Abstract
We investigated the potential role of an immune reaction in mediating the dominant engraftment of 1 cord blood unit in 14 patients who received a double-unit cord blood transplantation (CBT). In 10 patients, dominant engraftment of a single donor unit emerged by day 28 after CBT. In 9 of these 10 patients, a significant subset of CD8+ CD45RO+/−CCR7− T cells, present in peripheral blood mononuclear cells and derived from the engrafting cord blood unit, produced interferon-γ (IFN-γ) in response to the nonengrafting unit. No significant population of IFN-γ–secreting cells was detectable when posttransplantation peripheral blood mononuclear cells were stimulated against cells from the engrafted unit (P < .001) or from a random human leukocyte antigen disparate third party (P = .003). Three patients maintained persistent mixed chimerism after CBT, and no significant IFN-γ–secreting cells were detected after similar stimulations in these patients (P < .005). Our data provide the first direct evidence in human double-unit CBT recipients that immune rejection mediated by effector CD8+ T cells developing after CBT from naive precursors is responsible for the failure of 1 unit to engraft. Future investigations based on these findings may result in strategies to predict a dominant unit and enhance graft-versus-leukemia effect.
Introduction
The introduction of double-unit cord blood transplantation (CBT) has significantly decreased graft failure rates and transplantation-related mortality (TRM) among adults and large children undergoing CBT for hematologic malignancies.1-3 In the great majority of cases in which 2 cord blood units are administered as a stem cell source for transplantation, 1 unit emerges as the sole source of long-term hematopoiesis.1,2,4 To date, no unit specific factors, such as viability, infused total nucleated cell count (TNC), CD34+ or CD3+ cell counts, sex mismatch, ABO blood group, degree of human leukocyte antigen (HLA) mismatch, or order of infusion have been identified that reliably predict which unit will emerge as dominant.5 Thus, the biologic basis by which a single unit becomes dominant has not been established.
Studies of hematopoietic stem cell transplantation (HCT) in humans and animal models have demonstrated that donor T cells promote engraftment and that host T cells specific for alloantigens on donor cells as well as natural killer (NK) cells or donor-specific antibodies can lead to rejection of infused stem cells.6-11 Cord blood products contain naive but functional immune cells in addition to stem cells; thus, the transplantation of 2 cord blood units into a third party recipient provides a unique in vivo circumstance in which immunologic interactions between each of the cord blood units and the host may be initiated in vivo and contribute to selective engraftment of a single unit. Compared with single-unit CBT and stem cell transplantations from other donor sources, double-unit CBT is associated with an increased incidence of mild to moderate acute graft-versus-host disease (GVHD) and may result in a decreased rate of relapse.1,2,12-14 These observations suggest that understanding the immunologic interactions between the 2 units and the host could yield insights into the mechanisms of GVHD and graft-versus-leukemia (GVL) responses.
We hypothesized that the dominant engraftment of a single unit is mediated by an immune response of the dominant unit against the nonengrafting unit. We used flow cytometric techniques to directly analyze developing reactivity of T cells isolated from the recipient after transplantation against cells derived from each cord blood donor. Our studies investigated patients undergoing myeloablative conditioning and CBT with 2 unmanipulated cord blood units, patients undergoing myeloablative conditioning and CBT with 1 unmanipulated and 1 CD34+ selected and ex vivo expanded unit (without addback of T cells), and patients undergoing reduced-intensity conditioning (RIC) and CBT with 2 unmanipulated units. We demonstrate that in each setting, when single unit dominance occurs, CD8+ effector T cells derived from the dominant cord blood unit and specific for alloantigens present on the nonengrafting unit develop early after transplantation. These results provide direct evidence that dominant engraftment of a single unit after double CBT in humans is the result of immune-mediated rejection of the nonengrafting unit.
Methods
Patients and cord blood
Fourteen patients undergoing double-unit CBT were enrolled on protocols that were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board, and all patients provided written informed consent. Random cord blood units were obtained from local hospitals under Institutional Review Board–approved protocols to obtain cord blood units for research purposes. Study procedures were performed in accordance with the principles of the Declaration of Helsinki.
Generation of LCLs
On the day of CBT, cord blood units were thawed and washed according to the method of Rubinstein et al.15 Residual cells in the wash supernatant were collected and CD19+cells were enriched using immunomagnetic selection with the AutoMacs PRO (Miltenyi Biotec). CD19+ cells were transformed with Epstein-Barr virus (EBV) and the resulting lymphoblastoid cell lines (LCLs) were cultured as described.16
Collection of post-CBT peripheral blood samples
On up to 5 occasions, each at least 1 week apart, between day 14 and day 100 after transplantation, 30 mL of heparinized peripheral blood was obtained from patients. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient separation (Histopaque; Sigma-Aldrich) and cryopreserved.
Chimerism analysis
Analysis of donor chimerism was performed on CD3+, CD56+, and CD33+ fractions of peripheral blood sorted by flow cytometry on days 7, 14, 21, 28, 56, and 80 after transplantation. Whole bone marrow chimerism was performed on days 28 and 100 after transplantation. Single donor dominance was defined as more than 90% single unit contribution of CD3+ cells combined with at least 70% single unit contribution of both CD56+ and CD33+ fractions by day 28 after transplantation. DNA was isolated using the QIAamp DNA Blood Mini or Micro Kits (QIAGEN). DNA amplification was performed with the PowerPlex16 Human Identity Kit (Promega), which amplifies 15 short tandem repeat loci and the Amelogenin marker in a single polymerase chain reaction amplification step. The fluorescent-labeled polymerase chain reaction products were run on an ABI PRISM 3130xl Genetic Analyzer, and the profiles analyzed using the GeneMapper ID software (Applied Biosystems). Testing was performed in the Clinical Immunogenetics Laboratory at the Seattle Cancer Care Alliance.
Antibodies and flow cytometric analysis
T-cell subsets were enumerated using a panel, including live dead fixable violet stain (LDFVS; Invitrogen), and the following monoclonal antibodies: CD3-PE, CD4-PERCPCY5.5, CD8-APCH7, CD45RO-APC, and CCR7PECY7 (all from BD Biosciences). NK-cell subsets were enumerated using a panel, including LDFVS (Invitrogen) and the following monoclonal antibodies: CD3-PE, CD16-PERCPCY5.5, and CD56-APC (BD Biosciences). Anti–interferon-γ (IFN-γ)–fluorescein isothiocyanate (FITC) and CD107A-FITC (BD Biosciences) were used for functional analyses of T and NK cells, respectively. All analyses were performed with an LSR II (BD Biosciences) instrument.
