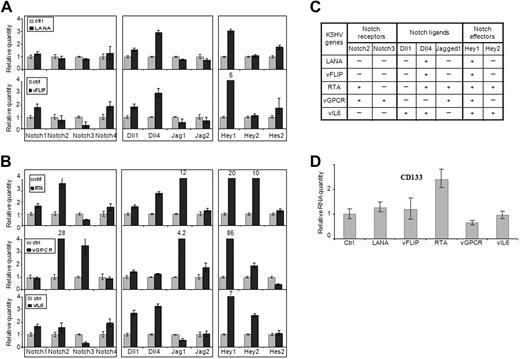
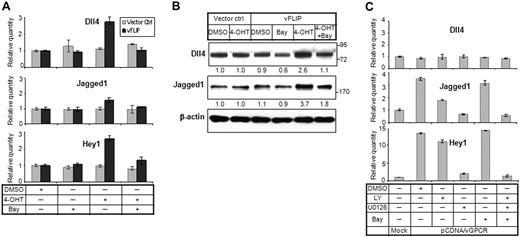
We next wished to determine whether KSHV viral proteins regulate Notch receptors and ligands. KS tumor cells have expression of latency proteins in nearly all cells. We thus first focused on latency proteins LANA and vFLIP. Expression vectors were introduced into 293T cells. To ensure the viral protein levels in transiently transfected 293T cells were comparable with those in KSHV-infected LECs, we performed a serial dilution of plasmid DNA and selected the optimal DNA amount (0.4 μg) for transfection (data not shown). Quantitative RT-PCR showed that LANA and vFLIP both induced Dll4, whereas vFLIP also showed modest increases in Notch4, Notch1, and Dll1 (Figure 3A). KSHV is latent in the majority of KS cells; thus, the regulation of Notch pathway by latency-related proteins, especially LANA and vFLIP, may be very important in initiating and sustaining tumor cell. We next examined the levels of Notch receptors and ligands in endothelial cells with inducible vFLIP vector transfected in primary endothelial cells.23 The results were confirmed for protein and mRNA measurements that Dll4 as well as Jagged1 were induced (Figure 4A-B). vFLIP has been shown to activate NF-κB and alter endothelial-cell phenotype to spindle-cell appearance similar to that of KS tumor cells.23,32 We thus wish to determine whether vFLIP induced Notch components via the NF-κB pathway. Endothelial cells expressing vFLIP were treated with NF-κB inhibitor Bay11-7085. The level of Dll4 and Jagged1 expression was measured (Figure 4A-B). vFLIP induced Dll4 and Jagged1, and this induction was attenuated by Bay11-7085 treatment.
Induction of Notch pathway genes by KSHV proteins. The 293T cells were transfected with expression vectors for latency phase genes (LANA or vFLIP; A) and lytic phase genes (RTA, vGPCR, or vIL-6; B). Empty pcDNA3 vector transfection was used as control. Expression of Notch receptors (left panel), Notch ligands (middle panel), and Notch pathway downstream genes (right panel) was analyzed by quantitative RT-PCR 72 hours after transfection. (C) The overall change of expression of Notch pathway genes after transfection was summarized. − indicates without significant induction; and +, with significant induction. (D) Expression of endothelial progenitor marker CD133 was analyzed by quantitative RT-PCR as in panels A and B.
Induction of Notch pathway genes by KSHV proteins. The 293T cells were transfected with expression vectors for latency phase genes (LANA or vFLIP; A) and lytic phase genes (RTA, vGPCR, or vIL-6; B). Empty pcDNA3 vector transfection was used as control. Expression of Notch receptors (left panel), Notch ligands (middle panel), and Notch pathway downstream genes (right panel) was analyzed by quantitative RT-PCR 72 hours after transfection. (C) The overall change of expression of Notch pathway genes after transfection was summarized. − indicates without significant induction; and +, with significant induction. (D) Expression of endothelial progenitor marker CD133 was analyzed by quantitative RT-PCR as in panels A and B.
