Proteasome inhibition represents a valid antitumor approach and its use has been validated in Waldenström macroglobulinemia (WM), where bortezomib has been successfully tested in clinical trials. Nevertheless, a significant fraction of patients relapses, and many present toxicity due to its off-target effects. Selective inhibition of the chymotrypsin-like (CT-L) activity of constitutive proteasome 20S (c20S) and immunoproteasome 20S (i20S) represents a sufficient and successful strategy to induce antineoplastic effect in hematologic tumors. We therefore studied ONX0912, a novel selective, irreversible inhibitor of the CT-L activity of i20S and c20S. Primary WM cells express higher level of i20S compared with c20S, and that ONX0912 inhibited the CT-L activity of both i20S and c20S, leading to induction of toxicity in primary WM cells, as well as of apoptosis through c-Jun N-terminal kinase activation, nuclear factor κB (NF-κB) inhibition, caspase cleavage, and initiation of the unfolded protein response. Importantly, ONX0912 exerted toxicity in WM cells, by reducing bone marrow (BM)–derived interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1) secretion, thus inhibiting BM-induced p-Akt and phosphorylated extracellular signal-related kinase (p-ERK) activation in WM cells. These findings suggest that targeting i20S and c20S CT-L activity by ONX0912 represents a valid antitumor therapy in WM.
Introduction
The multicatalytic ubiquitin-proteasome pathway plays an important role in the targeted degradation of a wide spectrum of proteins involved in the regulation of several cellular processes responsible for the maintenance of cellular homeostasis.1,2 The 26S constitutive proteasome has been identified in the majority of cell types. It consists of a 20S central core that exerts a proteolytic activity, and two 19S particles that represent the regulatory part of the complex. The 19S particles include 6 adenosine triphosphatase subunits that are responsible for the denaturation of target proteins and for the delivery of the substrate into the proteolytic 20S core. The 20S core contains 3 different catalytic activities known as chymotrypsin-like (CT-L), trypsinlike (T-L), or caspaselike (C-L), which are encoded by the β5, β2, and β1 subunits, respectively.2 Similarly, cells of hematopoietic origin express the immunoproteasome, which retains the structural subunits of the constitutive proteasome but exerts its enzymatic activities through the catalytic subunits low-molecular-mass polypeptide 7 (LMP7), mutational analysis of subunit iβ2 (MECL1), and LMP2, which form the 20Si core.3,–5 Specific targets for proteasome degradation include several proteins involved in cell-cycle regulation, cell proliferation, programmed cell death, and stress response. These findings validate targeting the proteasome for cancer therapy. Indeed, a wide spectrum of compounds, both natural and synthetic, has been identified as proteasome inhibitors, with bortezomib being the first proteasome inhibitor to enter clinical trials and receive approval for the treatment of patients with multiple myeloma (MM).6,7
A recent report has shown that B-cell malignancies are characterized by a preferential expression of the immunoproteasome 20S (i20S), and that a selective inhibition of the CT-L activity of the proteasome, both at the constitutive and immunoproteasome level, is sufficient to exert an antineoplastic effect in hematologic malignancies.8 In the present study, we show that primary Waldenström macroglobulinemia (WM) cells are characterized by higher expression of the i20S immunoproteasome subunits compared with constitutive proteasome 20S (c20S) subunits and that they contain a higher i20S content compared with normal CD19+ B cells. We therefore investigated for the first time the antitumor activity of the novel orally bioavailable and selective peptide epoxyketone proteasome inhibitor ONX0912 in WM. Our findings demonstrate that ONX0912 inhibits the chymotrypsin-like activity of both the immunoproteasome (LMP7) and the constitutive proteasome (β5) in WM cells, leading to induction of cytotoxicity in primary WM cells, as well as of programmed cell death in a caspase-dependent and -independent manner, as shown by activation of c-Jun N-terminal kinase, inhibition of nuclear factor κB (NF-κB), and initiation of the unfolded protein response. Importantly, ONX0912 exerted cytotoxicity in WM cells, even in the context of bone marrow milieu. We also showed that combination of ONX0912 and bortezomib acted synergistically in inhibiting the i20S and c20S CT-L activities, NF-κB activity, and caspase and poly(adenosine diphosphate ribose) polymerase (PARP) cleavage, thus inducing synergistic cytotoxicity in WM cells. Taken together, these findings provide the preclinical rationale for testing ONX0912 in Waldenström macroglobulinemia.
Methods
Cells
Primary WM cells were obtained from bone marrow (BM) samples from previously treated WM patients using CD19+ microbead selection (Miltenyi Biotec) with more than 90% purity, as confirmed by flow cytometric analysis with monoclonal antibody reactive to human CD20-PE (BD Biosciences). The WM and immunoglobulin M (IgM)–secreting low-grade lymphoma cell lines (BCWM.1, MEC.1, RL) were used in this study.9 Peripheral blood mononuclear cells (PBMCs) were obtained from healthy subjects by Ficoll-Hypaque density sedimentation, and CD19+ selection was performed as described.9 All cells were cultured at 37°C in RPMI-1640 containing 10% fetal bovine serum (Sigma Chemical), 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO). Approval for these studies was obtained from the Dana-Farber Cancer Institute Institutional Review Board. Informed consent was obtained from all patients and healthy volunteers in accordance with the Declaration of Helsinki protocol.
Reagents
ONX0912 (formerly PR-047) and proteasome active site binding protein (PABP) (both provided by Onyx Pharmaceuticals) were diluted in dimethyl sulfoxide, stored at 4°C, and diluted in culture medium immediately before use. The maximum final concentration of dimethyl sulfoxide (< 0.1%) did not affect cell proliferation and did not induce cytotoxicity on the cell lines and primary cells tested (data not shown). Bortezomib was obtained from Hospital Pharmacy. The c-Jun NH2 kinase (JNK) inhibitor SP600215 was purchased from Calbiochem. Salubrinal was purchased from Axxora. The pan-caspase inhibitor Z-VAD-fmk was purchased from Promega. Recombinant interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF-1) were purchased from R&D Systems.
Growth inhibition assay
The inhibitory effect of ONX0912 on the growth of WM cells, IgM-secreting cell lines, and primary cells was assessed by measuring 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International) dye absorbance, as previously described.9
DNA synthesis
DNA synthesis was measured by [3H]-thymidine (Perkin Elmer) uptake, as previously described.9
Flow cytometric analysis
Cell-cycle analysis was profiled by flow cytometry using propidium iodide staining (5 μg/mL; Sigma Chemical) after 24-hour culture with PR-047, as described.9
DNA fragmentation assay
Cell Death Detection enzyme-linked immunosorbent assay (ELISA; Roche Applied Science) was used to quantitate DNA fragmentation per the manufacturer's instructions.
Immunoblotting
WM and IgM-secreting cell lines were harvested and lysed using lysis buffer (Cell Signaling Technology) reconstituted with 5mM NaF, 2mM Na3VO4, 1mM polymethylsulfonyl fluoride, 5 μg/mL leupeptine, and 5 μg/mL aprotinin. Whole-cell lysates (50 μg/lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinyldene fluoride membrane (Bio-Rad Laboratories). The antibodies used for immunoblotting included anti–phospho-Akt (p-Akt; Ser473), -Akt, –phosphorylated extracellular signal-related kinase 1/2 (p-ERK1/2; Thr202/Tyr204), -ERK1/2, –caspase-3, –caspase-8, –caspase-9, -PARP, -β-catenin, –glucose-regulated protein of 94 kDa (-GRP94), –protein kinase-like endoplasmic reticulum kinase (-PERK), –phosphorylated eukaryotic initiation factor 2 (–p-EIF2α), –binding immunoglobulin protein (-BiP), –protein disulfide isomerase (-PDI), –activating transcription factor 6 (-ATF6), –phosphorylated mitogen activated protein kinase kinase 7 (–p-MKK7), –phosphorylated stress-activated protein kinase (p-SAPK)/JNK, -p27kip1, -p21Cip1, –cyclin D1, –cyclin D2, –cyclin E, -nucleolin, –p-p65, -p65, -p105, -p50, -p100, -p52, -RelB, –phosphorylated inhibitor of κB (p-IκB; Cell Signaling Technology); –α-tubulin, and –β-actin antibodies (Santa Cruz Biotechnology).
IL-6 and IGF-1 detection
IL-6 and IGF-1 concentrations were quantified by ELISA (Quantikine human IL-6 ELISA; Quantikine human IGF-1 ELISA; R&D Systems) according to the manufacturer's instructions.
Proteasome constitutive immunosubunit ELISA assay
The proteasome constitutive immunosubunit ELISA was performed as previously described.8 Briefly, human constitutive proteasome (c20S) and immunoproteasome 20S (i20S) subunits (Boston Biochemical); monoclonal antibodies anti-β1, -β2, -LMP7, -LMP2 (BioMol International), -MECL1 (Santa Cruz Biotechnology), and -β5 (Covance custom product); and horseradish peroxidase–conjugated antibodies (Jackson ImmunoResearch Laboratories and Zymed) were used. Baseline expression of each c20S and i20S subunit, and their modulation upon ONX0912 treatment, was tested on cell lysates prepared by incubating cell pellets in TE buffer (20mM tris(hydroxymethyl)aminomethane, pH 8.0, 5mM ethylenediaminetetraacetic acid). Cell lysates were then incubated with PABP (5μM) for 2 hours at 25°C. Samples were denatured with 8M guanidine hydrochloride (Fisher Scientific) and subunits bound to PABP were captured with streptavidin-conjugated sepharose beads (GE Healthcare). Individual subunits were probed with antibodies specific to each subunit. Each subunit was measured as nanograms per microgram of total protein, according to the SuperSignal ELISA Pico Kit.
20S proteasome activity
The chymotrypsin-like activity of the 20S proteasome of primary WM tumor cells was determined by measurement of fluorescence generated from the cleavage of the fluorogenic substrate suc-LLVY-amc, as described.10
NF-κB activity
NF-κB activity was investigated using the Active Motif TransAM kits, a DNA-binding ELISA-based assay (Active Motif North America). Briefly, BCWM.1 cells were treated with ONX0912 (10nM) or bortezomib (10nM) alone or in combination for 4 hours, and stimulated with tumor necrosis factor-α (TNF-α, 10 ng/mL) during the last 20 minutes of culture. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts, following the manufacturer's procedure, as described.10
Effect of PR-047 on paracrine WM cell growth in the BM
To evaluate growth stimulation and signaling in WM cells adherent to bone marrow stromal cells (BMSCs), 3 × 104 BCWM.1 cells were cultured in BMSC-coated 96-well plates for 48 hours in the presence or absence of ONX0912. DNA synthesis was measured as described.9
Statistical analysis
Statistical significance of differences in drug-treated versus control cultures was determined using Student t test. The minimal level of significance was P value less than .05. Drug synergism was analyzed by isobologram analysis using the CalcuSyn software program (Biosoft), as described.9
Results
WM primary cells are characterized by higher expression of the immunoproteasome
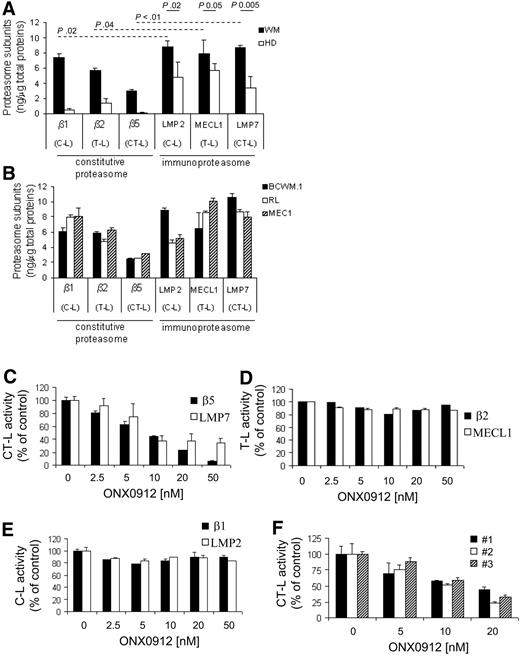
The 20S proteolytic cores of the constitutive proteasome and immunoproteasome have 3 different enzymatic activities: chymotrypsin-like (CT-L), trypsinlike (T-L), and caspaselike (C-L), which are encoded by the β5, β2, and β1 subunits, respectively, and by the LMP7, MECL1, and LMP2 subunits, respectively. We first examined the expression level of each 20S core subunit of the constitutive proteasome and immunoproteasome, in primary bone marrow–derived CD19+ WM cells, WM, and low-grade lymphoma IgM-secreting cell lines. Peripheral blood–derived CD19+ cells were used as normal controls. We found that primary tumor CD19+ bone marrow–derived WM cells have significantly higher level of the immunoproteasome compared with the constitutive proteasome (Figure 1A). Importantly, WM primary cells were characterized by significantly higher proteasome subunit expression compared with their normal cellular counterpart (Figure 1A). Similar results were confirmed in BCWM.1 cells, as well as other low-grade lymphoma IgM-secreting cells, such as MEC.1 and RL (Figure 1B). We next evaluated the activity of ONX0912, a selective CT-L inhibitor, in targeting the CT-L activity in WM cells. Cells were treated with increasing concentrations of ONX0912 (2.5-50nM) for 2 hours, and exhibited significant inhibition of the CT-L subunits of both constitutive proteasome (β5) and immunoproteasome (LMP7) (Figure 1C) in a dose-dependent manner, with minimal inhibition of the trypsin T-L and caspase activities (Figure 1D-E), suggesting that the selectivity of ONX0912 for the CT-L activity of the proteasome together with the weak activity on other protease classes may contribute to a better tolerability in vivo. Importantly, ONX0912-induced inhibition of the CT-L proteasome activity was confirmed in primary CD19+ WM cells (Figure 1F).
Primary WM cells express higher level of the immunoproteasome, and ONX0912 targets the CT-L activity in WM cells. Distribution of the caspaselike (C-L), trypsinlike (T-L), and chymotrypsin-like (CT-L) subunits of the constitutive proteasome (β1, β2, β5) and immunoproteasome (LMP2, MECL1, LMP7) was assessed by ELISA on protein lysates obtained from primary cells (average of 5 WM patients is shown; WM), and from peripheral blood–derived CD19+ cells of healthy subjects (average of 4 healthy donors is shown; HD) (A), BCWM.1 cells, and IgM-secreting low-grade lymphoma cells (RL, MEC.1; B). Anti-β1, -β2, -β5, -LMP2, -MECL1, and -LMP7 primary, and horseradish peroxidase–conjugated secondary antibodies were used. Each subunit was measured as nanograms per microgram of total protein, according to the SuperSignal ELISA Pico Kit manufacturer's instructions. (C-E) BCWM1 cells have been treated with ONX0912 (2.5-50nM) for 2 hours and ONX0912 effects on CT-L, T-L, and C-L activities of the proteasome have been evaluated as described earlier in the legend. (F) Primary CD19+ tumor cells from 3 patients with WM were incubated for 2 hours in the presence of diluent or ONX0912 (5-20nM). The chymotrypsin-like (CT-L) activity of the 20S proteasome of BCWM.1 was determined by measurement of fluorescence generated from the cleavage of the fluorogenic substrate suc-LLVY-amc. ONX0912-induced modulation of CT-L activity has been expressed as fold of untreated samples. In all panels, error bars represent SD.
Primary WM cells express higher level of the immunoproteasome, and ONX0912 targets the CT-L activity in WM cells. Distribution of the caspaselike (C-L), trypsinlike (T-L), and chymotrypsin-like (CT-L) subunits of the constitutive proteasome (β1, β2, β5) and immunoproteasome (LMP2, MECL1, LMP7) was assessed by ELISA on protein lysates obtained from primary cells (average of 5 WM patients is shown; WM), and from peripheral blood–derived CD19+ cells of healthy subjects (average of 4 healthy donors is shown; HD) (A), BCWM.1 cells, and IgM-secreting low-grade lymphoma cells (RL, MEC.1; B). Anti-β1, -β2, -β5, -LMP2, -MECL1, and -LMP7 primary, and horseradish peroxidase–conjugated secondary antibodies were used. Each subunit was measured as nanograms per microgram of total protein, according to the SuperSignal ELISA Pico Kit manufacturer's instructions. (C-E) BCWM1 cells have been treated with ONX0912 (2.5-50nM) for 2 hours and ONX0912 effects on CT-L, T-L, and C-L activities of the proteasome have been evaluated as described earlier in the legend. (F) Primary CD19+ tumor cells from 3 patients with WM were incubated for 2 hours in the presence of diluent or ONX0912 (5-20nM). The chymotrypsin-like (CT-L) activity of the 20S proteasome of BCWM.1 was determined by measurement of fluorescence generated from the cleavage of the fluorogenic substrate suc-LLVY-amc. ONX0912-induced modulation of CT-L activity has been expressed as fold of untreated samples. In all panels, error bars represent SD.
ONX0912 exerts antitumor activity in WM cells and other IgM-secreting low-grade lymphoma cells
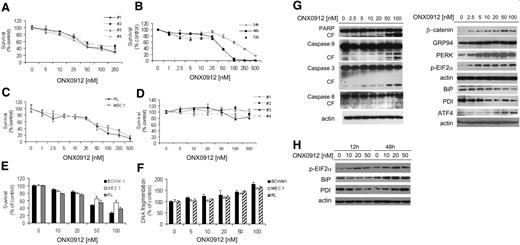
The efficacy of ONX0912-dependent proteasome inhibition in targeting clonal IgM-secreting cells was tested in primary WM CD19+ cells, normal PBMC-derived CD19+ cells, and WM and IgM low-grade lymphoma cell lines (BCWM, RL, MEC.1). We first evaluated the cytotoxic effect of ONX0912 (5-250nM) on primary WM bone marrow–derived CD19+ cells by MTT assay, and found that ONX0912 induced cytotoxicity in a dose-dependent manner (median inhibitory concentration [IC50]: 50-100nM; Figure 2A). Similar results were confirmed on WM and IgM-secreting low-grade lymphoma cell lines, where ONX0912 induced a dose-dependent cytotoxicity (IC50: 50nM, at 48 hours; Figure 2B-C). In contrast, ONX0912 did not exert cytotoxicity on normal PBMC-derived CD19+ cells isolated from 4 healthy volunteers (Figure 2D). We have previously shown that ONX0912-dependent inhibition of CT-L activity occurs after 2 hours, whereas induction of cytotoxicity has been observed at 24 hours, with an increasing effect at 48 and 72 hours. Given the irreversible nature of the proteasome inhibition exerted by ONX0912 in WM and IgM-secreting low-grade lymphoma cell lines, this could potentially indicate that cell death is either a delayed effect of inhibited CT-L activity, or that it depends on other deferred mechanisms. We therefore performed a washout experiment, where cells where treated with ONX0912 for 2 hours, and subsequently washed and replaced with fresh medium in absence or ONX0912. We found that there is induction of cytotoxicity, indicating that ONX0912-dependent cytotoxicity may result from both CT-L proteasome inhibition and delayed effects due to other ONX0912-induced mechanisms (Figure 2E).
ONX0912 exerts cytotoxicity on primary WM cells as well as on IgM-secreting low-grade lymphoma cells. Cytotoxicity was assessed by MTT assay in primary WM bone marrow–derived CD19+ cells (A; ONX0912 5-250nM; 48 hours); BCWM.1 cells (B; ONX0912 1-500nM; 24-48-72 hours); IgM-secreting low-grade lymphoma cells RL, MEC.1 (C; ONX0912 1-500nM; 48 hours); and freshly isolated primary peripheral blood–derived CD19+ cells from 4 healthy donors (D; ONX0912 5-500nM; 48 hours). (E) BCWM.1, RL, and MEC.1 were cultured with ONX0912 (10-100nM) for 2 hours, followed by a 48-hour washout period. Cytotoxicity was assessed by MTT assay at 48 hours. (F) BCWM.1, RL, and MEC.1 were cultured with ONX0912 (10-100nM) for 48 hours and percentage of cells undergoing apoptosis was assessed by DNA fragmentation. (G) BCWM.1 cells were cultured with ONX0912 (2.5-100nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti-PARP, –caspase-9, –caspase-3, –caspase-8, –β-catenin, -GRP94, -PERK, –phosphorylated EIF2α (p-EIF2α), -BiP, -PDI, -ATF, and –β-actin antibodies. (H) BCWM.1 cells were cultured with ONX0912 (10-50nM) for 12 and 48 hours. Whole-cell lysates were subjected to Western blotting using anti–phosphorylated EIF2α (p-EIF2α), -BiP, -PDI, and –β-actin antibodies. In all panels, error bars represent SD.
ONX0912 exerts cytotoxicity on primary WM cells as well as on IgM-secreting low-grade lymphoma cells. Cytotoxicity was assessed by MTT assay in primary WM bone marrow–derived CD19+ cells (A; ONX0912 5-250nM; 48 hours); BCWM.1 cells (B; ONX0912 1-500nM; 24-48-72 hours); IgM-secreting low-grade lymphoma cells RL, MEC.1 (C; ONX0912 1-500nM; 48 hours); and freshly isolated primary peripheral blood–derived CD19+ cells from 4 healthy donors (D; ONX0912 5-500nM; 48 hours). (E) BCWM.1, RL, and MEC.1 were cultured with ONX0912 (10-100nM) for 2 hours, followed by a 48-hour washout period. Cytotoxicity was assessed by MTT assay at 48 hours. (F) BCWM.1, RL, and MEC.1 were cultured with ONX0912 (10-100nM) for 48 hours and percentage of cells undergoing apoptosis was assessed by DNA fragmentation. (G) BCWM.1 cells were cultured with ONX0912 (2.5-100nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti-PARP, –caspase-9, –caspase-3, –caspase-8, –β-catenin, -GRP94, -PERK, –phosphorylated EIF2α (p-EIF2α), -BiP, -PDI, -ATF, and –β-actin antibodies. (H) BCWM.1 cells were cultured with ONX0912 (10-50nM) for 12 and 48 hours. Whole-cell lysates were subjected to Western blotting using anti–phosphorylated EIF2α (p-EIF2α), -BiP, -PDI, and –β-actin antibodies. In all panels, error bars represent SD.
We next demonstrated that ONX0912 induced apoptosis in a dose-dependent manner, as assessed by DNA fragmentation (Figure 2F). Similar effects were obtained on other IgM-secreting low-grade lymphoma cell lines (RL, MEC.1; Figure 2F). We also examined the molecular mechanisms whereby ONX0912 induces cytotoxicity in WM, and demonstrated that ONX0912 induced caspase-8, -9, and -3 and PARP cleavage in a dose-dependent manner (Figure 2G). It is known that proteasome inhibition eradicates tumor cells, partly by initiating the unfolded protein response (UPR), a signaling cascade activated by the accumulation of misfolded proteins in the endoplasmic reticulum (ER).11 Previous reports indicate that induction of ER stress in WM cells may represent a valid therapeutic option in WM.12 We therefore sought to investigate the effect of ONX0912 in modulating the expression of unfolded protein response (UPR) components in WM cells as one of the mechanisms of cytotoxicity in WM cells. We found that ONX0912 induced accumulation of β-catenin, consistent with a previous report.13 In addition, ONX0912 induced up-regulation of UPR components such as GRP94 and PERK, followed by PERK-dependent phosphorylation of EIF-2α. Consistent with terminal UPR induction by ONX0912, ATF4 protein level was increased in WM ONX0912-treated cells.
We observed a ONX0912-dependent down-modulation of PDI and BiP, at 12 hours (Figure 2G), and hypothesized that early exposure time (12 hours) induces down-modulation of BiP and PDI, leading to reduced cell survival and induction of apoptosis in the treated cells; however, longer exposure (48 hours) could result in up-regulation of BiP and PDI due to induction of UPR. We therefore treated cells with ONX0912 (10-50nM) for 12 and 48 hours, and cell lysates were subjected to Western blot. We found down-modulation and up-regulation of PDI and BiP, at 12 and 48 hours, respectively. In parallel, p-EIF2α protein expression increases upon ONX0912 treatment, at either 12 or 48 hours (Figure 2H). These findings suggest that ONX0912 first induces down-modulation of BiP/PDI, resulting in reduced cell survival and induction of apoptosis, which may be independent of pEIF2α modulation, whereas at longer treatment exposure, ONX0912 induces UPR, as demonstrated by increased pEIF2α, together with up-regulation of BiP and PDI.
To better delineate the role played by caspases and ER stress in ONX0912-indeuced cytotoxicity, WM cells were exposed to the pan-caspase inhibitor Z-VAD-fmk (25-50μM), or the ER stress–induced apoptosis protector salubrinal (5-10μM), in the presence or absence of ONX0912 (25-50-100nM). We found that Z-VAD-fmk did not totally overcome ONX0912-induced cytotoxicity (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Similar results were obtained in presence of salubrinal (supplemental Figure 1B). We next tested the protective effect of Z-VAD and salubrinal in ONX0912-treated cells and found that this combination does not completely rescue cells from ONX0912-induced cell toxicity (supplemental Figure 1C), indicating that ONX0912 triggers apoptosis also through other mechanisms different from caspase activation or ER stress modulation.
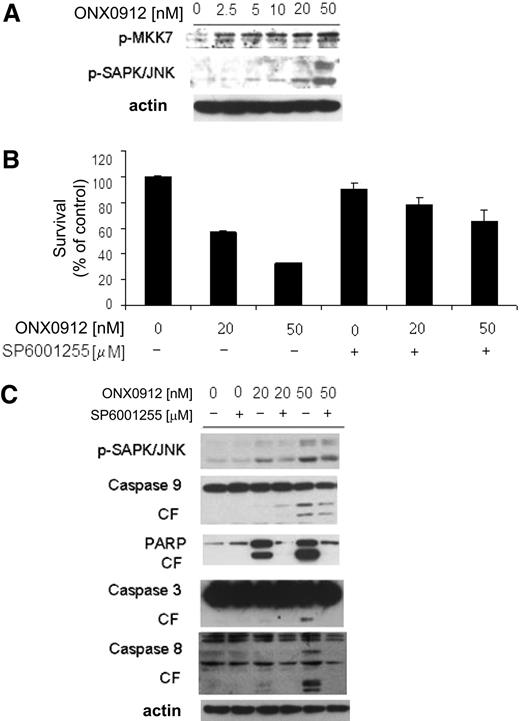
We therefore sought to determine other mechanisms as regulators of ONX0912-induced cytotoxicity. We showed that ONX0912 treatment also triggered MKK7-induced c-Jun N-terminal kinase (JNK) activation in WM cells, as shown by up-regulation of phosphorylated MKK7 (p-MKK7) and p-JNK1/2 (Figure 3A). To better define the role of JNK activity in mediating ONX0912-induced WM cytotoxicity, WM cells were treated with ONX0912 in presence or absence of the JNK inhibitor SP600125. ONX0912 (25-50nM)–induced cytotoxicity was inhibited upon SP600125 treatment (Figure 3B), together with an inhibition of ONX0912-dependent caspases-3, -8, and -9 and PARP cleavage (Figure 3C).
ONX0912-induced apoptosis is partially mediated by activation of JNK. (A) BCWM.1 cells were cultured with ONX0912 (2.5-50nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–p-MKK7, –p-SAP/JNK, and -actin antibodies. (B) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) in presence or absence of the JNK inhibitor SP600125 (10μM) and cytotoxicity was assessed by MTT assay. (C) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM), in presence or absence of SP600125 (10μM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–p-SAPK/JNK, -PARP, –caspase-9, –caspase-3, –caspase-8, and –β-actin. In all panels, error bars represent SD.
ONX0912-induced apoptosis is partially mediated by activation of JNK. (A) BCWM.1 cells were cultured with ONX0912 (2.5-50nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–p-MKK7, –p-SAP/JNK, and -actin antibodies. (B) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) in presence or absence of the JNK inhibitor SP600125 (10μM) and cytotoxicity was assessed by MTT assay. (C) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM), in presence or absence of SP600125 (10μM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–p-SAPK/JNK, -PARP, –caspase-9, –caspase-3, –caspase-8, and –β-actin. In all panels, error bars represent SD.