IFN-γ intracellular staining and IFN-γ capture assay and T-cell expansion
PBMCs (1.5-2.0 × 106) collected from patients at intervals between 14 and 100 days after transplantation were separately stimulated with 5 × 105 LCLs generated from both transplanted cord units as well as third party units and incubated for 5 hours in 1 mL of medium containing RPMI-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen) supplemented with 10% fetal bovine serum (Hyclone Laboratories), 2mM l-glutamine (Invitrogen), 35 IU/mL penicillin/streptomycin (Invitrogen) at 37°C in a humidified 5% CO2 incubator. For IFN-γ intracellular staining, brefeldin A (5 μg/mL; Sigma-Aldrich) was added after 1 hour. Unstimulated controls were also included. The cells were stained with LDFVS, washed and stained with antibodies for CD3, CD4, CD8, CCR7, and CD45RO, then fixed and permeabilized (Cytofix/Cytoperm; BD Biosciences), and stained with 10 μL of anti–IFN-γ-FITC. In some experiments, LCLs were preincubated with an HLA class I monoclonal antibody (W6/32) to block recognition of class I molecules or with an isotype control monoclonal antibody, each at a concentration of 50 μg/mL. LCLs were then incubated with PBMCs for 5 hours and stained for IFN-γ as described earlier in this section. For the IFN-γ capture assay, PBMCs were stimulated similarly and capture was performed using the IFN-γ secretion assay cell enrichment and detection kit (Miltenyi Biotec).17 Cells were selected using the FACSAria II (BD Biosciences) and cultured for 14 days in RPMI-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen), 10% human serum, 2mM l-glutamine (Invitrogen), 35 IU/mL penicillin/streptomycin (Invitrogen), 50μM β-mercaptoethanol, 1 ng/mL interleukin-15, 50 units/mL interleukin-2, 30 ng/mL OKT3, and allogeneic PBMCs (1000:1) irradiated with 3500 cGy and LCLs (200:1) irradiated with 8000 cGy.
NK-activation assay using increased CD107a expression
Aliquots of PBMCs (1.5-2.0 × 106) collected from patients at intervals between 14 and 100 days after transplantation were stimulated with 2 × 105 K562 cells, and with 2 × 105 LCLs generated from both transplanted cord units and incubated with 20 μL of anti-CD107a-FITC for 5 hours in 1 mL of medium containing RPMI-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (Invitrogen) containing 10% fetal bovine serum (Hyclone Laboratories), 2mM l-glutamine (Invitrogen), 35 IU/mL penicillin/streptomycin (Invitrogen) at 37°C in a humidified 5% CO2 incubator. A total of 6 μL of monensin (Golgi-Stop; BD Biosciences) was added after 1 hour. Unstimulated controls were also included. The cells were washed and stained with LDFVS and then washed and stained with monoclonal antibodies for CD3, CD16, and CD56.
Statistics
The percentage of IFN-γ–secreting cells in different cohorts was compared using the Mann-Whitney test.
Results
Patient and graft characteristics
Fourteen patients who underwent a double-unit CBT for a hematologic malignancy at the Seattle Cancer Care Alliance between June 2007 and September 2008 were the subjects of this study. The patient characteristics and infused cell doses (total nucleated cells, CD34+, and CD3+) derived from each cord blood unit are summarized in Table 1. Nine patients underwent myeloablative conditioning with fludarabine 75 mg/m2, cyclophosphamide 120 mg/kg, and total body irradiation (TBI) 13.2 Gy. Five patients underwent RIC with fludarabine 200 mg/m2, cyclophosphamide 50 mg/kg, and TBI 2 Gy (n = 2), TBI 2 Gy and antithymocyte globulin 90 mg/kg (n = 1), or TBI 3 Gy (n = 1). Among patients undergoing myeloablative conditioning, 5 patients were transplanted with 2 unmanipulated cord units and 4 patients were transplanted with 1 unmanipulated unit and 1 unit from which CD34+ cells were selected and expanded ex vivo using an engineered Notch ligand as previously described.18,19 Of note, there were no mature T cells infused with the expanded graft. Cyclosporine and mycophenolate mofetil were administered to all patients as prophylaxis for GVHD. Typing for unit selection was performed at low resolution for HLA A and B and high resolution for HLA DRB1. HLA typing of the patients and each of the cord units is provided in Table 2.
Patient details and infused unit characteristics
| Patient no. . | Transplantation type* . | Age, y . | Weight, kg . | Disease . | Status . | TNC × 107/kg . | CD34+ × 105/kg . | CD3+ × 106/kg . | Days to engraftment . | Dominant unit . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit 1 . | Unit 2 . | Total . | Unit 1 . | Unit 2 . | Total . | Unit 1 . | Unit 2 . | Total . | ||||||||
| 1 | MUU | 29 | 103 | AML | CR2 | 1.2 | 1 | 2.2 | 1.3 | 0.5 | 1.8 | 4.7 | 2.7 | 7.4 | 45 | Mixed |
| 2 | MUU | 28 | 53 | ALL | CR1 | 2.1 | 1.4 | 3.5 | 0.9 | 2.6 | 3.5 | 5.6 | 4.3 | 9.9 | 23 | 2 |
| 3 | MUU | 24 | 78 | CML | CP1 | 2.2 | 2 | 4.2 | 1.3 | 0.9 | 2.2 | 8.3 | 7.9 | 16.2 | 44 | 2 |
| 4 | MUU | 18 | 68 | MDS | 1.5 | 1.6 | 3.1 | 1.1 | 2.5 | 3.6 | 5 | 5 | 10 | 30 | 1 | |
| 5 | MUU | 39 | 64 | AML | CR2 | 3.6 | 1.7 | 5.3 | 3.8 | 1 | 4.8 | 11 | 5 | 16 | 16 | 1 |