vGPCR and vFLIP proteins regulate Notch pathway through ERK and NF-κB, respectively. (A) HUVECs stably transfected with vFLIP-ERTAM plasmid or control vector23 were grown on 6-well plates and induced by 4-OHT (100nM) for 48 hours. The cells were treated with DMSO or Bay (10μM) for 2 hours. Cells without 4-OHT induction were also treated with Bay (10μM) for 2 hours before harvest. RNA was isolated and subjected to quantitative RT-PCR. Expression of Dll4, Jagged1, and Hey1 was analyzed. (B) Cells were treated in the same way as in panel A and subjected to Western blot for Dll4 and Jagged1. (C) 293T cells were transfected with vGPCR plasmid for 72 hours. Before harvest, the vGPCR-transfected cells were treated by PI3K inhibitor LY (LY294002, 15μM), ERK inhibitor U0126 (15μM), and NF-κB inhibitor Bay (Bay 11-7085, 10μM), or a combination of the 3. Cells were treated by LY and U0126 for 5 hours and Bay for 2 hours. RNA was isolated and subjected to quantitative RT-PCR. Expression of Dll4, Jagged1, and Hey1 was analyzed.
vGPCR and vFLIP proteins regulate Notch pathway through ERK and NF-κB, respectively. (A) HUVECs stably transfected with vFLIP-ERTAM plasmid or control vector23 were grown on 6-well plates and induced by 4-OHT (100nM) for 48 hours. The cells were treated with DMSO or Bay (10μM) for 2 hours. Cells without 4-OHT induction were also treated with Bay (10μM) for 2 hours before harvest. RNA was isolated and subjected to quantitative RT-PCR. Expression of Dll4, Jagged1, and Hey1 was analyzed. (B) Cells were treated in the same way as in panel A and subjected to Western blot for Dll4 and Jagged1. (C) 293T cells were transfected with vGPCR plasmid for 72 hours. Before harvest, the vGPCR-transfected cells were treated by PI3K inhibitor LY (LY294002, 15μM), ERK inhibitor U0126 (15μM), and NF-κB inhibitor Bay (Bay 11-7085, 10μM), or a combination of the 3. Cells were treated by LY and U0126 for 5 hours and Bay for 2 hours. RNA was isolated and subjected to quantitative RT-PCR. Expression of Dll4, Jagged1, and Hey1 was analyzed.
KSHV lytic cycle proteins regulate Notch and Notch ligands
RTA initiates viral replication by inducing the majority of virally encoded proteins. RTA localizes to the viral gene promoters by interacting with Notch signal–regulating proteins RBP-J.9 We thus investigated the expression of RTA-regulated notch components by introducing the expression vector in 293T cells. Again, an appropriate DNA amount (0.1 μg) for transfection was chosen to ensure that the viral protein levels in transiently transfected 293T cells were comparable with those in TPA-induced KSHV-infected LECs. Induction of Notch2, Jagged1, and Dll4 was observed (Figure 3B). Furthermore, RTA induced Notch target proteins Hey1 and Hey2, and progenitor cell marker CD133 (Figure 3D). Hey1 induction was 20-fold over the empty vector. RTA may regulate gene expression directly by recruiting coactivator complex at RBP-J binding sites.
vGPCR is a transforming protein that also induces KS-like tumors in various transgenic mouse lines.6 vGPCR ectopic expression in endothelial cells and 293T cell line showed induction of Notch2, Notch3, and Jagged1 (Figures 3B, 4C). vGPCR induced the most profound induction of Hey1 (86-fold). vGPCR induces signaling via several pathways, including phosphoinositide-3 kinase (PI3K) and extracellular signal-regulated kinase (ERK).33 We thus tested whether inhibitors of various pathways modulate the regulation of Notch components. This indeed was the case. ERK inhibitor abolished the induction of Dll4, Jagged1, and Hey1 (Figure 4C). PI3K inhibitor decreased Jagged1 levels but had no effect on downstream target Hey1 (Figure 4C). Thus, the most prominent pathway used by vGPCR to regulate Notch components is through the ERK pathway.
