Abstract
The NLRP3 inflammasome can be activated by pathogen-associated molecular patterns or endogenous danger-associated molecular patterns. The activation of the NLRP3 inflammasome results in proteolytic activation and secretion of cytokines of the interleukin-1 (IL-1) family. The precise mode of activation of the NLRP3 inflammasome is still elusive, but has been postulated to be mediated by reactive oxygen species (ROS) generated by an NADPH oxidase. Using primary cells from chronic granulomatous disease (CGD) patients lacking expression of p22phox, a protein that is required for the function of Nox1-4, we show that cells lacking NADPH oxidase activity are capable of secreting normal amounts of IL-1β. Thus, we provide evidence that activation of the NLRP3 inflammasome does not depend on ROS generated from an NADPH oxidase.
Introduction
Inflammasomes are specialized intracellular protein complexes responsible for regulating the proteolytic activation of proinflammatory cytokines of the interleukin-1 (IL-1) family.1 The central components of inflammasomes, the Nod-like receptor (NLR) proteins, act as sensors for exogenous (microbial) or endogenous danger signals. Importantly, inappropriate activation of inflammasomes, due to activating mutations in the NLRs or an excess of danger signals can lead to autoinflammatory disorders such as chronic infantile neurologic cutaneous and articular syndrome, or the more common reactions in gout.1 The best-studied NLR, NLRP3, forms inflammasomes that mediate the release of IL-1β and related cytokines by, for example, macrophages. The NLRP3 inflammasome can be activated by pathogen-associated molecular patterns such as lipopolysaccharide (LPS) or muramyl dipeptide, or endogenous danger-associated molecular patterns such as uric acid.1 The precise mode of activation of the NLRP3 inflammasome is still elusive, but it has been postulated, in reports by Dostert et al, to be dependent on reactive oxygen species (ROS) generated by an NADPH oxidase.2,3 In these reports, the authors show an inhibitory effect on IL-1β secretion by knockdown of p22phox, a protein that is part of the catalytic core of several NADPH oxidases.
The catalytic core of NADPH oxidases contains a member of the Nox family of proteins.4 All members of the Nox1-4 subfamily form a heterodimer with the common p22phox subunit. In the case of Nox2, p22phox functions as a docking site for the regulatory protein p47phox, and indirectly for p67phox, and p40phox; the small GTPase Rac binds to p67phox and serves as a switch for Nox2 activation.4 For Nox1 and 3, p47phox and p67phox homologs are probably involved in this activation.4 Nox4 is believed not to be regulated by p47phox and p67phox or their homologs, and p22phox is not a conditio sine qua non for its activity but Nox4 activity seems to be clearly enhanced by association with p22phox4 . The best-studied NADPH oxidase is the phagocyte NADPH oxidase, a microbicidal enzyme that contains Nox2 (gp91phox) and p22phox and generates large amounts of superoxide. Defects in the phagocyte NADPH oxidase lead to chronic granulomatous disease (CGD), a condition that predisposes to recurrent bacterial and fungal infections and is the result of mutations in genes encoding the proteins that constitute this enzyme.5 In this report, we tested the secretion of IL-1β by peripheral blood mononuclear cells (PBMCs) of CGD patients carrying mutations in p22phox and found that IL-1β secretion was independent of Nox oxidase activity.
Methods
Venous blood was collected from healthy donors and from patients, after obtaining informed consent. Blood studies had been approved by the Sanquin Research institutional medical ethical committees in accordance with the standards laid down in the 1964 Declaration of Helsinki. Heparinized peripheral blood of p22phox patients and controls was diluted 1:10 in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) medium (132mM NaCl, 20mM HEPES, 6mM KCl, 1mM MgSO4, 1.2mM K2HPO4, 1mM CaCl2, 5mM glucose [all from Sigma-Aldrich], and 2% (vol/vol) human serum albumin [Sanquin Reagents], pH 7.4) and cultured overnight in the presence of uric acid (100 μg/mL; InvivoGen), silica (100 μg/mL; Alfa Aesar), imiquimod (10 μg/mL; InvivoGen), or LPS (10 μg/mL; InvivoGen), after which the IL-1β concentration was determined in the supernatant by an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (Sanquin Reagents).
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood over isotonic Percoll, as described.6 Cells were cultured overnight in HEPES medium at a concentration of 5 × 106/mL in the presence of uric acid (100 μg/mL) or silica (100 μg/mL), after which the IL-1β concentration was determined in the supernatant by ELISA. When treated with N-acetyl-L-cysteine (NAC; 25mM; Sigma-Aldrich) or diphenylene iodonium (DPI; 20 μM; Sigma-Aldrich), PBMCs were incubated for 6 hours in the presence of the indicated stimuli, after which the IL-1β concentration was determined in the supernatant by ELISA.