| 6 | RIC | 56 | 100 | AML | CR2 | 3.1 | 1.5 | 4.6 | 6.4 | 1.1 | 7.5 | 7.6 | 6 | 13.6 | 7‡ | 1 |
| 7 | RIC | 67 | 83 | AML | CR1 | 2.3 | 1.8 | 4.1 | 1.6 | 1.3 | 2.9 | 6.8 | 4.2 | 11 | 8‡ | 2 |
| 8 | RIC | 64 | 88 | AML | CR2 | 1.7 | 1.4 | 3.1 | 0.7 | 0.9 | 1.6 | 3.6 | 3.6 | 7.2 | 6‡ | 1 |
| 9 | RIC | 62 | 80 | AML/MDS | MDS | 2.2 | 1.9 | 4.1 | 2.8 | 0.9 | 3.7 | 6.4 | 5.5 | 11.9 | Death | 2 |
| 10 | RIC | 46 | 57 | CLL | PR | 1.9 | 1.8 | 3.7 | 0.5 | 0.7 | 1.2 | 5.5 | 4.8 | 10.3 | 24 | Mixed |
| 11 | MUE | 30 | 72 | AML | CR2 | 2.7 | 0.6 | 3.3 | 3.4 | 11.2 | 14.6 | 4.2 | NA† | 4.2 | 13§ | 1 |
| 12 | MUE | 29 | 79 | ALL | CR1 | 1.5 | 3.2 | 4.7 | 0.6 | 84 | 84.6 | 3 | NA† | 3 | NA | Rejection |
| 13 | MUE | 42 | 66 | AML | CR3 | 3 | 2.3 | 5.3 | 2.6 | 29 | 31.6 | 7 | NA† | 7 | 21 | 1 |
| 14 | MUE | 26 | 78 | AML/ALL | CR1 | 1.9 | 9.1 | 11 | 1.6 | 97 | 98.6 | 3.6 | NA† | 3.6 | 7§ | Mixed |
| Patient no. . | Transplantation type* . | Age, y . | Weight, kg . | Disease . | Status . | TNC × 107/kg . | CD34+ × 105/kg . | CD3+ × 106/kg . | Days to engraftment . | Dominant unit . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit 1 . | Unit 2 . | Total . | Unit 1 . | Unit 2 . | Total . | Unit 1 . | Unit 2 . | Total . | ||||||||
| 1 | MUU | 29 | 103 | AML | CR2 | 1.2 | 1 | 2.2 | 1.3 | 0.5 | 1.8 | 4.7 | 2.7 | 7.4 | 45 | Mixed |
| 2 | MUU | 28 | 53 | ALL | CR1 | 2.1 | 1.4 | 3.5 | 0.9 | 2.6 | 3.5 | 5.6 | 4.3 | 9.9 | 23 | 2 |
| 3 | MUU | 24 | 78 | CML | CP1 | 2.2 | 2 | 4.2 | 1.3 | 0.9 | 2.2 | 8.3 | 7.9 | 16.2 | 44 | 2 |
| 4 | MUU | 18 | 68 | MDS | 1.5 | 1.6 | 3.1 | 1.1 | 2.5 | 3.6 | 5 | 5 | 10 | 30 | 1 | |
| 5 | MUU | 39 | 64 | AML | CR2 | 3.6 | 1.7 | 5.3 | 3.8 | 1 | 4.8 | 11 | 5 | 16 | 16 | 1 |
| 6 | RIC | 56 | 100 | AML | CR2 | 3.1 | 1.5 | 4.6 | 6.4 | 1.1 | 7.5 | 7.6 | 6 | 13.6 | 7‡ | 1 |
| 7 | RIC | 67 | 83 | AML | CR1 | 2.3 | 1.8 | 4.1 | 1.6 | 1.3 | 2.9 | 6.8 | 4.2 | 11 | 8‡ | 2 |
| 8 | RIC | 64 | 88 | AML | CR2 | 1.7 | 1.4 | 3.1 | 0.7 | 0.9 | 1.6 | 3.6 | 3.6 | 7.2 | 6‡ | 1 |
| 9 | RIC | 62 | 80 | AML/MDS | MDS | 2.2 | 1.9 | 4.1 | 2.8 | 0.9 | 3.7 | 6.4 | 5.5 | 11.9 | Death | 2 |
| 10 | RIC | 46 | 57 | CLL | PR | 1.9 | 1.8 | 3.7 | 0.5 | 0.7 | 1.2 | 5.5 | 4.8 | 10.3 | 24 | Mixed |
| 11 | MUE | 30 | 72 | AML | CR2 | 2.7 | 0.6 | 3.3 | 3.4 | 11.2 | 14.6 | 4.2 | NA† | 4.2 | 13§ | 1 |
| 12 | MUE | 29 | 79 | ALL | CR1 | 1.5 | 3.2 | 4.7 | 0.6 | 84 | 84.6 | 3 | NA† | 3 | NA | Rejection |
| 13 | MUE | 42 | 66 | AML | CR3 | 3 | 2.3 | 5.3 | 2.6 | 29 | 31.6 | 7 | NA† | 7 | 21 | 1 |
| 14 | MUE | 26 | 78 | AML/ALL | CR1 | 1.9 | 9.1 | 11 | 1.6 | 97 | 98.6 | 3.6 | NA† | 3.6 | 7§ | Mixed |
AML indicates acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; TNC, total nucleated cells; and NA, not applicable.
Transplantation types are MUU (myeloablative, both units unmanipulated), RIC (reduced-intensity conditioning), and MUE (myeloablative, unmanipulated and expanded).
Unit was CD34+ selected and ex vivo expanded. The negative fraction after CD34+ selection was not added back at the time of transplantation, and no T cells from this unit were infused.
Initial neutrophil recovery was the result of transient autologous recovery of the myeloid (CD33+) lineage.
Initial neutrophil recovery was the result of transient production of the myeloid (CD33+) lineage from the CD34+ selected/expanded cord blood unit.
Patient and unit HLA typing, chimerism, and IFN-γ assay results
| Patient no./regimen . | HLA typing . | Chimerism . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A . | A . | B . | B . | C . | C . | DRB1 . | DRB1 . | Day* . | Percentage CD3 . | Percentage CD56 . | Percentage CD33 . | Percentage IFN-γ–secreting cells† . | |
| Patients establishing single donor dominance | |||||||||||||
| 2/Myelo | 15 | ||||||||||||
| Unit 1 | 6802 | 3101 | 57 | 35 | 07 | 04 | 1503 | 1001 | 100 | 100 | 100 | 0.2 | |
| Unit 2 | 3201 | 0201 | 5702 | 3503 | NP | NP | 1503 | 0403 | 0 | 0 | 0 | 1.29 | |
| Patient | 3201 | 3101 | 5703 | 35TDS | 0210 | 04KBG | 1503 | 1202 | 0 | 0 | 0 | ||
| 3/Myelo | 28 | ||||||||||||
| Unit 1 | 0201 | 0201 | 4801 | 5101 | NP | NP | 0411 | 1406 | 100 | 100 | 100 | 0.0 | |
| Unit 2 | 0201 | 0206 | 3906 | 5102 | 0702 | 0801 | 0802 | 1406 | 0 | 0 | 0 | 0.97 | |
| Patient | 0201 | 0201 | 0801 | 5101 | 07WTR | 1509 | 1402 | 1406 | 0 | 0 | 0 | ||
| 4/Myelo | 26 | ||||||||||||
| Unit 1 | 0201 | 68FKZ | 5601 | 4008 | 0102 | 0304 | 0101 | 0404 | 100 | 100 | 100 | 0.056 | |
| Unit 2 | 0201 | 6801 | 5601 | 4002 | 0102 | 1502 | 0101 | 0401 | 0 | 0 | 0 | 3.1 | |
| Patient | 0201 | 68FKZ | 5601 | 4008 | 0102 | 0304 | 01AH | 0404 | 0 | 0 | 0 | ||
| 5/Myelo | 14 | ||||||||||||
| Unit 1 | 02 | 2423 | 51 | 51 | 0304 | 1502 | 0801 | 0811 | 100 | 100 | 88 | 0 | |
| Unit 2 | 26 | 24 | 51 | 39 | 14 | 07 | 0801 | 0407 | 0 | 0 | 12 | 0.034 | |
| Patient | 2501 | 2402 | 5101 | 5101 | 0102 | 1402 | 0801 | 0407 | 0 | 0 | 0 | ||
| 6/RIC | 14 | ||||||||||||
| Unit 1 | 0201 | 0301 | 3503 | 1801 | 0401 | 0701 | 1112 | 1104 | 100 | 100 | 88 | 0 | |
| Unit 2 | 02 | 03 | 35 | 4901 | NP | NP | 0101 | 1104 | 0 | 0 | 12 | 1.27 | |