vIL-6 is also a lytic cycle protein. It binds glycoprotein 130 (gp130) and induces signaling via signal transducer and activator of transcription 3 (STAT3) pathway in contrast to cellular IL-6, which binds IL-6R-gp130 heterodimer to induce signaling via the same pathway. gp130 binding ligands, including cellular IL-6, Gro-α, and oncostatin-M, all of which are produced in KS cell lines, have previously been reported in neural cell lineage to regulate Notch pathway, and even induce stem cell-like phenotype.34-36 IL-6 and vIL-6 may thus have similar effects in KS cells. We thus ectopically expressed vIL-6 in 293T cells. vIL-6 induced the expression of Notch4, Dll1, and Dll4 as well as downstream targets Hey1 and Hey2 (Figure 3B). Thus, vIL-6 regulates Notch components, consistent with its known biochemical and biologic functions.
Notch provides survival signal in KSHV-transformed endothelial cells
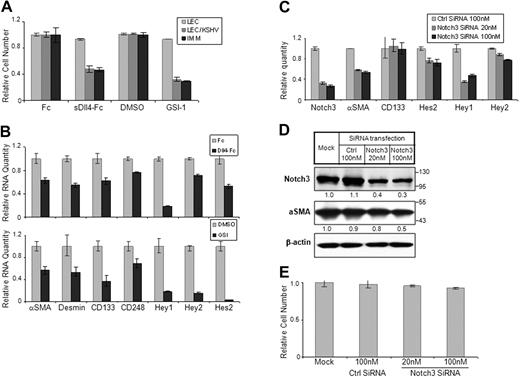
The significance of Notch pathway in KS tumor cell lines that lack KSHV infection has been investigated previously showing that Notch inhibitors induce cell-cycle arrest followed by cell death.10 It is probable that KSHV-infected endothelial cells also show a similar response. We thus investigated the activity of a notch inhibitor, GSI-1, that blocks the final cleavage of Notch and thus blocks release and nuclear localization of NICD. GSI-1 was highly active in LEC/KSHV but did not cause any cellular toxicity to LECs or endothelial cells (Figure 5A; and data not shown). GSI-1 activity is not restricted to Notch activation only. It also blocks the activation of several other proteins. We thus investigated the activity of the soluble form of Dll4 (sDll4-Fc) that blocks interaction between endogenous Notch receptors and ligands but cannot activate Notch receptors.37 Soluble Dll4 showed a marked decrease in LEC/KSHV cell survival with no cellular toxicity to LECs, consistent with the results with Notch inhibition (Figure 5A). We further determined that sDll4 and GSI-1 reduced the basal levels of Notch downstream target expression using quantitative RT-PCR (Figure 5B). Notch inhibitors GSI-1 and sDll4 also down-regulated the expression of CD133, indicating that the endothelial precursor phenotype is predominantly mediated through Notch pathway during latency (Figure 5E). Notch3 inhibition using siRNA (Figure 5C) alone was sufficient to down-regulate smooth muscle specific α-actin expression and reduce Hey1 levels, but not endothelial progenitor cell marker CD133 (Figure 5C-D). Notch3 down-regulation also did not induce any cellular toxicity to LEC/KSHV (Figure 5E). Thus, Notch components that induce endothelial cell precursor pathway may endow these precursor cell characteristics, promote cell renewal, and contribute to KS pathogenesis.
Notch signaling in KS cell survival and mural cell characteristic. (A) LEC, LEC/KSHV, and KS-IMM cells were treated by sDll4-Fc (4 μg/mL) or GSI-1 (1μM) for 48 hours. Purified human Fc fragment (4 μg/mL) and DMSO were used as control for sDll4-Fc and GSI-1, respectively. Cell viability was analyzed by MTT assay and normalized to control. (B) Cells were treated in the same way as in panel A but were harvested and subjected to quantitative RT-PCR. Expression of Notch pathway downstream genes was analyzed. (C) LEC/KSHV cells were transfected by Notch3 siRNA (20nM or 100nM) for 72 hours. Nonsilencing siRNA from QIAGEN (100 nM) was used as negative control. Cells were harvested, and Notch3 RNA level change after siRNA transfection was analyzed by quantitative RT-PCR. Expression level changes of putative Notch3 downstream genes were also analyzed. (D) Cells were treated in the way same as in panel C and subjected to Western blotting for Notch3 and α-SMA. The relative protein level of Notch3 and α-SMA was quantitated by ImageJ and normalized to β-actin level (E) Cells were treated as in panel C and subjected to MTT assay. Cell viability was normalized to mock transfection.