ONX0912 inhibits proliferation in WM and IgM-secreting low-grade lymphoma cells
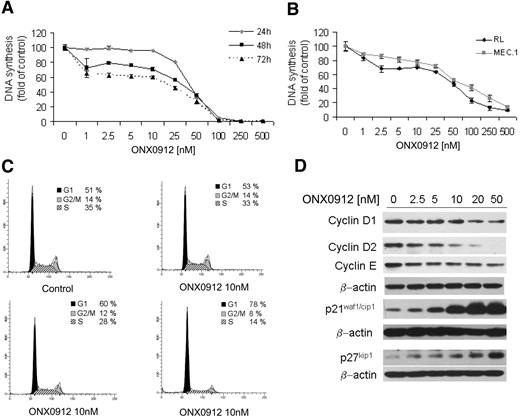
We next examined the effect of ONX0912 on reducing WM cell proliferation by targeting cell cycle profiling. WM and IgM-secreting cell lines were cultured for 24, 48, or 72 hours in the presence of ONX0912 (1-500nM). ONX0912 inhibited BCWM.1 proliferation in a dose-dependent manner, as measured by [3H]-thymidine uptake assay, with an IC50 of 37.5nM at 48 hours (Figure 4A). ONX0912 demonstrated similar activity on all cell lines tested, with IC50 of 50nM at 48 hours (Figure 4B). The effect of ONX0912 in modulating cell-cycle progression was evaluated using propidium iodide staining and flow cytometric analysis in WM cells cultured in absence or presence of ONX0912 (10, 20, 50nM). We found that ONX0912, in a dose-dependent manner, induced G1 cell-cycle arrest and a concomitant reduction of cells in S phase. Specifically, G1-phase cells increased from 51% in the untreated setting to 53%, 60%, and 78% in those cells exposed to ONX0912 10, 20, and 50nM, respectively. Similarly, S-phase cells decreased from 35% in the control to 33%, 28%, and 14% in those cells exposed to ONX0912 10, 20, and 50nM, respectively (Figure 4C). ONX0912-induced G1/S-phase transition arrest was supported by the down-regulation of positive cell-cycle regulators, such as cyclin D1, cyclin D2, cyclin E, and by the up-regulation of negative cell-cycle regulators, such as p21waf1/cip1 and p27kip1 (Figure 4D).
ONX0912 exerts antiproliferative effects on primary WM cells as well as on IgM-secreting low-grade lymphoma cells. (A-B) DNA synthesis was measured by thymidine uptake assay in BCWM.1 (A) and IgM-secreting cell lines, RL and MEC.1 (B), and treated with ONX0912 (1-500nM) for 24, 48, and 72 hours (A) or for 48 hours (B). (C) BCWM.1 cells were treated with ONX0912 (10-50nM) for 24 hours, and cell-cycle profiling was performed by propidium iodide staining and flow cytometric analysis. (D) BCWM.1 cells were cultured with ONX0912 (10-20-50nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–cyclin D1, –cyclin D2, –cyclin E, -p21waf1/cip1, -p27kip1, and –β-actin.
ONX0912 exerts antiproliferative effects on primary WM cells as well as on IgM-secreting low-grade lymphoma cells. (A-B) DNA synthesis was measured by thymidine uptake assay in BCWM.1 (A) and IgM-secreting cell lines, RL and MEC.1 (B), and treated with ONX0912 (1-500nM) for 24, 48, and 72 hours (A) or for 48 hours (B). (C) BCWM.1 cells were treated with ONX0912 (10-50nM) for 24 hours, and cell-cycle profiling was performed by propidium iodide staining and flow cytometric analysis. (D) BCWM.1 cells were cultured with ONX0912 (10-20-50nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti–cyclin D1, –cyclin D2, –cyclin E, -p21waf1/cip1, -p27kip1, and –β-actin.
ONX0912 inhibits the canonical and noncanonical NF-κB pathways
NF-κB pathway plays a pivotal role in regulating growth and survival of plasma cell malignancies, and inhibition of NF-κB represents one of the mechanisms of action for proteasome inhibitors.14 We therefore sought to investigate whether ONX0912 could target this pathway.
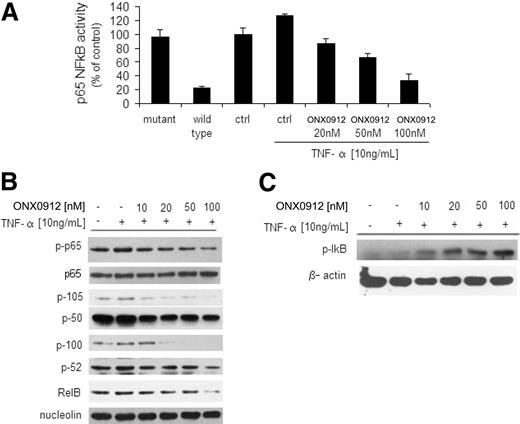
We first investigated the effect of ONX0912 on the p65NF-κB DNA binding activity, studying nuclear extracts from treated cells using the Active Motif assay. We showed that TNF-α treatment induced NF-κB recruitment to the nucleus in BCWM.1 cells, which was inhibited by ONX0912 in a dose-dependent manner (Figure 5A). Moreover, immunoblotting from nuclear extracts demonstrated that p65 phosphorylation and p50/p105NF-κB expression were inhibited by ONX0912 (Figure 5B). We next examined whether ONX0912 could target the noncanonical NF-κB pathway. Immunoblotting of nuclear extracts showed that ONX0912 inhibited the expression of p100/p52 and RelB, which are mostly activated through the noncanonical pathway (Figure 5B).15 We next investigated the role of ONX0912 on the expression of NF-κB negative regulator IκB in the cytoplasmic compartment, and found that ONX0912 up-regulated its expression (Figure 5C). Taken together, these data demonstrate that ONX0912 regulates both canonical and noncanonical pathways of NF-κB in WM cells.
ONX0912 leads to inhibition of the canonical and noncanonical NF-κB pathway. (A) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means ± SD of triplicate experiments. (B) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, -p65, -p105, -p50, -p100, -p52, -RelB, and -nucleolin antibodies. (C) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and TNF-α (100 ng/mL) was added for the last 20 minutes. Cytoplasmic extracts were subjected to Western blotting using anti–p-IκB and -actin antibodies.
ONX0912 leads to inhibition of the canonical and noncanonical NF-κB pathway. (A) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means ± SD of triplicate experiments. (B) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, -p65, -p105, -p50, -p100, -p52, -RelB, and -nucleolin antibodies. (C) BCWM.1 cells were cultured with ONX0912 (0-100nM) for 4 hours, and TNF-α (100 ng/mL) was added for the last 20 minutes. Cytoplasmic extracts were subjected to Western blotting using anti–p-IκB and -actin antibodies.
Effect of ONX0912 and bortezomib in inducing WM cell cytotoxicity
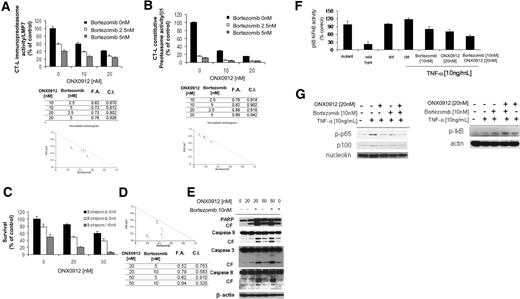
ONX0912 exerts a different inhibitory profile on proteasome activities compared with bortezomib.16 Previous reports indicate that bortezomib targets mainly the CT-L activity and to a lesser degree the C-L activity,17 whereas ONX0912 affects mainly the CT-L,16 but the effect of ONX0912 and bortezomib in inhibiting the immunoproteasome and the constitutive proteasome activities has not been evaluated. Moreover, previous reports demonstrate synergism between different classes of proteasome inhibitors.17 We therefore sought to determine the effect of ONX0912 and bortezomib, used either as single agents or in combination, in targeting the CT-L activity of both constitutive proteasome and immunoproteasome. WM cells were treated with ONX0912 (10-20nM), bortezomib (2.5-5nM), alone or combination, for 2 hours. ONX0912 showed a significant increase in inhibiting CT-L activity of both the i20S (LMP7; Figure 6A) and the c20S (β5; Figure 6B) when combined with bortezomib in WM cells. Specifically, ONX0912 (10nM) induced inhibition of the LMP7 activity in 40% of the treated cells, which was increased to 62% and 73% in the presence of bortezomib at 2.5nM (combination index [CI], 0.870) and 5nM (CI, 0.812), respectively, indicating additive effect (Figure 6A). Similar effect was also observed using ONX0912 and bortezomib in targeting the β5 activity (Figure 6B).
Mechanisms whereby ONX0912/bortezomib combination enhances WM cell cytotoxicity. (A-B) BCWM.1 cells were treated with ONX0912 (10nM, 20nM) in presence or absence of bortezomib (2.5nM, 5nM) for 2 hours, and effects on chymotrypsin-like activity (CT-L) of the immunoproteasome (LMP7; A) and constitutive proteasome (β5; B) were evaluated by ELISA on protein lysates. Proteasome activity is expressed as fold of control (untreated cells). CalcuSyn software was used to determine presence or absence of synergism between ONX0912 and bortezomib in targeting the CT-L enzymatic activities. Combination indices (CIs) and fractions affected (FAs) of the combination of ONX0912 and bortezomib and isobolograms are shown below each panel. All experiments were repeated in triplicate. (C) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) for 48 hours, in the presence or absence of bortezomib (5nM, 10nM). Cytotoxicity was assessed by MTT assay. (D) Representative isobologram of ONX0912 and bortezomib, with the CalcuSyn software demonstrating synergy for the combination. Combination indices (CIs) and fractions affected (FAs) of the combinations of ONX0912 and bortezomib are shown. All experiments were repeated in triplicate. (E) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) in the presence or absence of bortezomib (10nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti-PARP, –caspase-9, –caspase-3, –caspase-8, and –β-actin antibodies. (F) BCWM.1 cells were cultured with either ONX0912 (20nM), bortezomib (10nM), or the combination for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means ± SD of triplicate experiments. (G) BCWM.1 cells were cultured with either ONX0912 (20nM), bortezomib (10nM), or the combination for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Cytoplasmic and nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, –NF-κBp100, -nucleolin, –p-IκB, and –α-tubulin antibodies.
Mechanisms whereby ONX0912/bortezomib combination enhances WM cell cytotoxicity. (A-B) BCWM.1 cells were treated with ONX0912 (10nM, 20nM) in presence or absence of bortezomib (2.5nM, 5nM) for 2 hours, and effects on chymotrypsin-like activity (CT-L) of the immunoproteasome (LMP7; A) and constitutive proteasome (β5; B) were evaluated by ELISA on protein lysates. Proteasome activity is expressed as fold of control (untreated cells). CalcuSyn software was used to determine presence or absence of synergism between ONX0912 and bortezomib in targeting the CT-L enzymatic activities. Combination indices (CIs) and fractions affected (FAs) of the combination of ONX0912 and bortezomib and isobolograms are shown below each panel. All experiments were repeated in triplicate. (C) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) for 48 hours, in the presence or absence of bortezomib (5nM, 10nM). Cytotoxicity was assessed by MTT assay. (D) Representative isobologram of ONX0912 and bortezomib, with the CalcuSyn software demonstrating synergy for the combination. Combination indices (CIs) and fractions affected (FAs) of the combinations of ONX0912 and bortezomib are shown. All experiments were repeated in triplicate. (E) BCWM.1 cells were cultured with ONX0912 (20nM, 50nM) in the presence or absence of bortezomib (10nM) for 12 hours. Whole-cell lysates were subjected to Western blotting using anti-PARP, –caspase-9, –caspase-3, –caspase-8, and –β-actin antibodies. (F) BCWM.1 cells were cultured with either ONX0912 (20nM), bortezomib (10nM), or the combination for 4 hours, and then TNF-α (10 ng/mL) was added for the last 20 minutes. NF-κBp65 transcription factor binding to its consensus sequence on the plate-bound oligonucleotide was studied from nuclear extracts. Wild-type and mutant are wild-type and mutated consensus competitor oligonucleotides, respectively. All results represent means ± SD of triplicate experiments. (G) BCWM.1 cells were cultured with either ONX0912 (20nM), bortezomib (10nM), or the combination for 4 hours, and TNF-α (10 ng/mL) was added for the last 20 minutes. Cytoplasmic and nuclear extracts were subjected to Western blotting using anti–p-NF-κBp65, –NF-κBp100, -nucleolin, –p-IκB, and –α-tubulin antibodies.
We next investigated whether the ONX0912/bortezomib-dependent effect on targeting the CT-L activity could lead to either additive or synergistic induction of cytotoxicity on WM cells. BCWM.1 cells were cultured with ONX0912 (20-50nM) for 48 hours, in the presence or absence of bortezomib (5-10nM). ONX0912 showed significant cytotoxic effects when combined with bortezomib, as demonstrated using MTT assays at 48 hours (Figure 6C). ONX0912 (20nM) induced cytotoxicity in 15.4% of BCWM.1 cells, which was increased to 52.1% and 78.8% in the presence of bortezomib at 5nM (CI, 0.75) and 10nM (CI, 0.58), respectively, indicating additive and synergistic effect, respectively. Similar results were observed when ONX0912 50nM was tested in presence of bortezomib 5nM and 10nM, with CIs of 0.91 and 0.32, respectively. Isobologram analysis, fractions affected, and the combination indexes for each of these combinations are summarized in Figure 6D. To better define the mechanisms of combined ONX0912 plus bortezomib-induced WM cytotoxicity, we investigated the effect of ONX0912 (20-50nM), either alone or in combination with bortezomib 10nM, using immunoblotting after 12 hours of treatment. Interestingly, we demonstrated that PARP and caspase-9, -3, and -8 cleavage were significantly higher using the combination compared with each agent alone (Figure 6E).
Finally, we sought to investigate whether the combination of the 2 proteasome inhibitors would lead to synergistic modulation of NF-κB pathway. We first investigated the effect of ONX0912, either alone or in combination with bortezomib, on the NF-κBp65 DNA binding activity, studying nuclear extracts from treated cells using the Active Motif assay. We showed that TNF-α treatment induced NF-κB recruitment to the nucleus in BCWM.1 cells, which was inhibited to a greater extent by ONX0912 than bortezomib, and more significantly by the combination of the 2 proteasome inhibitors (Figure 6F). Moreover, immunoblotting from nuclear extracts demonstrated that p65 phosphorylation was inhibited by ONX0912, either alone or in combination with bortezomib, more than by bortezomib as single agent (Figure 6G). Next, proteins isolated from the cytoplasmic compartment were examined, and we found that the NF-κB inhibitory protein IκB was more significantly increased upon ONX0912 plus bortezomib than single-agent exposure (Figure 6G).
ONX0912 targets WM cells in the context of bone marrow milieu
Because the BM microenvironment confers growth and induces drug resistance in malignant cells,18 we investigated whether ONX0912 inhibits WM cell growth in the context of the BM milieu. BCWM.1 cells were cultured with ONX0912 (2.5-50nM) in the presence or absence of BMSCs for 48 hours. The viability of BMSCs assessed by MTT was not affected by ONX0912 treatment (data not shown). Using [3H]-thymidine uptake assay, adherence of BCWM.1 cells to BMSCs triggered a 37% increase in proliferation, which was inhibited by ONX0912 in a dose-dependent manner (Figure 7A).
Adherence to primary BMSCs does not protect against ONX0912-induced cytotoxicity. (A) BCWM.1 cells were cultured with control media and with ONX0912 (2.5-100nM), for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiments. (B-C) Primary WM bone marrow stromal cells were cultured with increasing doses of ONX0912 (10-100nM) for 8 hours. Conditioned media were collected and IL-6 (B) and IGF-1 (C) concentrations were measured by IL-6 (A) and IGF-1 (C) ELISA assays. (D) BCWM.1 cells were cultured with control media or ONX0912 (10-100nM) for 8 hours in presence or absence of primary BMSCs. Whole-cell lysates from WM cells were subjected to Western blotting using anti–p-AKT, -AKT, –p-ERK, -ERK, and –α-tubulin antibodies. (E-F) Primary WM bone marrow stromal cells were cultured with ONX0912 (20-50nM), neutralizing antibody anti–IL-6 (anti–IL-6: 0.15μg/mL), and neutralizing antibody anti–IGF-1 (anti–IGF-1: 5 μg/mL) for 8 hours. Conditioned media were collected; IL-6 (E) and IGF-1 (F) concentrations were measured by IL-6 (E) and IGF-1 (F) ELISA assays. (G) BCWM.1 cells and primary WM BMSCs were cultured either alone or in a coculture system, in presence of primary WM BMSCs, with ONX0912 (20-50nM) and anti–IL-6 (0.15 μg/mL), anti–IGF-1 (5 μg/mL), or exogenous recombinant IL6 (25 ng/mL) or IGF1 (25 ng/mL) for 48 hours. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean ± SD of triplicate experiment.
Adherence to primary BMSCs does not protect against ONX0912-induced cytotoxicity. (A) BCWM.1 cells were cultured with control media and with ONX0912 (2.5-100nM), for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiments. (B-C) Primary WM bone marrow stromal cells were cultured with increasing doses of ONX0912 (10-100nM) for 8 hours. Conditioned media were collected and IL-6 (B) and IGF-1 (C) concentrations were measured by IL-6 (A) and IGF-1 (C) ELISA assays. (D) BCWM.1 cells were cultured with control media or ONX0912 (10-100nM) for 8 hours in presence or absence of primary BMSCs. Whole-cell lysates from WM cells were subjected to Western blotting using anti–p-AKT, -AKT, –p-ERK, -ERK, and –α-tubulin antibodies. (E-F) Primary WM bone marrow stromal cells were cultured with ONX0912 (20-50nM), neutralizing antibody anti–IL-6 (anti–IL-6: 0.15μg/mL), and neutralizing antibody anti–IGF-1 (anti–IGF-1: 5 μg/mL) for 8 hours. Conditioned media were collected; IL-6 (E) and IGF-1 (F) concentrations were measured by IL-6 (E) and IGF-1 (F) ELISA assays. (G) BCWM.1 cells and primary WM BMSCs were cultured either alone or in a coculture system, in presence of primary WM BMSCs, with ONX0912 (20-50nM) and anti–IL-6 (0.15 μg/mL), anti–IGF-1 (5 μg/mL), or exogenous recombinant IL6 (25 ng/mL) or IGF1 (25 ng/mL) for 48 hours. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean ± SD of triplicate experiment.
The phosphoinositide-3 kinase (PI3K)/Akt pathway is implicated in promoting growth and survival of tumor B cells, including WM.19,20 We therefore examined the effect of ONX0912 on Akt activation in WM cells. Previous reports have demonstrated that bortezomib induced up-regulation of p-Akt in MM and WM cells10,21 ; similarly, ONX0912 up-regulated p-Akt and p-ERK in treated cells (data not shown). Recent reports have indicated that inhibition of the chymotrypsin-like activity of the immunoproteasome (LMP7) inhibits cytokine release.22 We therefore tested the activity of ONX0912 in modulating p-Akt and p-ERK signaling pathways in WM cells in the context of BM milieu, by determining the effect of ONX0912 in IL-6 and IGF-1 secretion from primary BMSCs and found that ONX0912 reduced secretion of both IL-6 and IGF-1 from the BM milieu in a dose-dependent manner (Figure 7B-C). Because IL-6 and IGF-1 are known to induce Akt and ERK phosphorylation,23 we next investigated whether ONX0912 could target p-Akt and p-ERK in WM cells in the context of BM microenvironment, as a result of ONX0912-reduced IL-6 and IGF-1 secretion from the BM milieu. BCWM.1 were treated with increasing doses of ONX0912 (2.5-50nM) for 6 hours, in presence or absence of primary BMSCs. The adherence of BCWM.1 to BMSCs, however, induced Akt and ERK phosphorylation in BCWM.1 cells, which was inhibited by ONX0912 in a dose-dependent manner (Figure 7D). These data indicate that ONX0912 may trigger significant antitumor activity against WM cells, even in the presence of the BM milieu.
We next measured the efficacy of ONX0912 in inhibiting IL-6 and IGF-1 secretion from primary WM BMSCs, using neutralizing IL-6 and IGF-1 antibodies as controls. We demonstrated that ONX0912-induced inhibition of IL-6 and IGF-1 in BMSC-conditioned medium was comparable with the effect obtained using anti–IL-6– and anti–IGF-1–neutralizing antibodies (Figure 7E-F). We next sought to test the efficacy of ONX0912 in overcoming BMSC-induced growth, compared with the effect obtained upon anti–IL-6– and anti–IGF-1–neutralizing antibodies, and found that ONX0912 inhibited BMSC-induced WM cell growth as effectively as using anti–IL-6– and anti–IGF-1–neutralizing antibodies (Figure 7G), suggesting that ONX0912 may trigger significant antitumor activity against WM cells in the presence of the BM milieu due to its inhibitory effect on IL-6 and IGF-1 secretion from the BM microenvironment. Moreover, addition of exogenous IL-6 and IGF-1 partially blocked ONX0912-depenent cell growth inhibition (Figure 7G). Absence of anti–IL-6– and anti–IGF-1–induced cytotoxicity on BMSCs was observed (data not shown).
Discussion
WM is characterized by the presence of lymphoplasmacytic cells in the bone marrow (BM) and the secretion of IgM monoclonal protein in the serum, indicating that WM cells present a high protein turnover.24,25 Protein metabolism is a tightly regulated process, and inhibition of its turnover may lead to apoptosis in malignant cells, such as with proteasome inhibitors.2,26
One of the most extensively studied proteasome inhibitors is bortezomib (Millennium Inc). Bortezomib reversibly inhibits the ubiquitin-26S proteasome pathway, which regulates the turnover of a vast number of intracellular proteins, and has become an exciting target in a variety of malignancies, most notably multiple myeloma.27 The proper functioning of this system is crucial for cell cycle regulation, gene transcription, and signal transduction. Based on its activity in multiple myeloma, single-agent bortezomib was tested in WM in phase 2 trials and achieved 40% to 80% responses.28
Nevertheless, a significant number of patients develop resistance to therapy or have neurologic toxicity due to its inhibition of nonproteasome targets28 ; therefore, preclinical evaluation of new proteasome inhibitors is needed to improve patient outcome. Subsequently, a new irreversible, parenterally administered, peptide epoxyketone proteasome inhibitor, carfilzomib, has been developed. Its antitumor activity has been demonstrated in vitro in MM,29 and it has shown promising activity in a phase 2 clinical trial in patients with relapsed refractory MM.30 Recently, ONX0912, a new orally bioavailable analog of carfilzomib with a selective inhibitory effect of the CT-L activity of both immunoproteasome and constitutive proteasome, has been developed to improve dosing flexibility and patient convenience over intravenously administered agents.16 Importantly, it has been recently demonstrated that a selective and specific dual inhibition of the CT-L activity of the i20S (LMP7) and c20S (β5) represents a sufficient and successful strategy to induce antineoplastic effect in hematologic tumors, as shown by using carfilzomib in T-cell leukemia, Burkitt lymphoma, and multiple myeloma, without causing cytotoxicity in nontransformed cells.8 In contrast, inhibition of all the proteasome enzymatic activities induces cytotoxicity in nontransformed cells, which may be responsible for a significant induction of peripheral neuropathy as well as of any other toxicity. Therefore new proteasome inhibitors with a selected CT-L inhibitory activity, such as ONX0912, have been developed.16 Preclinical pharmacology and in vitro characterization of ONX0912 have demonstrated a favorable toxicologic profile and an irreversible dose-dependent inhibition of the CT-L activity of the 20S and 20Si, with more than 80% proteasome inhibition in most tissues upon ONX0912 doses 4- to 10-fold below the maximum tolerated dose.16
In the present studies, we first characterized the distribution of the i20S and c20S subunits in WM primary cells and in their normal cellular counterpart; primary cells express a significantly higher level of i20S subunits compared with c20S, and expression level of i20S and c20S components is significantly higher than in normal cells. We next evaluated for the first time the antitumor activity of ONX0912 in WM; it inhibits the CT-L activity of both i20S (LMP7) and c20S (β5), which are both significantly higher in WM cells compared with normal cells, and resulted in inhibition of proliferation and induction of cytotoxicity in WM cells by inhibiting cell-cycle progression and inducing apoptosis in a caspase-dependent and -independent manner, as evidenced by activation of c-Jun N-terminal kinase, inhibition of NF-κB, and initiation of the unfolded protein response. Unfolded protein response (UPR) represents an adaptive mechanism that cells adopt under physiologic conditions, which leads to accumulation and activation of misfolded proteins in the endoplasmic reticulum (ER), thus resulting in cell survival.11 In contrast, prolonged ER stress, as occurs with proteasome inhibition,31,32 may override the prosurvival mechanisms of the initiated UPR, leading to apoptosis. In the present study, we found that ONX0912 induced down-modulation of BiP and PDI, leading to reduced cell survival and induction of apoptosis in the treated cells; however, longer exposure could exert a protective effect in treated cells. This could potentially represent a mechanism of resistance to ONX0912 treatment; nevertheless, we observed that ONX0912 induced up-regulation of p-eIF2α even at a late time point. This could possibly represent a way of survival in these cells due to attenuation of p-eIF2α, as recently reported.33
It has been previously reported that bortezomib-induced proteasome inhibition results in up-regulation of AKT phosphorylation.10,21 Importantly, we found that ONX0912 inhibited bone marrow stromal cell–induced phosphorylation of AKT and ERK in WM cells, indicating that ONX0912 may trigger significant antitumor activity against WM cells, even in the presence of the BM milieu, by reducing the BM paracrine growth of WM cells. Because we observed that ONX0912 does not target p-Akt and p-ERK in WM cells in the absence of bone marrow stromal cells, we hypothesize that the efficacy of ONX0912 in reducing BMSC-induced up-regulation of Akt and ERK signaling cascades in WM cells may result from the inhibition of BMSC-derived cytokines such as IL-6 and IGF-1, which are known to be activators of both PI3K/Akt and MAPK/ERK signaling pathways.22
These preclinical findings demonstrate that ONX0912 targets WM cells, due to its anti–CT-L activity of both immunoproteasome and constitutive proteasome, providing the framework for testing this novel irreversible CT-L inhibitor in this disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Jennifer Stedman for reviewing the paper.
This study was supported in part by R21 1R21CA126119-01 and the International Waldenström Macroglobulinemia Foundation (IWMF). This work was supported by the Michelle and Steven Kirsch laboratory for Waldenström and the Heje fellowship for Waldenström.
Authorship
Contribution: A.M.R. and I.M.G. designed the research; A.M.R., K.C.A., and I.M.G. wrote the paper; A.M.R., A.S., M.A., H.T.N., F.A., A.K.A., P.Q., P.M., and J.R. performed research; and A.M.R., M.A., S.D., and I.M.G. analyzed the data.
Conflict-of-interest disclosure: M.A. and S.D. are employed by Onyx Pharmaceuticals. K.C.A. is a member of the Speakers Bureau and has received honoraria and research funding from Millennium, Celgene, and Novartis. I.M.G. is a member of the Speakers Bureau and has received honoraria from Millennium, Celgene, and Novartis, is on the Ad Board for Celgene, and has received research funding from Millennium. A.M.R., A.S., H.T.N., A.K.A., F.A., P.Q., P.M., and J.R. declare no competing financial interests.
Correspondence: Irene M. Ghobrial, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 548A, Boston, MA, 02115; e-mail: irene_ghobrial@dfci.harvard.edu.

![Figure 7. Adherence to primary BMSCs does not protect against ONX0912-induced cytotoxicity. (A) BCWM.1 cells were cultured with control media and with ONX0912 (2.5-100nM), for 48 hours, in the presence or absence of BMSCs. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean (± SD) of triplicate experiments. (B-C) Primary WM bone marrow stromal cells were cultured with increasing doses of ONX0912 (10-100nM) for 8 hours. Conditioned media were collected and IL-6 (B) and IGF-1 (C) concentrations were measured by IL-6 (A) and IGF-1 (C) ELISA assays. (D) BCWM.1 cells were cultured with control media or ONX0912 (10-100nM) for 8 hours in presence or absence of primary BMSCs. Whole-cell lysates from WM cells were subjected to Western blotting using anti–p-AKT, -AKT, –p-ERK, -ERK, and –α-tubulin antibodies. (E-F) Primary WM bone marrow stromal cells were cultured with ONX0912 (20-50nM), neutralizing antibody anti–IL-6 (anti–IL-6: 0.15μg/mL), and neutralizing antibody anti–IGF-1 (anti–IGF-1: 5 μg/mL) for 8 hours. Conditioned media were collected; IL-6 (E) and IGF-1 (F) concentrations were measured by IL-6 (E) and IGF-1 (F) ELISA assays. (G) BCWM.1 cells and primary WM BMSCs were cultured either alone or in a coculture system, in presence of primary WM BMSCs, with ONX0912 (20-50nM) and anti–IL-6 (0.15 μg/mL), anti–IGF-1 (5 μg/mL), or exogenous recombinant IL6 (25 ng/mL) or IGF1 (25 ng/mL) for 48 hours. Cell proliferation was assessed using [3H]-thymidine uptake assay. All data represent mean ± SD of triplicate experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/115/20/10.1182_blood-2009-09-243402/5/m_zh89991050660007.jpeg?Expires=1765898276&Signature=ERlqDdVm9ZqwAqse8s8zjoKYz7G51rlV61grOtfExeRWKhU870DyXZ82YZwcWpslxB0a9hAyi4OaNtBXRomwhnwk6lGNmiyra5gYks8MIUNeuSdybQsgc9D7g0a7Lht75SoEJz4DpHOTTWoCURrzCDIA9gbENEWVD4xr~GzMN178bSaxrLmYygane6xDX4eGkuWvO4SQ8Dq~dGNr000hT3kPNgwhT6R4kEoyE3XkPwGPoP9X2uN-AYoUTA8roFAsa8f4uv0QR8rHk-3eZagOsvyTfsvJhdcvUxo87TtcmHv8m7s8-dM4cthsMd8bSte0vSaYaNJsaiYBFGvtIXMrrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)