| Patient | 0201 | 0301 | 3503 | 4901 | 1203 | 07WTR | 14BUYP | 1104 | 0 | 0 | 0 | ||
| 7/RIC | 28 | ||||||||||||
| Unit 1 | 02 | 01 | 27 | 51 | NP | NP | 0103 | 0404 | 94 | 95 | 73 | 0 | |
| Unit 2 | 0201 | 0301 | 2705 | 5101 | 0102 | 0202 | 0103 | 1101 | 4 | 4 | 0 | 2.06 | |
| Patient | 0201 | 2901 | 27EKN | 5108 | 0102 | 1602 | 0103 | 1303 | 2 | 1 | 27 | ||
| 8/RIC | 28 | ||||||||||||
| Unit 1 | 0302 | 0301 | 0801 | 07ANVB | 07WTR | 07WCP | 0301 | 1001 | 100 | 82 | 88 | 0 | |
| Unit 2 | 0301 | 0301 | 0801 | 07ANVB | 07WTR | 07WCP | 0301 | 0101 | 0 | 0 | 4 | 6.22 | |
| Patient | 0302 | 0301 | 0801 | 0702 | 0702 | 0702 | 0301 | 0901 | 0 | 18 | 8 | ||
| 9/RIC | 28 | ||||||||||||
| Unit 1 | 03 | 02 | 07 | 51 | 07 | 07 | 1501 | 0803 | 100 | 100 | 100 | 0.033 | |
| Unit 2 | 03 | 02 | 07 | 51 | 07 | 15 | 1501 | 0801 | 0 | 0 | 0 | 1.13 | |
| Patient | 0301 | 0201 | 0702 | 5101 | 0702 | 0202 | 1501 | 0803 | 0 | 0 | 0 | ||
| 11/Myelo ex | 14 | ||||||||||||
| Unit 1 | 24 | 23 | 3508 | 49 | 04 | 07 | 0403 | 1501 | 100 | 100 | 100 | 0 | |
| Unit 2 | 24 | 23 | 51 | 4901 | 07 | 07 | 1302 | 1501 | 0 | 0 | 0 | 1.36 | |
| Patient | 2402L | 2301 | 3801 | 4901 | 1203 | 07WTR | 0403 | 1501 | 0 | 0 | 0 | ||
| 13/Myelo ex | 28 | ||||||||||||
| Unit 1 | 0101 | 0301 | 3503 | 4001 | 0304 | 1203 | 1001 | 0404 | 100 | 100 | 100 | 0.061 | |
| Unit 2 | 0101 | 0301 | 4102 | 4001 | 17MN | 0304 | 1201 | 0404 | 0 | 0 | 0 | 2.5 | |
| Patient | 01CJT | 03AJ | 1302 | 4001 | 0602 | 0304 | 0407 | 0404 | 0 | 0 | 0 | ||
| Patients maintaining mixed chimerism | |||||||||||||
| 1/Myelo | 80 | ||||||||||||
| Unit 1 | 3301 | 7401 | 7801 | 07 | 16 | 07 | 0302 | 0901 | 68 | — | 0 | 0.058 | |
| Unit 2 | 3301 | 23 | 7801 | 07 | 1601 | 0702 | 0302 | 1101 | 32 | — | 100 | 0.36 | |
| Patient | 3301 | 0301 | 7801 | 0702 | 1601 | 0702 | 0302 | 1001 | 0 | — | 0 | ||
| 10/RIC | 80 | ||||||||||||
| Unit 1 | 2402 | 0201 | 1801 | 5701 | NP | NP | 1103 | 0701 | 35 | 56 | 47 | 0 | |
| Unit 2 | 24 | 02 | 5001 | 51 | 06 | 14 | 1103 | 0701 | 34 | 44 | 53 | 0.074 | |
| Patient | 2402 | 02 | 18RRG | 5101 | 1203 | 1502 | 1103 | 0701 | 31 | 0 | 0 | ||
| 14/Myelo ex | 80 | ||||||||||||
| Unit 1 | 68 | 24 | 40 | 39 | 03 | 07 | 0802 | 0404 | 95 | 17 | 77 | 0.12 | |
| Unit 2 | 6803 | 29 | 44 | 39 | 07 | 16 | 0407 | 0404 | 5 | 83 | 23 | 0.15 | |
| Patient | 68FKZ | 2501 | 4002 | 3901 | 0304 | 1203 | 0407 | 0404 | 0 | 0 | 0 | ||
| Patient experiencing graft rejection | |||||||||||||
| 12/Myelo ex | 28 | ||||||||||||
| Unit 1 | 24 | 24 | 40 | 08 | 03 | 07 | 0301 | 0404 | 0 | 0 | 0 | 3.18 | |
| Unit 2 | 2402 | 03 | 4001 | 5001 | 0304 | 0602 | 0301 | 0404 | 0 | 0 | 0 | 1.41 | |
| Patient | 01CJT | 03AJ | 1302 | 4001 | 0602 | 0304 | 0407 | 0404 | 100 | 100 | 100 | ||
| Patient no./regimen . | HLA typing . | Chimerism . | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A . | A . | B . | B . | C . | C . | DRB1 . | DRB1 . | Day* . | Percentage CD3 . | Percentage CD56 . | Percentage CD33 . | Percentage IFN-γ–secreting cells† . | |
| Patients establishing single donor dominance | |||||||||||||
| 2/Myelo | 15 | ||||||||||||
| Unit 1 | 6802 | 3101 | 57 | 35 | 07 | 04 | 1503 | 1001 | 100 | 100 | 100 | 0.2 | |
| Unit 2 | 3201 | 0201 | 5702 | 3503 | NP | NP | 1503 | 0403 | 0 | 0 | 0 | 1.29 | |
| Patient | 3201 | 3101 | 5703 | 35TDS | 0210 | 04KBG | 1503 | 1202 | 0 | 0 | 0 | ||
| 3/Myelo | 28 | ||||||||||||
| Unit 1 | 0201 | 0201 | 4801 | 5101 | NP | NP | 0411 | 1406 | 100 | 100 | 100 | 0.0 | |
| Unit 2 | 0201 | 0206 | 3906 | 5102 | 0702 | 0801 | 0802 | 1406 | 0 | 0 | 0 | 0.97 | |
| Patient | 0201 | 0201 | 0801 | 5101 | 07WTR | 1509 | 1402 | 1406 | 0 | 0 | 0 | ||
| 4/Myelo | 26 | ||||||||||||
| Unit 1 | 0201 | 68FKZ | 5601 | 4008 | 0102 | 0304 | 0101 | 0404 | 100 | 100 | 100 | 0.056 | |
| Unit 2 | 0201 | 6801 | 5601 | 4002 | 0102 | 1502 | 0101 | 0401 | 0 | 0 | 0 | 3.1 | |
| Patient | 0201 | 68FKZ | 5601 | 4008 | 0102 | 0304 | 01AH | 0404 | 0 | 0 | 0 | ||
| 5/Myelo | 14 | ||||||||||||
| Unit 1 | 02 | 2423 | 51 | 51 | 0304 | 1502 | 0801 | 0811 | 100 | 100 | 88 | 0 | |
| Unit 2 | 26 | 24 | 51 | 39 | 14 | 07 | 0801 | 0407 | 0 | 0 | 12 | 0.034 | |
| Patient | 2501 | 2402 | 5101 | 5101 | 0102 | 1402 | 0801 | 0407 | 0 | 0 | 0 | ||
| 6/RIC | 14 | ||||||||||||
| Unit 1 | 0201 | 0301 | 3503 | 1801 | 0401 | 0701 | 1112 | 1104 | 100 | 100 | 88 | 0 | |
| Unit 2 | 02 | 03 | 35 | 4901 | NP | NP | 0101 | 1104 | 0 | 0 | 12 | 1.27 | |
| Patient | 0201 | 0301 | 3503 | 4901 | 1203 | 07WTR | 14BUYP | 1104 | 0 | 0 | 0 | ||
| 7/RIC | 28 | ||||||||||||
| Unit 1 | 02 | 01 | 27 | 51 | NP | NP | 0103 | 0404 | 94 | 95 | 73 | 0 | |
| Unit 2 | 0201 | 0301 | 2705 | 5101 | 0102 | 0202 | 0103 | 1101 | 4 | 4 | 0 | 2.06 | |
| Patient | 0201 | 2901 | 27EKN | 5108 | 0102 | 1602 | 0103 | 1303 | 2 | 1 | 27 | ||
| 8/RIC | 28 | ||||||||||||
| Unit 1 | 0302 | 0301 | 0801 | 07ANVB | 07WTR | 07WCP | 0301 | 1001 | 100 | 82 | 88 | 0 | |
| Unit 2 | 0301 | 0301 | 0801 | 07ANVB | 07WTR | 07WCP | 0301 | 0101 | 0 | 0 | 4 | 6.22 | |
| Patient | 0302 | 0301 | 0801 | 0702 | 0702 | 0702 | 0301 | 0901 | 0 | 18 | 8 | ||
| 9/RIC | 28 | ||||||||||||
| Unit 1 | 03 | 02 | 07 | 51 | 07 | 07 | 1501 | 0803 | 100 | 100 | 100 | 0.033 | |
| Unit 2 | 03 | 02 | 07 | 51 | 07 | 15 | 1501 | 0801 | 0 | 0 | 0 | 1.13 | |
| Patient | 0301 | 0201 | 0702 | 5101 | 0702 | 0202 | 1501 | 0803 | 0 | 0 | 0 | ||
| 11/Myelo ex | 14 | ||||||||||||
| Unit 1 | 24 | 23 | 3508 | 49 | 04 | 07 | 0403 | 1501 | 100 | 100 | 100 | 0 | |
| Unit 2 | 24 | 23 | 51 | 4901 | 07 | 07 | 1302 | 1501 | 0 | 0 | 0 | 1.36 | |
| Patient | 2402L | 2301 | 3801 | 4901 | 1203 | 07WTR | 0403 | 1501 | 0 | 0 | 0 | ||
| 13/Myelo ex | 28 | ||||||||||||
| Unit 1 | 0101 | 0301 | 3503 | 4001 | 0304 | 1203 | 1001 | 0404 | 100 | 100 | 100 | 0.061 | |
| Unit 2 | 0101 | 0301 | 4102 | 4001 | 17MN | 0304 | 1201 | 0404 | 0 | 0 | 0 | 2.5 | |
| Patient | 01CJT | 03AJ | 1302 | 4001 | 0602 | 0304 | 0407 | 0404 | 0 | 0 | 0 | ||
| Patients maintaining mixed chimerism | |||||||||||||
| 1/Myelo | 80 | ||||||||||||
| Unit 1 | 3301 | 7401 | 7801 | 07 | 16 | 07 | 0302 | 0901 | 68 | — | 0 | 0.058 | |
| Unit 2 | 3301 | 23 | 7801 | 07 | 1601 | 0702 | 0302 | 1101 | 32 | — | 100 | 0.36 | |
| Patient | 3301 | 0301 | 7801 | 0702 | 1601 | 0702 | 0302 | 1001 | 0 | — | 0 | ||
| 10/RIC | 80 | ||||||||||||
| Unit 1 | 2402 | 0201 | 1801 | 5701 | NP | NP | 1103 | 0701 | 35 | 56 | 47 | 0 | |
| Unit 2 | 24 | 02 | 5001 | 51 | 06 | 14 | 1103 | 0701 | 34 | 44 | 53 | 0.074 | |
| Patient | 2402 | 02 | 18RRG | 5101 | 1203 | 1502 | 1103 | 0701 | 31 | 0 | 0 | ||
| 14/Myelo ex | 80 | ||||||||||||
| Unit 1 | 68 | 24 | 40 | 39 | 03 | 07 | 0802 | 0404 | 95 | 17 | 77 | 0.12 | |
| Unit 2 | 6803 | 29 | 44 | 39 | 07 | 16 | 0407 | 0404 | 5 | 83 | 23 | 0.15 | |
| Patient | 68FKZ | 2501 | 4002 | 3901 | 0304 | 1203 | 0407 | 0404 | 0 | 0 | 0 | ||
| Patient experiencing graft rejection | |||||||||||||
| 12/Myelo ex | 28 | ||||||||||||
| Unit 1 | 24 | 24 | 40 | 08 | 03 | 07 | 0301 | 0404 | 0 | 0 | 0 | 3.18 | |
| Unit 2 | 2402 | 03 | 4001 | 5001 | 0304 | 0602 | 0301 | 0404 | 0 | 0 | 0 | 1.41 | |
| Patient | 01CJT | 03AJ | 1302 | 4001 | 0602 | 0304 | 0407 | 0404 | 100 | 100 | 100 | ||
Myelo indicates myeloablative transplantation with both units unmanipulated; Myelo ex, myeloablative transplantation with 1 unmanipulated and 1 expanded unit; NP, typing not performed; and —, quantity not sufficient for analysis.
Day indicates day after transplantation on which PBMCs were collected for IFN-γ secretion assay. Chimerism assays were performed on the same day.
Numbers reported indicate the percentage of CD8+CD45RO+/−CCR7− IFN-γ cells detected when PBMCs were stimulated against the unit in that row.
Hematopoietic recovery results from engraftment of a single cord blood unit
Twelve of the 14 patients exhibited engraftment of neutrophils, defined as the first of 3 consecutive days with absolute neutrophil count greater than 500/μL, at a median of 15 days after transplantation (range, 7-45 days after transplantation). Three patients undergoing RIC had transient early recovery (days 6-8) of autologous neutrophils demonstrated by chimerism analysis of peripheral blood CD33+ cells. Two patients receiving 1 CD34+ selected/expanded unit and 1 unmanipulated unit had transient early neutrophil recovery (days 7 and 13) derived from the expanded unit, as demonstrated by chimerism analysis of peripheral blood CD33+ cells. Four of these 5 patients (ID 6,7, 8, and 11) developed myeloid hematopoiesis derived completely from 1 transplanted cord blood unit by day 14 after transplantation, whereas 1 patient (ID 14) maintained persistent contribution to myeloid hematopoiesis from both of the transplanted cord blood units. One patient died from regimen-related toxicity before engraftment, and 1 patient experienced rejection of both transplanted cord blood units.
In 10 of the 14 patients, analysis of the CD3+, CD56+, and CD33+ cells in the peripheral blood between days 14 and 28 demonstrated that engraftment of these lineages was provided solely or predominantly by only 1 of the transplanted cord blood units (Table 2). Six of these patients received myeloablative conditioning (4 with both units unmanipulated and 2 with 1 expanded unit and 1 unmanipulated unit), and 4 of these patients received RIC. The dominance of 1 unit did not correlate with differences in the infused number of TNC, CD34+, or CD3+ cells contained in the grafts (Table 1), and single unit engraftment was sustained throughout the period of follow-up for all patients (median, 539 days among survivors; range, 203-629 days). In 3 of the 14 patients, a major proportion of the CD3+, CD56+, or CD33+ cells were derived from both cord blood units even at day 80 after transplantation and in the second of these patients, host CD3 cells was composed of approximately 30% of the overall T-cell numbers (Table 2). The first patient maintained mixed chimerism until relapsing 205 days after transplantation, the second patient is alive without relapse 313 days after transplantation with persistent mixed T-cell chimerism, and the third patient maintained mixed chimerism until death resulting from sepsis 198 days after transplantation.
CD8+ T cells acquire a phenotype consistent with antigen exposure early after CBT
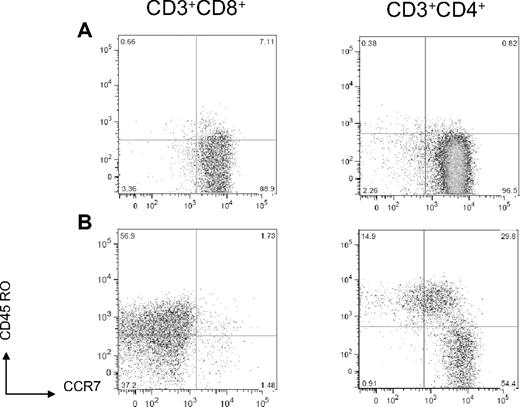
The absolute number of T cells infused with cord blood stem cell grafts is relatively low. The early recovery of T cells can be derived from proliferation of naive T cells infused with the stem cell grafts either in response to homeostatic cytokines or antigen exposure and from new naive T cells that arise from thymic education of progenitor cells.20 We analyzed the composition and phenotype of T cells that were present in 26 cord blood units after thaw and before infusion on the day of transplantation and compared these results with the phenotype of T cells that were present in the early after transplantation period (between days 14 and 28) of double CBT recipients. The median CD4+/CD8+ ratio in thawed cord blood units before infusion was 2.78 (range, 1.82-5.56; n = 26), and, consistent with previous reports,21 the majority of cord blood CD4+ and CD8+ T cells analyzed in 6 fresh research cord units expressed a naive C45RO−CCR7+ antigen inexperienced phenotype (Figure 1A). In CBT recipients, CD8+ T cells typically outnumbered CD4+ cells early after transplantation with a median CD4+/CD8+ ratio of 0.38 (range, 0.05-6.25). Moreover, we found that the CD8+ T cells present in vivo at early time points after transplantation predominately expressed a CD45RO+/−CCR7− effector memory or effector phenotype (median, 92.5% of cells; range, 66.7%-99.9% of cells), whereas CD4+ T cells were significantly more CD45RO−CCR7+ (naive; median, 25.5% of cells; range, 10%-65.5% of cells) or CD45RO+CCR7+ (central memory) phenotype (median, 24.6% of cells; range, 8%-38.9% of cells; Figure 1B). The phenotype of the T-cell subsets early after transplantation was comparable in all patients regardless of conditioning regimen. These results are consistent with antigen exposure after CBT or with alteration in phenotype of T cells as a consequence of homeostatic proliferation in the lymphopenic environment.22,23
Conversion from naive to effector/effector memory T-cell phenotype after double-cord CBT. (A) CD8+ and CD4+ T cells in a representative unmanipulated fresh cord blood express a naive phenotype (CCR7+CD45RO−). (B) In a representative sample of day 14 posttransplantation PBMCs, CD8+ T cells express a primarily effector/effector memory phenotype (CCR7−CD45RO+/−), and CD4+ T cells express primarily naive (CCR7+CD45RO−) and central memory (CCR7+CD45RO+) phenotypes.
Conversion from naive to effector/effector memory T-cell phenotype after double-cord CBT. (A) CD8+ and CD4+ T cells in a representative unmanipulated fresh cord blood express a naive phenotype (CCR7+CD45RO−). (B) In a representative sample of day 14 posttransplantation PBMCs, CD8+ T cells express a primarily effector/effector memory phenotype (CCR7−CD45RO+/−), and CD4+ T cells express primarily naive (CCR7+CD45RO−) and central memory (CCR7+CD45RO+) phenotypes.
IFN-γ–secreting CD8+ T cells from the engrafting unit specifically recognize the nonengrafting unit after CBT
We next investigated whether CD8+ and/or CD4+ T cells that developed in the recipient after double CBT were capable of specific recognition of cells derived from either cord blood unit. Unlike effector T cells, naive T cells do not produce IFN-γ immediately after encountering cognate antigen but rather require a period of proliferation and subsequent differentiation to acquire this function.24 Thus, we designed a multiparameter flow cytometry assay to detect IFN-γ–producing effector/memory T cells in PBMCs after a brief 5-hour stimulation with LCLs derived from each of the cord blood units. A detectable IFN-γ response was not observed when naive T cells from 4 fresh cord blood samples were stimulated with a total of 18 LCLs from major histocompatibility complex-mismatched unrelated cord blood units, confirming that the duration of this assay was not sufficient for potentially alloreactive naive T cells to differentiate into IFN-γ–producing effector cells (data not shown).
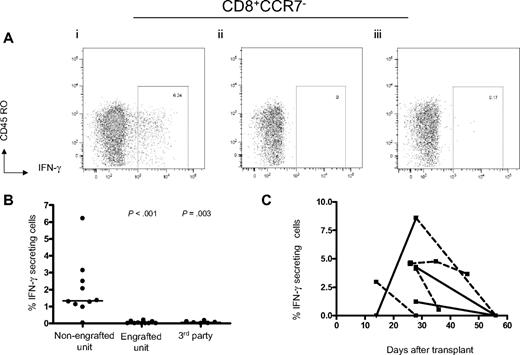
An intracellular cytokine flow cytometry assay was used to analyze the reactivity of the engrafted T cells directly against each of the cord blood units. In 9 of the 10 patients where there was predominant engraftment from a single unit, a significant CD8+ CD45RO+/−CCR7− IFN-γ–secreting population (median, 1.36%; range, 0.97%-6.22%) was detected in the PMBCs obtained between 14 and 28 days after transplantation (before the onset of acute GVHD) after stimulation with LCLs from the nonengrafted unit (Table 2; Figure 2A-B). We did not detect a significant frequency of IFN-γ–secreting CD4+ T cells in any of these assays (data not shown). No IFN-γ–secreting CD8+ T-cell population above background was observed after stimulation with LCLs from the engrafted unit (median, 0%; range, 0%-0.2%, P < .001), which excludes recognition of EBV antigens expressed by the nonengrafting unit LCLs because the engrafting unit would also present EBV antigens in the context of shared HLA alleles (Figure 2B). In 11 patients from whom sufficient PBMCs were available, we included third party LCLs derived from persons who were HLA-mismatched with both cord blood units and did not observe recognition of these control target cells (median, 0.06%; range, 0%-0.17%, P = .003), making it doubtful that the specific response to the nonengrafted unit was the result of in vitro activation of naive T cells (Figure 2B).
Development of IFN-γ–secreting CD8+ T cells recognizing alloantigens expressed by the nonengrafting unit after double-unit CBT. (A) After a 5-hour stimulation, CD8+CD45RO+/−CCR7− IFN-γ–staining cells from PBMCs samples collected 14 to 28 days after transplantation are demonstrable against LCLs generated from the nonengrafting unit (i), but not the dominant unit (ii) or a random third party unit (iii). (B) IFN-γ–secreting CD8+ T cells are detectable among 9 of 10 patients establishing single donor dominance. (C) Alloreactive IFN-γ–secreting CD8+ T cells decline at later times after CBT. ■ represents time point at which the sample was drawn; solid lines, no administration of steroids for GVHD in the interval between sample assays; dotted lines, administration of steroids for GVHD in the interval between sample assays. Because 3 patients died before second PBMCs samples were collected, serial data for only 6 patients are shown.
Development of IFN-γ–secreting CD8+ T cells recognizing alloantigens expressed by the nonengrafting unit after double-unit CBT. (A) After a 5-hour stimulation, CD8+CD45RO+/−CCR7− IFN-γ–staining cells from PBMCs samples collected 14 to 28 days after transplantation are demonstrable against LCLs generated from the nonengrafting unit (i), but not the dominant unit (ii) or a random third party unit (iii). (B) IFN-γ–secreting CD8+ T cells are detectable among 9 of 10 patients establishing single donor dominance. (C) Alloreactive IFN-γ–secreting CD8+ T cells decline at later times after CBT. ■ represents time point at which the sample was drawn; solid lines, no administration of steroids for GVHD in the interval between sample assays; dotted lines, administration of steroids for GVHD in the interval between sample assays. Because 3 patients died before second PBMCs samples were collected, serial data for only 6 patients are shown.
Concurrent chimerism studies showed that 100% of the CD3+ cells were from a single cord blood unit at the time the blood was drawn in 9 of the 10 patients. IFN-γ–secreting T cells were captured and expanded from 3 patients where sufficient PBMCs were obtained to confirm the derivation of the T cells reacting with the nonengrafting unit. Chimerism studies on this selected subset of reactive T cells demonstrated that 100% were derived from the engrafted cord blood unit. In 1 patient in whom sufficient sample was available, the stimulations were repeated in the presence of isotype control or W6/32 antibody that will bind to and block recognition of HLA class I molecules. After class I blockade only, no IFN-γ–secreting CD8+ T cells were detectable, consistent with recognition of mismatched class I major histocompatibility complex or of a minor histocompatibility (H) antigen presented by a shared class I allele (data not shown).
We retested PBMCs obtained at later time points after transplantation from 5 patients to evaluate if the specific CD8+ T-cell responses against the nonengrafting cord blood cells persisted. T cells specific for the nonengrafted cord blood cells declined at later time points in all patients and were below the sensitivity of this direct assay in 3 of the 5 patients (Figure 2C). Notably, all 5 of these patients developed acute GVHD between 21 and 34 days after transplantation that required systemic prednisone treatment, which may have contributed to the decline in alloreactivity.
Similar assays did not detect NK cells (CD3−CD56+) that produced IFN-γ in response to LCLs from either cord blood unit. We also stained NK cells (CD3−CD56+CD16+/−) in PBMCs collected between day 12 and day 100 from 9 of the patients for up-regulation of cell-surface CD107a after stimulation for 5 hours with LCLs from each cord blood unit or with the K562 cell line. CD107a up-regulation was not detected in any samples in response to stimulation against either cord blood LCL, although a subset of NK cells did respond to K562 during the first 100 days after transplantation. The frequency of NK cells that responded to K562 increased with samples obtained at time points later after transplantation (data not shown).
Cord blood CD8+ T cells are mutually tolerant in patients maintaining mixed chimerism after double CBT
In the 3 patients with persistent engraftment of CD33+ and CD3+ cells from both cord blood units, no significant IFN-γ–secreting CD8+ T cells were detected when posttransplantation PBMCs were stimulated against LCLs generated from either of the transplanted units or from random third-party donors (median, 0.09%; range, 0%-0.36%; P < .005 vs percentage of IFN-γ–secreting cells detectable against the nondominant unit among single donor dominant patients). Similar to patients who developed engraftment of only 1 cord blood unit, no CD4+ or NK-cell responses were detected in patients with mixed chimerism when posttransplantation PBMCs were stimulated with LCLs generated from each of the transplanted units.
Host IFN-γ–secreting CD8+ T cells specific for both cord blood units are present after graft rejection
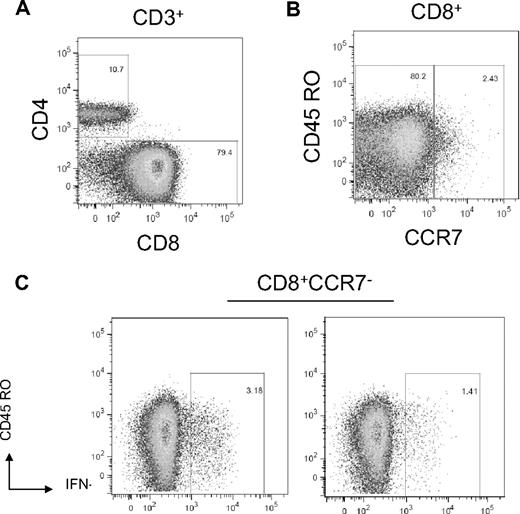
One patient (ID 12) failed to engraft after myeloablative conditioning and the infusion of 1 unmanipulated unit and 1 ex vivo expanded unit. A salvage-mismatched unrelated donor peripheral blood stem cell transplantation was attempted, but the patient died 86 days after the initial transplantation. After the double-unit CBT, the patient's neutrophil count remained below the limit of detection. On day 24 after transplantation, the patient had a white blood cell count of 160/μL consisting entirely of lymphocytes. Chimerism analysis of peripheral blood subsets on day 28 showed that the CD3+, CD33+, and CD56+ cells were 100% host origin, and bone marrow cells obtained on day 32 were also 100% host, consistent with graft rejection. The phenotype of the CD3+ cells in the blood showed that 79% were effector CD8+CD45RO+/−CCR7− cells. A 5-hour stimulation of PBMCs obtained on day 26 with LCLs from both the expanded unit and the unmanipulated unit elicited a significant frequency (3.18% and 1.41%, respectively) of IFN-γ+CD8+ T cells to each unit (Figure 3). We did not detect any IFN-γ production by the CD4+ T cells in this patient, although the absolute number of CD4 cells in the blood at the time of the assay was very low.
Host CD8+ T cells reactive with cord blood alloantigens are present in 9 patients rejecting double CBT. Day 26 posttransplantation PBMCs were stimulated against LCLs generated from the transplanted cord units. (A) The majority of CD3+ cells are CD8+. (B) The CD8+ cells express primarily effector/effector memory phenotype (CD45RO+/−CCR7−). (C) Host CD8+CD45RO+/−CCR7− cells stain positive for intracellular IFN-γ staining after stimulation with each of the transplanted cord unit.
Host CD8+ T cells reactive with cord blood alloantigens are present in 9 patients rejecting double CBT. Day 26 posttransplantation PBMCs were stimulated against LCLs generated from the transplanted cord units. (A) The majority of CD3+ cells are CD8+. (B) The CD8+ cells express primarily effector/effector memory phenotype (CD45RO+/−CCR7−). (C) Host CD8+CD45RO+/−CCR7− cells stain positive for intracellular IFN-γ staining after stimulation with each of the transplanted cord unit.
Discussion
Transplantation of umbilical cord blood stem cells provides a potentially curative therapy for many patients who do not have access to a suitably HLA-matched related or unrelated donor.25-27 In initial cord blood transplantation trials in which cord blood cells from a single donor were infused, graft failure was a significant problem and correlated with the low dose of CD34+ cells per kilogram that was administered.27-30 More recently, coinfusion of 2 cord blood units has enabled the administration of a higher overall CD34+ cell dose, resulting in improved engraftment and survival among adults and large children compared with historical results using single cord blood units for transplantation.1,2,31 These improved outcomes are seen despite the finding that only 1 unit is responsible for sustained donor engraftment in the majority of recipients. The mechanism(s) for the dominant engraftment of 1 of the transplanted cord blood units has not been conclusively identified.
Our data provide the first direct evidence in human double-unit CBT recipients that immune recognition by T cells from 1 cord blood unit probably contributes to the failure of the second unit to engraft. We used a brief in vitro stimulation to avoid measuring potentially alloreactive naive T cells and detected a significant IFN-γ–producing CD8+ T-cell response against the nonengrafting unit early after transplant in 9 of 10 patients in whom only 1 cord blood unit engrafted. IFN-γ+ CD8+ T cells were not detected in posttransplantation PBMCs when third-party LCLs that were HLA-mismatched with both the engrafted and nonengrafted units were used as stimulators. In addition, stimulation of T cells from random cord units with HLA-disparate LCLs did not elicit an IFN-γ response consistent with the requirement for naive T cells to differentiate to acquire the ability to rapidly produce IFN-γ in response to cognate antigen. We did not detect a T-cell response against either cord blood unit in 3 patients who maintained dual donor chimerism. Finally, in 1 patient in whom sufficient cells were available for testing, class I blockade with W6/32 antibody abrogated detection of CD8+ IFN-γ–secreting T cells. Collectively, these data demonstrate that naive CD8+ T cells in 1 cord blood unit are probably activated in vivo by antigens expressed by the second cord blood unit and expand and differentiate into mature effector T cells that mediate rejection of the second cord blood unit.
IFN-γ–secreting CD8+ T cells expressed a CD45RO+/−CCR7− phenotype, consistent with an antigen-experienced effector or effector memory cell, and were only detected transiently after CBT. The inability to detect a durable memory T-cell response by this direct assay could be the result of the dilution of the reactive T cells to levels below the sensitivity of the direct ex vivo assay by the recovery of lymphocyte numbers at later times after transplantation. In addition, all 5 patients who were assayed at later time points developed acute GVHD requiring systemic prednisone treatment (1 or 2 mg/kg prednisone), which could have resulted in the preferential deletion of activated alloreactive T cells. Finally, all patients were receiving mycophenolate mofetil and cyclosporine after transplantation, and it is possible that these agents interfered with the transition of effector cells to the memory pool.
Our studies did not identify alloreactive CD4+ T cells, which was surprising because the majority of the cord blood units were mismatched at 1 or more class II alleles. It is premature to conclude that CD4+ T cells are not involved in the graft-versus-graft (GVG) reaction because the frequency of CD4+ T cells in the blood early after CBT is low and the sensitivity of the direct ex vivo assay for intracellular IFN-γ may not be sufficient to detect a response. CD4+ T cells might also be more susceptible to the immunosuppressive drugs administered to these patients, and less probable to proliferate to sufficient numbers for detection. Thus, further studies that include the use of short-term in vitro culture to expand alloreactive T cells from the CD4+ subset are warranted. We did not detect alloreactive NK cells that responded specifically to the nonengrafted cord blood cells. However, NK cells might play a role in determining dominant engraftment of 1 cord blood unit in cases where killer immunoglobulin-like receptor (KIR)/KIR ligand differences between the cord blood units might favor unidirectional NK alloreactivity.32
The alloreactive CD8+ T-cell response detected to the nonengrafting cord blood unit could be specific for major or minor allogeneic determinants because the double cord blood grafts were mismatched with each other at 1 or more HLA alleles, and probably differ in multiple minor H antigens that could be presented by HLA alleles that are shared between the cord blood units. Preliminary data have suggested a decreased relapse rate with double-unit CBT, and it is conceivable that the immune interaction between the 2 units may contribute to an enhanced GVL effect. For example, major or minor H antigens expressed on hematopoietic stem cells of the losing unit might be shared by host leukemic stem cells, resulting in an enhanced GVL effect.33 Identification of the antigens to which T cells in the dominant unit are responding is in progress and may be useful for identifying minor H antigens that are expressed on hematopoietic lineage cells, including leukemic cells. Moreover, if the dominant unit can be predicted before transplantation, a nonengrafting unit sharing host antigens not present on the dominant unit might be selected to enhance GVL.
IFN-γ–secreting alloreactive CD8+ T cells were not detected in 4 patients who engrafted after CBT. Three of these patients had hematopoietic engraftment from both cord blood units despite at least 1 class I HLA mismatch between the units and the potential for alloreactivity to develop. The mechanisms for bidirectional tolerance between cord blood units is not understood but could result from the immunosuppressive drugs administered after CBT or the presence in the cord blood graft of CD4+ T regulatory cells that develop in utero and promote tolerance against noninherited maternal alloantigens that by chance might be shared by the other cord blood unit.34
A preliminary report in an immunodeficient mouse xenograft model provided indirect evidence for an immune interaction after infusion of 2 human cord blood units and is consistent with our demonstration that a T-cell–mediated GVG reaction occurs after double CBT. Eldjerou et al35 infused small aliquots of 2 cord blood units into NOD/SCID IL2R-γ null mice that were the same units administered to patients as a double-unit CBT. In 11 of 12 cases, the dominant unit that engrafted in the mice was the same as the single unit that engrafted in the patient. Single unit dominance did not occur when CD34+ selected products were transplanted into the mice but was restored with the add-back of the CD34− product.35 Similarly, Yahata et al demonstrated that infusing 2 CD34+ selected cord units into NOD/SCID IL2R-γ null mice resulted in ongoing mixed chimerism, but adding back the mononuclear cells to both units resulted in single unit dominance.36
Our results in human CBT and the results in murine models do not exclude a role for nonimmune factors in the engraftment potential of a given cord blood unit. Ex vivo expansion studies performed by our group demonstrate highly variable proliferative potential of CD34+ cells between different cord units, suggesting that there may be intrinsic and as yet not understood variability in the engraftment potential of different units. Variability in the capacity of cord blood cells to home to the stem cell niche may be significant, and it is probable that a minimum threshold of viable CD34+ cells is necessary for engraftment. Host factors, including prior therapy, intensity of conditioning, and quality of the stem cell niche, could also affect the interaction between the units and the capacity of units to engraft.
The findings presented here have implications for stem cell transplantation with multiple cord blood units. Currently, selecting units for CBT requires consideration of both unit size and degree of HLA matching. Although larger units may overcome increasing degrees of mismatching, clinical outcomes are improved in patients receiving 6 of 6 (low resolution A and B, high resolution DRB1 typing) HLA-matched units,26 suggesting that higher resolution matching at all loci might further improve outcomes. However, given current cord blood bank inventories, very few patients will have an adequately sized 6 of 6 HLA-matched unit. The demonstration here that a GVG immune-mediated mechanism underlies the emergence of a dominant unit suggests that an immunologically favored smaller well-matched unit might be supplemented with a larger unit to help promote and ensure the long-term engraftment of the better matched unit. Studies to investigate if a predictive assay for unit dominance in double-unit CBT might be developed based on a quantitative mixed lymphocyte reaction are under way. The results may provide a framework for improving transplantation outcome and expand the potential therapeutic role of double-unit CBT.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shalini Pereira, Denise Ziegler, Adrienne Papermaster, Ivy Riffkin, and Mary Joy Lopez for their assistance in preparation of the manuscript.
This work was supported by the University of Washington Institute of Translational Health Sciences (grant UL1RR025014; J.A.G., C.D.), Fred Hutchinson Cancer Research Center Core Center for Excellence in Hematology Pilot Grant (grant DK56465; J.A.G., C.D.), and National Institutes of Health (grants R24 HL74445, K23 HL077446, C.D.; and T32 CA 009515-24, J.A.G.). C.D. is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (CI# 35-07).
National Institutes of Health
Authorship
Contribution: J.A.G., C.J.T., T.J.M., S.R.R., and C.D. designed the research; J.A.G., C.J.T., and T.J.M. performed the research; J.A.G., C.J.T., S.R.R., I.D.B., and C.D. analyzed data; and J.A.G., C.J.T., S.R.R., S.H., I.D.B., and C.D. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan A. Gutman, University of Colorado Health Sciences Center, Division of Medical Oncology, 1665 Aurora Ct, Room 2251A, Aurora, CO 80045; e-mail: jgutman@fhcrc.org.