Notch signaling in KS cell survival and mural cell characteristic. (A) LEC, LEC/KSHV, and KS-IMM cells were treated by sDll4-Fc (4 μg/mL) or GSI-1 (1μM) for 48 hours. Purified human Fc fragment (4 μg/mL) and DMSO were used as control for sDll4-Fc and GSI-1, respectively. Cell viability was analyzed by MTT assay and normalized to control. (B) Cells were treated in the same way as in panel A but were harvested and subjected to quantitative RT-PCR. Expression of Notch pathway downstream genes was analyzed. (C) LEC/KSHV cells were transfected by Notch3 siRNA (20nM or 100nM) for 72 hours. Nonsilencing siRNA from QIAGEN (100 nM) was used as negative control. Cells were harvested, and Notch3 RNA level change after siRNA transfection was analyzed by quantitative RT-PCR. Expression level changes of putative Notch3 downstream genes were also analyzed. (D) Cells were treated in the way same as in panel C and subjected to Western blotting for Notch3 and α-SMA. The relative protein level of Notch3 and α-SMA was quantitated by ImageJ and normalized to β-actin level (E) Cells were treated as in panel C and subjected to MTT assay. Cell viability was normalized to mock transfection.
In vivo activity of sDll4 in KSHV-transformed cells
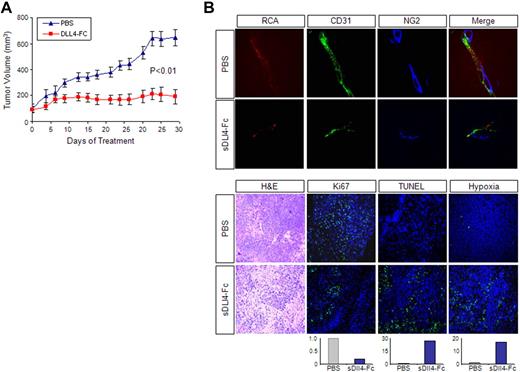
We next tested the activity of sDll4 in vivo. KS-IMM cells were implanted in athymic mice and allowed to establish tumors before randomization to sDll4-Fc or control group. Treatment with sDll4-Fc at a dose of 10 mg/kg 3 times a week (intraperitoneally) starting at a basal tumor volume of approximately 100 mm3 showed a marked reduction in tumor growth (Figure 6A). Tissue analysis at the completion of the study showed that the blood vessels in the sDll4-Fc–treated group were thin and lacked perfusion. Furthermore, there was a marked reduction in the recruitment of pericytes to the newly forming tumor vessels (NG2 staining, Figure 6B). In addition, the treatment group showed reduced cell proliferation (Ki-67 staining), increased tumor cell death (TUNEL assay), and increased areas of hypoxia (Figure 6B). Based on the in vitro and in vivo studies, sDll4-Fc may exert direct cytotoxicity to KS tumor cells and modify tumor vascular response, resulting in reduced tumor growth.
sDll4-Fc inhibits KS tumor growth in vivo. (A) Athymic mice were implanted with 2 × 106 KS-IMM cells. When tumor sizes were approximately 100 mm3, mice were randomly assigned to treatment groups (6 per group) (day 0). Mice were treated by intraperitoneal injection of sDll4-Fc (10 mg/kg) or PBS, 3 times a week for 31 days. Tumor volume was measured 3 times a week. The P value was calculated by Student t test. (B) Just before tissue harvest, mice were infused with RCA-Lectin and hypoxyprobe. Tumor tissue structure was examined by hematoxylin and eosin staining. Perfused vessels were localized by RCA-Lectin, microvascular endothelial cells were localized by CD31 staining, and pericytes were localized by NG2 staining. Nuclei were counterstained with 6-diamidino-2-phenylindole dihydrochloride. Proliferating cells within the tumor were assessed by immunostaining with anti-Ki67 antibody. Apoptosis was examined with TUNEL assay. Hypoxia was assessed by immunostaining with anti-Hypoxyprobe antibody. Quantitation was performed with the use of Bioquant Image Analysis (Bioquant), and the relative fluorescence level is shown below the panel. The confocal images were taken at an original magnification ×60.