Results and discussion
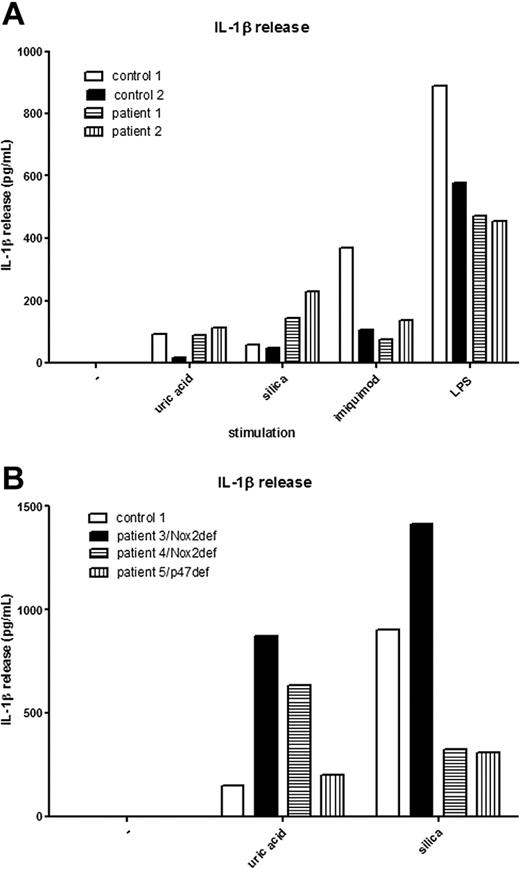
Recently, we had the opportunity to test the secretion of IL-1β in 2 CGD patients carrying mutations in CYBA, the gene encoding the p22phox protein. These 2 patients carry different homozygous mutations in CYBA, an insertion of a C at position c.166_167, resulting in a premature stop codon in exon 3, and a missense mutation at position c.70G>A, resulting in an amino acid substitution of a glycine to an arginine at position 24.7 Expression of Nox2 and p22phox was undetectable in both patients, and ROS production in response to phorbol myristate acetate was reduced to below the detection limit and less than 5% of normal, respectively.7 We tested the secretion of IL-1β in blood samples of these patients after stimulation with NLRP3 activators, such as uric acid, silica, imiquimod, and LPS. Strikingly, we found normal secretion of IL-1β in the blood samples of these patients, despite their genetic defects in p22phox (Figure 1A). This proves that IL-1β can be effectively secreted in patient samples that lack expression of p22phox-containing NADPH oxidases and contradicts the hypothesis that ROS generation by Nox1-4 is important for NLRP3 inflammasome activation.
Normal IL-1β secretion in p22phox and Nox2-deficient patients. (A) Peripheral blood of p22phox patients and controls was diluted 1:10 and cultured overnight in the presence of uric acid (100 μg/mL), silica (100 μg/mL), imiquimod (10 μg/mL), or LPS (10 μg/mL). (B) PBMCs from Nox2-deficient patients (patients 3 and 4), a p47phox-deficient patient (patient 5), and a control were cultured overnight in the presence of uric acid (100 μg/mL) or silica (100 μg/mL).
Normal IL-1β secretion in p22phox and Nox2-deficient patients. (A) Peripheral blood of p22phox patients and controls was diluted 1:10 and cultured overnight in the presence of uric acid (100 μg/mL), silica (100 μg/mL), imiquimod (10 μg/mL), or LPS (10 μg/mL). (B) PBMCs from Nox2-deficient patients (patients 3 and 4), a p47phox-deficient patient (patient 5), and a control were cultured overnight in the presence of uric acid (100 μg/mL) or silica (100 μg/mL).
Furthermore, we found IL-1β secretion also to be unaffected in PBMCs isolated from the blood of 2 patients carrying a mutation in CYBB (the gene encoding Nox2), both mutations being substitutions (c.781-C>T and c.271C>T, respectively) that result in premature stop codons. Furthermore, we found IL-1β secretion in a patient carrying a homozygous mutation (a 2–base pair deletion, c.75_76delGT, resulting in a premature stop codon) in NCF1, the gene encoding p47phox (Figure 1B). Thus, for primary cells, Nox1-4 oxidase activity does not seem to be important for activation of the NLRP3 inflammasome. We suggest that the results of Dostert et al, which were all obtained from cell lines, are based on artefacts introduced by the short hairpin RNA (shRNA) method used to repress the expression of p22phox and/or on the use of aspecific inhibitors such as DPI.2,3
To determine whether the secretion of IL-1β was dependent on ROS from sources other than Nox1-4 in primary cells, we preincubated the PBMCs of healthy controls with the ROS scavenger NAC or the general flavoprotein inhibitor DPI and stimulated them with the indicated NLRP3 activators (Figure 2). Of interest, we found that these substances greatly reduced the amount of secreted IL-1β.
IL-1β secretion is ROS dependent in primary cells. PBMCs from healthy controls were pretreated with NAC or DPI and subsequently incubated with LPS (10 μg/mL), uric acid (100 μg/mL) or imiquimod (10 μg/mL). Numbers represent the means of 3 individual donors.
IL-1β secretion is ROS dependent in primary cells. PBMCs from healthy controls were pretreated with NAC or DPI and subsequently incubated with LPS (10 μg/mL), uric acid (100 μg/mL) or imiquimod (10 μg/mL). Numbers represent the means of 3 individual donors.
A recent publication by van de Veerdonk et al, which was published during the review process of our current report, supports our findings.8 In this report, the authors used primary cells from p47phox-deficient patients and found that NLRP3 inflammasome activation and subsequent IL-1β secretion is not inhibited in these cells. Collectively, our data indicate that ROS, although clearly not derived from Nox1-4 oxidase activity, do play an important role in NLRP3 inflammasome activation. A recent report by Zhou et al identified thioredoxin–interacting protein (TXNIP) as a ROS-sensitive activator of the NLRP3 inflammasome.9 Furthermore, TXNIP has also recently been described to interact with mitochondria,10 which are also an important source of ROS.11 Future experiments will have to be performed to establish the nature of the ROS that are needed to activate the NLRP3 inflammasome.
In conclusion, these results exclude a role for Nox1-4 in NLRP3 inflammasome activation in primary cells, but suggest that another source of ROS is involved in this process.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Contribution: R.v.B. performed research and wrote the manuscript; M.Y.K. analyzed data; M.J. and M.v.H. performed experiments; D.R. and T.W.K. suggested key experiments and supervised the writing of the manuscript; and T.K.v.d.B. designed the study and supervised the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robin van Bruggen, PhD, Department of Blood Cell Research, Sanquin Research, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: r.vanbruggen@sanquin.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal