Abstract
Imatinib mesylate is a rationally designed tyrosine kinase inhibitor that has revolutionized the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors. Although the efficacy and tolerability of imatinib are a vast improvement over conventional chemotherapies, the drug exhibits off-target effects. An unanticipated side effect of imatinib therapy is hypophosphatemia and hypocalcemia, which in part has been attributed to drug-mediated changes to renal and gastrointestinal handling of phosphate and calcium. However, emerging data suggest that imatinib also targets cells of the skeleton, stimulating the retention and sequestration of calcium and phosphate to bone, leading to decreased circulating levels of these minerals. The aim of this review is to highlight our current understanding of the mechanisms surrounding the effects of imatinib on the skeleton. In particular, it examines recent studies suggesting that imatinib has direct effects on bone-resorbing osteoclasts and bone-forming osteoblasts through inhibition of c-fms, c-kit, carbonic anhydrase II, and the platelet-derived growth factor receptor. The potential application of imatinib in the treatment of cancer-induced osteolysis will also be discussed.
Introduction
Imatinib mesylate (Glivec/Gleevec, STI571, CGP 57148B; Novartis) is a 2-phenylaminopyrimidine derivative that binds to the adenosine triphosphate (ATP)–binding site of a select group of protein tyrosine kinases, thereby precluding ATP-binding and inhibiting kinase activity.1 Imatinib was originally discovered during a small molecule screen for specific inhibitors of tyrosine kinase activity and was subsequently found to have specificity for BCR-Abl, c-kit, and platelet-derived growth factor receptor (PDGFR), suggesting its application in tumors and nonmalignant proliferative disorders characterized by dysregulated activity of these kinases.1 Imatinib was the first small-molecule tyrosine kinase inhibitor to be successfully used in the clinic. It is currently the “gold standard” treatment for Philadelphia chromosome–positive chronic myeloid leukemia (CML) in chronic phase and for gastrointestinal stromal tumors (GISTs), based on its inhibition of BCR-Abl and c-kit, respectively.
Although imatinib was designed to specifically target BCR-Abl, many off-target kinases are affected by imatinib. At pharmaceutically obtainable concentrations (Cmax = 4.6μM at 400 mg/day),2 imatinib inhibits the tyrosine kinases Abl (IC50 = 0.22μM),3 the abl-related gene product (IC50 = 0.5μM),4 collagen-induced discoiddin domain receptor 1 (IC50 = 0.34μM)5 and DDR2 (IC50 = 0.68μM),5 the PDGFR family members stem cell factor receptor (c-kit; 0.1μM),6 PDGFR-α and -β (IC50 = 0.1μM)6 and the macrophage colony stimulating factor (M-CSF) receptor, c-fms (IC50 = 1.4μM).7 In addition, imatinib has recently been determined to potently inhibit the nontyrosine kinase targets NAD(P)H:quinone oxidoreductase 2 (IC50 = 0.08μM)8,9 and some members of the carbonic anhydrase (CA) family of metalloproteases, including human CA (hCA) II (IC50 = 0.03μM) and hCA XIV (IC50 = 0.47μM).10
Altered calcium and phosphate metabolism in imatinib-treated patients
Considering the wide range of signaling pathways inhibited by imatinib, one might expect that imatinib therapy would be associated with severe adverse effects. However, imatinib is well tolerated, compared with traditional chemotherapies, with adverse effects that are commonly only mild to moderate, including edema, musculoskeletal pain, muscle cramps, nausea, diarrhea, and rash.2,11 An additional common and unexpected side effect of imatinib therapy is disturbed calcium and phosphate metabolism, as evidenced by decreased serum phosphate and calcium levels.
Altered phosphate metabolism was first reported in patients receiving imatinib therapy for GIST, CML, and sarcoma by Berman et al.12 These studies showed that a subgroup (25 of 49) of imatinib-treated patients had low serum phosphate levels, compared with healthy controls. This group received significantly higher doses of imatinib than patients with normal serum phosphate levels. Furthermore, this group of patients also exhibited significantly lower total calcium and, subsequently, significantly higher serum parathyroid hormone (PTH) levels than the group of imatinib-treated patients with normal phosphate levels. These findings were consistent with results from 2 clinical trials, which showed that 50% of patients receiving imatinib exhibited hypophosphatemia of grade 2 or above at some stage during imatinib therapy.13
Subsequent to this report, several other groups have reported altered phosphate metabolism as a side effect of imatinib treatment. A longitudinal study of GIST patients receiving imatinib for 3 to 24 months found that 10 of 11 patients had lower mean plasma phosphate levels during treatment compared with baseline levels.14 Similarly, a prospective study of 9 CML patients found that, relative to baseline, phosphate levels were significantly decreased after 3, 6, and 18 months of imatinib treatment.15,16 In a retrospective study of 17 CML patients receiving imatinib therapy over 17 to 69 months, there was a significant decrease in serum phosphate levels, compared with baseline.17 In the largest study to date, Osorio et al18 reported that 36 CML patients showed a significant decrease in serum phosphate levels after 3 months of imatinib treatment that was sustained until the final analysis at 12 months.
These studies strongly suggest that imatinib treatment results in decreased serum phosphate levels,14-18 resulting in hypophosphatemia in at least 50% of patients.12-14 In addition, these patients exhibit an increase in serum levels of PTH, secondary to decreased calcium levels, and increased serum 1,25 hydroxyvitamin D3.12,15-18
Homeostatic control of serum phosphate and calcium levels is achieved via 3 mechanisms: (1) resorption of phosphate and calcium by the kidneys, (2) absorption of dietary phosphate and calcium by the gut, and (3) dissolution of phosphate and calcium from bone. Therefore, it is conceivable that reduced levels of calcium and phosphate seen in imatinib-treated patients could result from one or a combination of the following mechanisms: decreased intestinal absorption of phosphate and calcium, increased urinary loss of phosphate and calcium, decreased dissolution of calcium and phosphate from bone, or sequestration of phosphate and calcium from extracellular fluid into the bone.
It is doubtful that the decreased serum calcium and phosphate levels result from gastrointestinal malabsorption. Although approximately 50% of imatinib-treated CML and GIST patients experience nausea and diarrhea,19,20 which could result in increased loss of electrolytes, the severity of these conditions is usually mild to moderate and probably does not result in a sustained loss of calcium and phosphate.
Several case reports suggest that imatinib therapy may be associated with renal dysfunction. Kitiyakara and Atichartakarn21 described a 67-year-old man with preexisting chronic renal failure who developed acute renal failure after commencement of treatment with cytarabine and imatinib for blast-phase CML. In another case study, a 58-year-old woman developed acute renal failure with tubular necrosis after starting imatinib therapy for CML.22 Another report described a patient with hematuria who commenced treatment with imatinib and subsequently developed proximal tubular renal dysfunction (partial Fanconi syndrome) and hypophosphatemia.23 The paucity of reports describing acute renal dysfunction with imatinib therapy suggests that this may only occur in patients with preexisting kidney problems.
Nonetheless, imatinib therapy is associated with decreased reabsorption of phosphate by the kidneys, suggesting that increased renal phosphate output may be causing the observed decreases in serum phosphate.23 Berman et al12 reported increased urinary phosphate output in patients undergoing imatinib therapy compared with normal controls. Another study reported that maximal tubular resorption of phosphate was significantly decreased, relative to baseline levels, at 3, 6, and 18 months of imatinib treatment of CML patients.15,16
The analysis of serum hormone levels in imatinib-treated patients suggests that the observed phosphaturia may be secondary to increased PTH. A decrease in serum calcium levels stimulates PTH secretion from the parathyroid gland. This hormone acts on bone-resorbing osteoclast cells in the bone marrow to increase dissolution of calcium and phosphate from the bone, increase renal resorption of calcium and excretion of phosphate, and stimulate the production of 1,25 dihydroxyvitamin D3 via the activation of 1-α hydroxylase enzyme.24 1,25 dihydroxyvitamin D3 stimulates increased phosphate and calcium resorption by the gut and reduces phosphate excretion by the proximal tubule in the kidneys.24 Hypophosphatemia caused by renal phosphate wasting is usually associated with decreased serum levels of 1,25 dihydroxyvitamin D3 and normal levels of PTH. However, in imatinib-treated patients, the decreased serum phosphate is associated with increased serum PTH and 1,25 dihydroxyvitamin D3.12,15,16,18 As PTH stimulates the proximal tubule in the kidney to decrease tubular resorption of phosphate, the increased urinary phosphate levels are probably secondary to increased serum PTH, rather than a direct inhibitory effect of imatinib on the kidneys. Although it cannot be ruled out that kidney problems may contribute to the decreased serum phosphate levels, there has been no evidence presented thus far to demonstrate a direct toxic effect of imatinib on the kidneys.
Decreased serum calcium and phosphate levels could result from sequestration of these minerals to bone via a decrease in bone resorption and/or an increase in bone deposition (Figure 1), as has been described in patients treated with the bisphosphonate zoledronic acid.25-27 In support of this hypothesis, there is growing evidence that patients undergoing imatinib therapy experience dysregulated bone remodeling. In the remainder of this review, we will discuss the possibility that these changes result from direct inhibitory effects on the bone-resorbing osteoclast cells and antiproliferative and prodifferentiation effects on bone-forming osteoblast cells. In addition, we will discuss the potential applications for this in the treatment of diseases characterized by dysregulated bone remodeling.
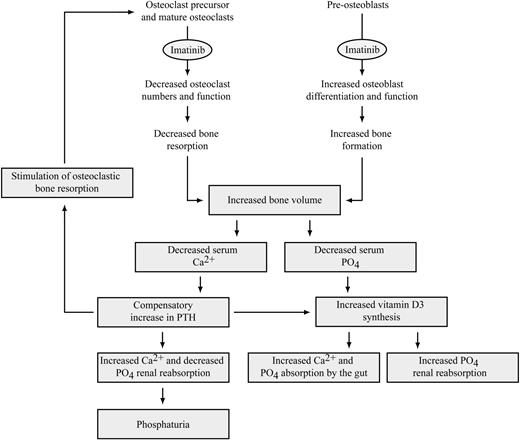
Proposed model for the effects of imatinib on bone metabolism and serum calcium and phosphate metabolism. Decreased dissolution of calcium and phosphate from the bone, or increased deposition of calcium and phosphate in newly formed bone, may result in decreased serum calcium and phosphate levels in imatinib-treated patients. The decrease in serum phosphate results in increased 1,25 dihydroxyvitamin D3 production, which in turn stimulates increased phosphate and calcium resorption by the gut and decreases phosphate excretion by the kidneys. Decreased serum calcium causes increased PTH production, increasing calcium reabsorption and phosphate excretion by the kidney and stimulating a further increase in 1,25 dihydroxyvitamin D3. PTH also stimulates bone resorption to release calcium and phosphate from bone; however, this may be inhibited by imatinib. Decreased absorption of phosphate and calcium resulting from gastrointestinal problems and decreased tubular resorption of phosphate may, in some cases, also contribute to the decreased levels of serum calcium and phosphate in imatinib-treated patients (not shown).
Proposed model for the effects of imatinib on bone metabolism and serum calcium and phosphate metabolism. Decreased dissolution of calcium and phosphate from the bone, or increased deposition of calcium and phosphate in newly formed bone, may result in decreased serum calcium and phosphate levels in imatinib-treated patients. The decrease in serum phosphate results in increased 1,25 dihydroxyvitamin D3 production, which in turn stimulates increased phosphate and calcium resorption by the gut and decreases phosphate excretion by the kidneys. Decreased serum calcium causes increased PTH production, increasing calcium reabsorption and phosphate excretion by the kidney and stimulating a further increase in 1,25 dihydroxyvitamin D3. PTH also stimulates bone resorption to release calcium and phosphate from bone; however, this may be inhibited by imatinib. Decreased absorption of phosphate and calcium resulting from gastrointestinal problems and decreased tubular resorption of phosphate may, in some cases, also contribute to the decreased levels of serum calcium and phosphate in imatinib-treated patients (not shown).
Effects of imatinib on bone
Recently, evidence has been published indicating that changes in bone parameters may occur in imatinib-treated patients. Studies from our group have shown that, relative to baseline, CML patients receiving imatinib for periods in excess of 17 months exhibited significantly increased iliac spine trabecular bone volumes.17 Almost half of the patients (8 of 17), including 4 patients who had a significant degree of osteoporosis at initial diagnosis, had an increase in trabecular bone volume of greater than 2-fold. The changes in trabecular bone volume inversely correlated with serum phosphate and total calcium levels. Conversely, no changes in trabecular bone volume were observed in a cohort of CML patients who responded to interferon-α therapy, suggesting that these changes were not related to decreased occupation of the marrow by tumor but were the result of the actions of imatinib.
In a subsequent study by Jönsson et al,28 regional bone mineral density (BMD) was examined in CML patients treated with imatinib for 24 to 73 months. Imatinib-treated patients had significantly higher areal lumbar spine and total hip BMD compared with normal aged-matched controls, as determined by dual-energy x-ray absorptiometry. In addition, peripheral quantitative computed tomography analysis revealed that the radial and tibial cortical BMD, but not trabecular BMD, was significantly higher in the imatinib-treated group than in the control group. This was associated with decreased serum phosphate and calcium, as well as a decrease in serum levels of bone formation markers.
In a prospective study, O'Sullivan et al16 measured BMD in 9 CML patients receiving imatinib therapy over a 24-month period. They observed a small, but significant, increase in vertebral BMD and a trend toward an increase in total body BMD by 12 months; however, no change in proximal femur BMD was observed.
Preliminary in vivo studies support the hypothesis that imatinib treatment results in increased bone volume. In skeletally immature 4-week-old male C3H mice, treatment with 110 mg/kg per day or 150 mg/kg per day imatinib for 10 weeks resulted in a small, but significant, increase in tibial trabecular BMD (assessed by dual-energy x-ray absorptiometry) and bone volume (BV/TV; assessed by micro-CT).29,30 This augmentation of BMD and BV/TV was associated with an increase in trabecular number and a decrease in trabecular separation.
Collectively, these data strongly suggest that imatinib alters bone remodeling in animals and patients. Although the mechanism(s) remain to be fully elucidated, these changes in bone remodeling parameters may be the result of a decrease in bone resorption that is not corrected by a concomitant decrease in osteoblast activity or, conversely, the result of an increase in osteoblast activity that is not balanced by an increase in osteoclast activity, or both. These effects would theoretically result in a decrease in the dissolution of calcium and phosphate from bone, or an increase in the sequestration of calcium and phosphate to bone, resulting in decreased serum levels of phosphate and calcium. In support of this hypothesis, there is growing evidence that imatinib has direct effects on the proliferation and activity of cells involved in maintaining bone homeostasis: the bone resorbing osteoclasts and the bone-forming osteoblasts.
Evidence for an antiosteoclastic effect of imatinib
Osteoclasts are large, multinucleated hematopoietic cells of the monocyte/macrophage lineage that are capable of resorbing mineralized bone. Secretion of protons and proteases from osteoclasts breaks down the mineral and protein components of the bone matrix, respectively. The formation of osteoclasts from monocyte/macrophage precursor cells in vitro is largely dependent on 2 factors: receptor activator of nuclear factor–κB ligand (RANKL), which drives osteoclast fusion, activity, and survival,31-33 and M-CSF, which is essential for the proliferation and survival of the osteoclast precursor cells and for survival of the mature osteoclast.34-38
In vitro, studies show that imatinib directly inhibits osteoclastogenesis, with treatment reducing the formation of tartrate-resistant acid phosphatase (TRAP)–positive osteoclasts from human CD14+ peripheral blood mononuclear cells, primary rat bone marrow cells, and primary rabbit bone marrow-derived monocytes at concentrations of 1μM and greater.39-41 In addition, imatinib inhibits the bone-resorptive activity of human osteoclasts at concentrations lower than those required to decrease osteoclast numbers, with 0.3μM imatinib and higher significantly decreasing their capacity to resorb a dentine substrate.39
There is also evidence that imatinib inhibits the survival of osteoclast precursors and mature osteoclasts in vitro. The proliferation, survival, and activity of primary human monocyte/macrophage lineage cells were inhibited by imatinib at concentrations of 1.5μM and greater.42,43 In cultures of murine bone marrow cells, imatinib treatment was found to dose-dependently decrease the number of viable cells, relative to vehicle controls.40,44 In addition, in pure cultures of mature osteoclasts, imatinib treatment reversed the antiapoptotic effect of M-CSF treatment, resulting in increased caspase-dependent apoptosis.41 In contrast, imatinib treatment had no effect on apoptosis of cells cultured in interleukin-1α (IL-1α), RANKL, or without cytokines.41
Consistent with these in vitro findings, analyses of serum markers of bone turnover suggest that osteoclast activity may be decreased in imatinib-treated patients. Berman et al12 observed that patients treated with imatinib had significantly lower levels of the bone resorption marker CTX-1 compared with healthy controls. This was particularly marked in patients with low serum phosphate.12 In addition, O'Sullivan et al16 found that serum CTX-1 levels were significantly decreased in a group of CML patients after 18 months of imatinib treatment. Decreases in serum osteoclast markers have also been observed in imatinib-treated normal mice. Preliminary results suggest that treatment of 4-week-old C3H mice with imatinib for 10 weeks resulted in a significant reduction in the serum levels of the osteoclast marker TRAP5b, compared with vehicle-treated controls,29,30 indicating that imatinib treatment mediated a decrease in osteoclast activity in these animals.
Although these observed changes in bone resorption markers suggest that imatinib may be inhibiting osteoclasts, directly or indirectly, no histologic studies have been carried out to determine changes in osteoclast numbers in imatinib-treated patients. In animal studies performed by our group, treatment of C57BL/6 mice with 100 mg/kg imatinib resulted in a significant decrease in the number of osteoclast-occupied resorption lacunae in the tibia, relative to vehicle controls.39 Similarly, preliminary data suggest that imatinib treatment of skeletally immature 4-week-old male C3H mice for 10 weeks resulted in a significant decrease in osteoclasts and resorption lacunae in the femora, but not in the vertebrae.29,30
Taken together, these results strongly suggest that imatinib is a direct inhibitor of osteoclast survival, differentiation, and activity. There are several known targets that may be contributing to the antiosteoclastogenic activity of imatinib, including c-fms, c-kit, carbonic anhydrase II (CAII), and PDGFR-α and -β (Figure 2).
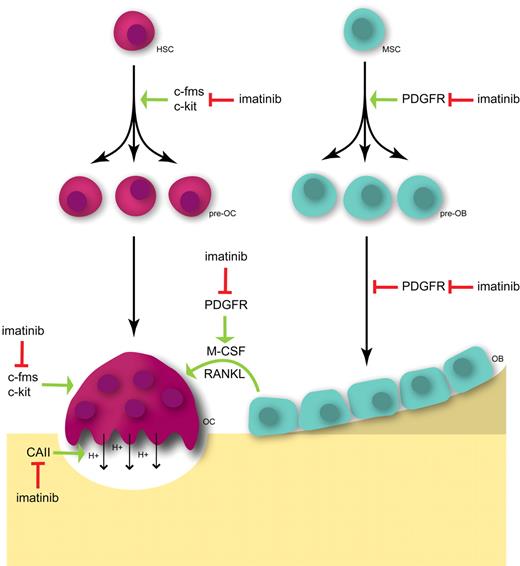
Hypothetical schema for the effects of imatinib on osteoclasts and osteoblasts. The proliferation and survival of osteoclast precursors (pre-OC) and the survival of mature osteoclasts (OC) are driven by M-CSF signaling through the receptor tyrosine kinase c-fms. The inhibition of c-fms signaling by imatinib decreases osteoclast numbers and activity. In addition, inhibition of c-kit may decrease pre-OC numbers and inhibit osteoclast activity. Imatinib also inhibits the proton-generating activity of CAII, preventing the dissolution of mineral from bone and, hence, inhibiting bone resorption. Further, the inhibition of PDGFR signaling on osteoblasts (OB) inhibits the production of osteoclastogenic cytokines, including M-CSF and RANKL. In addition, inhibition of PDGFR by imatinib in pre-osteoblasts (pre-OB) and osteoblasts (OB) inhibits cell proliferation but also relieves the inhibitory effects of PDGF on OB maturation, resulting in decreased cell numbers but increased OB activity. HSC indicates hemopoietic stem cell; and MSC, mesenchymal stem cell.
Hypothetical schema for the effects of imatinib on osteoclasts and osteoblasts. The proliferation and survival of osteoclast precursors (pre-OC) and the survival of mature osteoclasts (OC) are driven by M-CSF signaling through the receptor tyrosine kinase c-fms. The inhibition of c-fms signaling by imatinib decreases osteoclast numbers and activity. In addition, inhibition of c-kit may decrease pre-OC numbers and inhibit osteoclast activity. Imatinib also inhibits the proton-generating activity of CAII, preventing the dissolution of mineral from bone and, hence, inhibiting bone resorption. Further, the inhibition of PDGFR signaling on osteoblasts (OB) inhibits the production of osteoclastogenic cytokines, including M-CSF and RANKL. In addition, inhibition of PDGFR by imatinib in pre-osteoblasts (pre-OB) and osteoblasts (OB) inhibits cell proliferation but also relieves the inhibitory effects of PDGF on OB maturation, resulting in decreased cell numbers but increased OB activity. HSC indicates hemopoietic stem cell; and MSC, mesenchymal stem cell.
c-fms
c-fms is a transmembrane receptor that is a member of the type III receptor tyrosine kinase family that includes PDGFR-α and -β, c-kit, and flt3. c-fms is expressed by a variety of cell types, including primitive multipotent hematopoietic cells, mononuclear phagocyte progenitors, monocytes, tissue macrophages, osteoclasts, B cells, smooth muscle cells, neurons, and some cells of the female reproductive tract.45 Although imatinib was originally reported as having no effect on c-fms phosphorylation,6 subsequent work from our laboratory7 and those of others46-48 showed that it potently and selectively inhibits M-CSF–induced c-fms activity at therapeutically achievable concentrations. Imatinib inhibits the phosphorylation of c-fms in a murine hematopoietic cell line ectopically expressing human c-fms (FDC-cfms) at an IC50 of 1.42μM, without affecting total c-fms protein levels.7 In addition, imatinib has been shown to inhibit M-CSF–dependent, but not IL-3–dependent, proliferation of FDC-cfms cells and in Rat-2 and Bac1,2F5 fibroblasts ectopically expressing human c-fms at concentrations of 0.5 to 2μM and higher.7,46,48,49 This specific inhibition of c-fms phosphorylation/activity with imatinib treatment suggests a mechanism whereby imatinib may inhibit osteoclastogenesis.7
M-CSF is essential for osteoclastogenesis in vitro and in vivo. Signal transduction through c-fms regulates the proliferation, differentiation, activation, and survival of monocyte/macrophage lineage cells, including osteoclast precursors.34 In osteoclasts, M-CSF inhibits apoptosis and promotes cell proliferation, migration, and cytoplasmic spreading.35,36,38 Although RANKL is the primary promoter of osteoclast differentiation, M-CSF also plays a minor role through induction of the expression of osteoclast-associated genes, including RANK.37,50
The importance of M-CSF in osteoclast activity is illustrated by op/op mice, which are M-CSF–deficient.51,52 In these animals, a profound decrease in osteoclast numbers, resulting from defective osteoclast proliferation and differentiation, results in severe osteopetrosis and defective tooth eruption.51 Administering M-CSF to M-CSF−/− animals rapidly restores osteoclast numbers.52 Homozygous knockouts c-fms have an identical phenotype to op/op mice.53
The importance of c-fms signaling in osteoclast survival and activity, together with the similarities in the IC50 concentrations of imatinib required to inhibit c-fms and osteoclast formation and activity, suggests that inhibition of c-fms is a mechanism whereby imatinib inhibits osteoclastogenesis. In addition, other second-generation 2-phenylaminopyrimidine–derived tyrosine kinase inhibitors, including nilotinib and dasatinib, have been found to inhibit c-fms and osteoclastogenesis in vitro49,54,55 and in vivo (K.V., A.L.D., S.F., P. Diamond, C. G. Schultz, N. A. Sims, and A.C.W.Z., manuscript submitted July 2009). Inhibition of c-fms kinase activity therefore may account, in part, for the dysregulated bone remodeling observed in patients undergoing imatinib treatment.