sDll4-Fc inhibits KS tumor growth in vivo. (A) Athymic mice were implanted with 2 × 106 KS-IMM cells. When tumor sizes were approximately 100 mm3, mice were randomly assigned to treatment groups (6 per group) (day 0). Mice were treated by intraperitoneal injection of sDll4-Fc (10 mg/kg) or PBS, 3 times a week for 31 days. Tumor volume was measured 3 times a week. The P value was calculated by Student t test. (B) Just before tissue harvest, mice were infused with RCA-Lectin and hypoxyprobe. Tumor tissue structure was examined by hematoxylin and eosin staining. Perfused vessels were localized by RCA-Lectin, microvascular endothelial cells were localized by CD31 staining, and pericytes were localized by NG2 staining. Nuclei were counterstained with 6-diamidino-2-phenylindole dihydrochloride. Proliferating cells within the tumor were assessed by immunostaining with anti-Ki67 antibody. Apoptosis was examined with TUNEL assay. Hypoxia was assessed by immunostaining with anti-Hypoxyprobe antibody. Quantitation was performed with the use of Bioquant Image Analysis (Bioquant), and the relative fluorescence level is shown below the panel. The confocal images were taken at an original magnification ×60.
Discussion
We show that KS cell lines and KSHV latently infected endothelial cells express Notch receptors and ligands. Furthermore, induction of lytic cycle in KSHV-infected endothelial cells leads to up-regulation of Notch3, Dll4, Jagged1, and Jagged2. It has previously been shown that KS tumor tissues express Notch receptors Notch1, Notch2, and Notch4, Notch ligand Jagged1, and Notch target genes Hey1 and Hes1.10 KS tumor samples were not previously examined for Notch3, which is highly expressed in KS tumor and KSHV-transformed cells. Notch3 expression in vascular structures is normally limited to smooth muscle cells of small arteries. Moreover, Notch3 enhances its own expression and that of Jagged1, thus maintaining or amplifying the homotypic interaction with adjacent mural cells.24 Notch3 knockout mice show disarrayed vascular smooth muscle cells around the arteries.14 Notch3 expression in KS is consistent with previous studies showing expression of a SMA in the KS cell line.29 Dll4 is also highly expressed in KSHV-transformed cells. Dll4 in vascular structures are limited to arterial endothelium, in particular tip cells of sprouting blood vessels.38 Thus, up-regulation of Dll4 and its downstream target EphrinB2 in KS is consistent with arterial phenotypic vasculature, excessive vascular sprouting, and tight control of vascular lumen size in KS.25,37,39
Induction and activation of Notch components in KS tumor tissue and KSHV latently infected endothelial cells indicated that latency proteins of KSHV may regulate Notch components. This indeed was observed. vFLIP induced Dll4 and Jagged1 via the NF-κB pathway, which is consistent with the previous studies showing that vFLIP functions through the NF-κB pathway. The mechanism of how LANA regulates Dll4 needs further investigation.
Notch components were also induced when KSHV-infected cells were subjected to the lytic cycle. These findings suggest that certain lytic proteins may directly or indirectly regulate Notch components. It should be noted that some Notch components were induced with individual lytic viral proteins, whereas no significant induction was observed in KSHV-infected cells undergoing the lytic cycle. One possible explanation for this discrepancy is that other viral proteins induced during latency may attenuate the effects from lytic proteins. RTA is a critical viral protein that initiates lytic cycle by binding to NICD-interacting protein RBP-J.9 RTA thus regulates Notch downstream targets directly. We also demonstrate that RTA regulates Notch2, Dll4, and Jagged1 as well. One possible mechanism to regulate Notch components by RTA is through induction of other lytic cycle proteins. vGPCR and vIL-6 are among the most studied lytic cycle proteins, and both are revealed to regulate Notch components as well.