c-kit
In addition to c-fms, inhibition of signaling through c-kit by imatinib may affect osteoclastogenesis. c-kit is a transmembrane tyrosine kinase that binds soluble or membrane-bound isoforms of its ligand, stem cell factor (SCF; also known as mast cell growth factor, steel factor, c-kit ligand).56 Some in vitro evidence suggests that c-kit may play a role in osteoclastogenesis. c-kit is expressed by osteoclasts,57 and SCF has been shown to be mitogenic for hematopoietic stem cells58 and osteoclast precursors.59,60 Soluble SCF stimulates osteoclast precursor proliferation, resulting in increased osteoclast numbers,59,60 and promotes mature osteoclast activity.61 c-kit signals via microphthalmia transcription factor (Mitf), which lies downstream of c-kit,61 c-fms,62 and RANK.63 The essential role of Mitf in osteoclast formation is demonstrated by Mitfmi/mi mice, which exhibit severe osteopetrosis resulting from defective osteoclast fusion.64,65 Inhibition of c-kit signaling may therefore contribute to the antiosteoclastogenic properties of imatinib.
CAII
The recent identification of CAII as a nonkinase target of imatinib suggests another mechanism by which imatinib may inhibit bone resorption. The α-carbonic anhydrases are a family of zinc enzymes that are responsible for catalyzing the conversion of carbon dioxide and water to carbonic acid, which dissociates to form carbonate and a proton (H+).
CAII plays an important role in the dissolution of the mineralized bone matrix by the osteoclast. Bone resorption is carried out by acidification of the extracellular space formed adjacent to the bone surface at the resorptive surface of the osteoclast through the excretion of protons via a proton pump (H+-ATPase).66 CAII enables the acidification of the resorption lacunae by catalyzing the reaction, which produces protons in the cytoplasm of the osteoclast, providing the protons required to drive resorption of the mineralized matrix.66
Patients with autosomal-recessive CAII deficiency exhibit osteopetrosis, which is associated with cerebral calcification and renal tubular acidosis.67 Similarly, CAII-deficient mice have a slower growth rate than their wild-type littermates and exhibit renal tubular acidosis68 and mild osteopetrosis presenting as an increase in trabecular bone volume.69
In vitro, blocking of CAII expression with antisense RNA or DNA inhibits osteoclast formation, intravesicular acidification, and bone resorption in rat osteoclast and fetal mouse bone organ cultures.70,71 In addition, treatment of rat bone marrow osteoclasts and murine bone organ cultures with carbonic anhydrase inhibitors, such as acetazolamide, decreases osteoclast bone-resorptive activity.71,72
The major role that CAII plays in bone resorption through resorption pit acidification suggests that inhibition of this kinase by imatinib may be another mechanism whereby imatinib inhibits osteoclast activity.
PDGFR
PDGF is a potent mitogen and chemotactic agent for cells of mesenchymal origin. The PDGF isoforms A and B can form either homodimers or heterodimers, which bind to the receptors PDGFR-α (PDGF-AA, PDGF-BB, and PDGF-AB) and PDGFR-β (PDGF-BB only). Treatment with PDGF-BB and, to a lesser extent, PDGF-AA increases osteoclast number and bone resorption surface in cultured fetal mouse calvaria.73,74 In cultures of iliac crest-derived primary human bone cells, PDGF-BB treatment significantly increased osteoclastic bone resorption in vitro.75 The observed increase in osteoclast activity is probably the result of PDGF-BB–stimulating osteoblasts and stromal cells to release cytokines that have stimulatory effects on osteoclasts. For example, in cultures of murine cell lines and primary osteoblasts, PDGF-BB treatment has been shown to up-regulate the expression of several cytokines, including IL-6,76 which stimulates the production of RANKL by osteoblasts, and the osteoclast mitogen M-CSF.77 Thus, inhibition of PDGFR signaling by imatinib in vivo could indirectly have effects on osteoclastogenesis via inhibition of cytokine secretion by stromal cells and osteoblasts.
Effects of imatinib on osteoblasts
In addition to having inhibitory effects on osteoclasts, there is emerging evidence that imatinib can affect osteoblasts. Osteoblasts are bone marrow cells of mesenchymal origin that, in adult bone, are responsible for the synthesis of new bone matrix.
Studies from our laboratory17 and those of others44,78-80 suggest that imatinib decreases in vitro osteoblast proliferation while increasing their activity. In mineralization assays in vitro using stromal cells isolated from human bone explants, treatment with therapeutically achievable concentrations of imatinib significantly increased mineral deposition,17,80 decreased proliferation of stromal cells,17,78 and suppressed stromal cell clonogenicity.78 Similarly, imatinib increases mineralization by primary rat osteoblast cells and by the mouse osteoblast-like cell line MC3T3-E1.44 Treatment with therapeutically relevant concentrations of imatinib partially inhibited proliferation of primary rat osteoblasts, the human osteoblast-like cell line SaOS2, the murine stromal cell line ST2,44 and the mouse osteoblast-like cell line MC3T3-E1,79 and increased apoptosis in primary rat osteoblasts.44
There is some suggestion that patients undergoing imatinib therapy have altered serum levels of osteoblast markers. In CML patients, serum levels of the osteoblast markers osteocalcin and pre-collagen I propeptides (P1NP) have been shown to be significantly increased after 3 months of imatinib treatment,15,16 with elevated levels returning to baseline by 24 months.16 Studies by Tibullo et al80 found that increased serum osteocalcin levels in patients undergoing imatinib therapy were sustained until 24 months of treatment. These studies contrast those of Berman et al,12 who reported that patients treated with imatinib for an unspecified amount of time did not have significantly altered levels of bone-specific alkaline phosphatase, relative to normal controls. Moreover, the imatinib-treated patients had significantly lower levels of osteocalcin compared with aged-matched normal controls.12
Taken together, these results suggest that osteoblast activity may be transiently increased by imatinib treatment but subsequently decreases to levels at, or lower than, those at baseline over time. This is consistent with in vitro data showing that imatinib increases osteoblast activity while inhibiting osteoblast proliferation.17 One could hypothesize that, at the commencement of treatment, imatinib may increase the differentiation of the existing pool of osteoblast precursors, thereby increasing the number of committed osteoblasts. However, as imatinib inhibits mesenchymal cell proliferation, the number of osteoblast precursors and the number of osteoblasts would decrease over time. Histomorphometric analysis is therefore required to determine the temporal changes in osteoblasts and bone deposition rates occurring in patients undergoing imatinib therapy.
The proliferation and differentiation of osteoblasts are regulated through several signaling pathways, including known imatinib targets c-Abl and PDGFR. Whereas loss of signaling through c-Abl is thought to inhibit osteoblast activity,81 PDGFR signaling is known to be mitogenic to mesenchymal cells and to have a negative regulatory effect on osteoblastogenesis, and thus represents the best candidate to explain the mechanism whereby imatinib activates osteoblast differentiation while inhibiting mesenchymal cell proliferation (Figure 2).
PDGFR
In addition to inducing expression of osteoclastogenic cytokines by osteoblasts and stromal cells, PDGF is known to have direct effects on osteoblast proliferation and differentiation. Osteoblasts express PDGF receptors82 and PDGF-AA, which can stimulate cell proliferation in a paracrine or autocrine manner.83,84 PDGF-BB and, to a lesser extent, PDGF-AA are mitogenic for mesenchymal cells and inhibit osteoblast differentiation in vitro. Recombinant PDGF enhances proliferation of osteoblasts in rat calvarial cultures, isolated fetal rat calvarial osteoblasts, and mouse osteoblast-like MC3T3-E1 cells in vitro.74,85 In addition, inhibition of osteoblast differentiation by PDGF-BB has been observed in human osteoblast cultures, MC3T3-E1 cells, and fetal rat calvarial cultures,85-87 resulting in decreased mineralized nodule formation.85 Furthermore, PDGF-AA and -BB were found to decrease mineral apposition rate and bone formation rate in fetal rat calvarial organ cultures, suggesting an inhibition of mineralization activity.74 In support of these findings, targeted deletion of PDGFR-β in murine mesenchymal cells promotes osteoblast differentiation and function.88
As expected, imatinib is able to reverse the effects of PDGF on osteoblasts in vitro. Treatment of human bone trephine explant cultures and MC3T3-E1 cells with PDGF-BB inhibited mineralized matrix formation; this was prevented by pretreatment with imatinib.17,44 In rat primary osteoblasts, proliferation is activated by PDGF-BB.44 This is reversed by imatinib, resulting in enhanced mineralization on a per-cell basis.44 These results suggest that inhibition of PDGFR signaling may be one mechanism whereby imatinib inhibits osteoblast proliferation and activates differentiation in vitro.
Potential application of imatinib as bone cancer treatment
The observed effects of imatinib on osteoclast and osteoblast activity suggest a rationale for its use to treat conditions in which dysregulated bone remodeling is a feature, such as in bone metastases in breast cancer (BrCa), prostate cancer (PCa), and multiple myeloma.
The majority of bone lesions resulting from tumors, including BrCa and multiple myeloma, are primarily osteolytic. In contrast, lesions resulting from PCa skeletal metastases are mixed (ie, both osteoblastic and osteosclerotic), although they usually result in an overall increase in local bone volume.89 Irrespective of the lesion type, bone resorption is almost universally increased in patients with bone tumors.90 Moreover, studies suggest that bone resorption is necessary for the establishment of tumor cells in the bone through the local release of growth factors that promote establishment of the secondary tumor.91
In osteolytic tumors, there is evidence to suggest that tumor cells can act indirectly or directly to increase the recruitment, maturation, activity, and survival of osteoclasts, leading to an imbalance in bone remodeling and, therefore, bone loss. In addition, factors released from the bone during osteolysis stimulate cancer cell survival, thus instigating a “vicious cycle” resulting in decreased bone density and increased tumor burden in the bone marrow microenvironment.89 Thus, whereas direct induction of tumor cell death is the primary strategy for decreasing tumor size, antiosteoclastogenic treatments are a potential therapeutic strategy for limiting tumor progression by inhibiting tumor-associated osteolysis.
Several studies have examined the effects of imatinib therapy on the growth of BrCa tumor cells in the bone marrow environment in vivo. In a systemic MDA-MB-231 model of BrCa bone metastasis92 and in an intratibial MDA-MB-435 model of tumor-associated bone loss,93 daily imatinib therapy was found to decrease tumor size and tumor-associated osteolysis.
Similarly, in animal models of PCa bone metastases, imatinib treatment has been shown to decrease tumor burden and associated osteolysis. In an intratibial model of PCa-associated bone loss, treatment with 50 mg/kg imatinib significantly inhibited bone lysis.94-96 This was associated with reduced tumor incidence, tumor size, tumor cell proliferation, and tumor cell apoptosis.94-96
The rationale for these in vivo studies has primarily focused on inhibiting tumor cell proliferation and tumor-stroma interactions through the inhibition of paracrine and autocrine growth factor signaling. Indeed, evidence suggests that high concentrations of imatinib may inhibit tumor cell proliferation in some BrCa and PCa cell lines in vitro.97,98 In addition, orthotopic growth of the MDA-MB-231 BrCa cells is also inhibited by imatinib therapy.92 Therefore, a decrease in tumor-associated osteolysis may be an indirect effect of imatinib-mediated tumor cytotoxicity. Further studies are required to ascertain to what extent direct inhibition of osteolysis by imatinib contributes to decreased tumor-associated bone loss.
Assessing the effects of imatinib on tumor-associated osteolysis is further complicated by the fact that few clinical trials have directly examined the impact of imatinib therapy on tumor bone lysis. Although some studies have examined the efficacy of imatinib as a treatment for PCa or BrCa, these have primarily been undertaken in patient populations with nonmetastatic disease.99-103 In addition, most studies that have been carried out in patients with bone metastases have not examined indices of bone turnover,104,105 or have been complicated by the patients' concurrent use of bisphosphonate therapy.106 To date, only one clinical trial has reported the effects of imatinib on bone parameters in patients with bone metastases.107 In this study, imatinib was used in combination with docetaxol to treat patients with advanced hormone-refractory PCa with bone metastases. Although time to progression was no different between the treatment and placebo groups during the 42 days of the trial, the level of urinary collagen N-telopeptides was significantly decreased in the imatinib group. Although not definitive, these studies suggest that imatinib is decreasing bone loss by directly affecting osteoclast formation/activity. The potential application for imatinib as a direct inhibitor of osteoclast activity and tumor-associated osteolysis requires further investigation.
Conclusions
The long-term consequences of dysregulated bone remodeling by imatinib are not known. However, there is some suggestion that long-term inhibition of bone turnover may result in weakened bone through the accumulation of microfractures.108-110 Several studies indicate that long-term inhibition of bone turnover by bisphosphonate treatment may result in an increased accumulation of microfractures and, in some cases, decreased bone strength.111-113 We are currently performing longitudinal histologic assessment of bone biopsies from patients taking imatinib to determine the effects of this compound on osteoblast and osteoclast numbers and function, bone formation rates, and bone quality.
Alteration of the activity of bone cells by imatinib may have additional unanticipated side effects. For example, there are emerging data suggesting that imatinib therapy may result in decelerated growth in prepubescent patients. Three recent case reports have described retarded growth in juvenile CML patients undergoing imatinib therapy.114-116 This may be the result of accelerated growth plate closure, as has been observed in imatinib-treated rats117 resulting from an inhibition of chondrocyte activity and altered mineralization dynamics via the inhibition of PDGFR-β17,44,117 or DDR2.118 In addition, the effects of imatinib on osteoblasts suggest that imatinib may have unanticipated effects on hematopoiesis, as osteoblasts are a vital component of the hematopoietic stem cell niche.119 This warrants future studies to investigate the effects of imatinib therapy on this niche.
The significant incidence of acquired resistance to imatinib has led to the development of second-generation tyrosine kinase inhibitors, including dasatinib, nilotinib, and bosutinib, which have greater affinity for their target kinases. As the target profile of these second-generation inhibitors overlaps with that of imatinib, there is a potential for off-target skeletal effects of these compounds, which should be investigated.
In conclusion, there is a mounting body of evidence suggesting that dysregulated bone remodeling may be a side effect of imatinib treatment. This probably results from inhibition of osteoclasts, by blocking c-fms, c-kit, CAII, and PDGFR signaling, and activation of osteoblast activity through inhibition of PDGFR. Clearly, the suggestion that imatinib may increase bone strength, through positive effects on BMD and trabecular bone volume, warrants further investigation. These results suggest the possibility that imatinib may be clinically useful as an inhibitor of osteoclast activity in diseases associated with excessive bone resorption, such as cancer-induced bone loss.
Authorship
Contribution: K.V., S.F., A.L.D., and A.C.W.Z. wrote the manuscript; and T.P.H. critically reviewed the manuscript.
Conflict-of-interest disclosure: A.C.W.Z. and T.P.H. receive research funding from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Andrew C. W. Zannettino, Myeloma Research Program, Bone and Cancer Research Laboratories, Department of Haematology, Centre for Cancer Biology, Hanson Institute of Medical and Veterinary Science, GPO Box 14, Adelaide, SA, Australia 5000; e-mail: andrew.zannettino@imvssa.gov.au.