vGPCR induces Notch3 and Jagged1, primarily through the ERK pathway. vGPCR functions as a constitutively activated receptor with homology to human CXCR1 and CXCR2.6 vGPCR also induces growth factors and growth factor receptors, including VEGF and VEGFR2, resulting in autocrine and paracrine effects.40 VEGF and VEGFR2 are expressed at high levels in KS, even during latency,40 and are known to induce Notch and downstream signals.41 It is thus possible that a minority of KS cells undergoing lytic cycle may induce Notch components in adjacent cells. This is consistent with the fact that expression of vGPCR in transgenic animal models that drives tumor formation is only present in a minority of cells.42 In addition, vGPCR is the most potent inducer of Notch target Hey1, which may result from signal amplification via secondary effects.
Another KSHV lytic protein vIL-6 induces Notch4, Dll1, and Dll4, all of which are expressed only in arterial endothelial cells. vIL-6 binds gp130 with greater affinity than cellular IL-6 and activates the STAT3 pathway.43 Cellular IL-6 is expressed in abundance in KS tumor tissues that primarily have latent infection.44 Furthermore, cellular IL-6 has autocrine activity because KS cells express both IL-6 receptor-α and gp130. It is thus probable that cellular IL-6 induces the Notch components in latency similar to what is observed with lytic phase vIL-6.35,45 Induction of gp130 has been shown to induce stem cell self-renewal in embryonic cells,46 whereas many gp130 ligands, such as Gro-α36 and oncostatin M,34,47 are also expressed in KS cells and function as autocrine and paracrine factors. It is thus possible that gp130 signaling may also expand the KS stem cell population.
The significance of Notch signaling can vary from stem cell renewal, asymmetric cell division, transformation, cell proliferation to cell differentiation, and cell cycle arrest. Induction of CD133 in KSHV-infected endothelial cells and by specific viral proteins supports the possibility that KSHV induces dedifferentiation of endothelial cells to generate precursor cells with capacity to self-renew, differentiate to endothelial cells, or trans-differentiate to vascular smooth muscle cells.
Notch inhibitors, such as GSIs, induce apoptosis in KS cell lines and KSHV-transformed endothelial cells, and reduce tumor growth in vivo. GSIs, however, may also block processing of other proteins, such as EphrinB248 and epidermal growth factor receptor.49 We thus used Notch-specific inhibitor, a soluble form of Dll4 that competes for binding between most Notch ligands and receptors,37 to block Notch signaling in KS cells. There was a dramatic decline in cell viability that occurred through induction of apoptosis and inhibition of cell proliferation. Furthermore, sDll4 and GSI also down-regulated canonical Notch downstream targets. sDll4 and GSI also down-regulated CD133, a marker of precursor endothelial cells, supporting the hypotheis that KSHV-induced Notch pathway renders a precursor cell phenotype. sDll4 also showed profound inhibition of tumor growth in vivo combined with increased tumor cell apoptosis, decreased cell proliferation index, and reduced vessel perfusion. Notch pathway thus has a potential for further investigations in KS biology and novel therapeutic targeting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Wenming Gao (University of Southern California) and Dr Eric Ley (Cedars-Sinai Hospital) for technical support.
This work was supported in part by the National Cancer Institute (RO1 CA 079218-07, P.S.G.; and RO1CA085177, P.M.C.) and AIDS Malignancy Consortium (CA082057 and CA115284; J.J.).
National Institutes of Health
Authorship
Contribution: R.L., A.T., J.J., P.M.C., and P.S.G. designed the studies; R.L. and P.S.G. wrote the paper; and R.L., X.L., Y.Z., S.Z., J.-S.L., J.S.S., J.J., and P.S.G. performed research and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Parkash S. Gill, University of Southern California, Norris Hospital, NOR 6332, 1441 Eastlake Ave, Los Angeles, CA 90033; e-mail: parkashg@usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal