NPM-ALK (nucleophosmin-anaplastic lymphoma kinase) and TPM3-ALK (nonmuscular tropomyosin 3-anaplastic lymphoma kinase) are oncogenic tyrosine kinases implicated in the pathogenesis of human ALK-positive lymphoma. We report here the development of novel conditional mouse models for ALK-induced lymphomagenesis, with the use of the tetracycline regulatory system under the control of the EμSRα enhancer/promoter. The expression of either oncogene resulted in the arrest of the differentiation of early B cells and lymphomagenesis. We also observed the development of skin keratoacanthoma lesions, probably because of aberrant ALK expression in keratinocytes. The inactivation of the ALK oncogene on doxycycline treatment was sufficient to induce sustained regression of both hematopoietic tumors and skin disease. Importantly, treatment with the specific ALK inhibitor (PF-2341066) also reversed the pathologic states, showing the value of these mouse models for the validation of ALK tyrosine kinase inhibitors. Thus, our results show (1) that NPM-ALK and TPM3-ALK oncogenes are sufficient for lymphoma/leukemia development and required for tumor maintenance, hence validating ALK as potentially effective therapeutic target; and (2) for the first time, in vivo, the equal tumorigenic potential of the NPM-ALK and TPM3-ALK oncogenic tyrosine kinases. Our models offer a new tool to investigate in vivo the molecular mechanisms associated with ALK-induced lymphoproliferative disorders.
Introduction
The anaplastic lymphoma kinase (ALK) is associated with a subset of non-Hodgkin lymphomas characterized by the translocation of ALK gene located on chromosome 2p23, which is fused to 1 of 13 partners.1,,,,–6 The 2 most common fusion partners are the nucleophosmin 1 (NPM1) gene, on chromosome 5, and the nonmuscular tropomyosin 3 (TPM3) gene, on chromosome 1, that account for 75% and 15% of ALK-positive lymphomas, respectively. Two different ALK-positive lymphoproliferative disorders have been described and are now recognized as distinct entities: systemic anaplastic large cell lymphoma T/null (World Health Organization classification7 ) and a rare variety of diffuse large B-cell lymphoma often with plasmablastic features that we described in 1997.8 More recently, we have shown that ALK-positive diffuse large B-cell lymphoma was namely associated with the t(2;17)(p23;q23) involving Clathrin-ALK rearrangement.9 Occasional cases have been associated with the t(2;5)(p23;q35) translocations and found to express the NPM-ALK fusion protein.10,11
The oncogenic properties of NPM-ALK and TPM3-ALK fusion proteins have been shown in vitro, in fibroblastic and lymphoid cell lines,12,,–15 in vivo with xenografts of ALK-expressing cells,16,17 retroviral transduction,18,19 or transgenic mouse models.20,21 In particular, it has been established that to induce ALK in transgenic models, it is critical both to use the correct lineage-specific promoter and to induce significant levels of protein expression.22,23 Hence, the ideal mouse model should use conditional and lineage-specific promoter to closely mimic the human malignancy and to allow reversible expression of the transgene, as has been suggested.23,24 To achieve these objectives, we used the tetracycline regulatory system to generate conditional transgenic mice overexpressing NPM-ALK or TPM3-ALK oncogenes. To drive expression of the tetracycline-regulated transactivator (tTA) in the lymphoid lineage, we chose the EμSRα enhancer/promoter, as has been previously described.25,26 Here, we will show that double-transgenic mice develop an ALK-positive B-cell lymphoproliferative disorder, corresponding to an early B lymphoma/leukemia, after a short period of latency. Suppression of ALK fusion protein expression by administration of doxycycline (Dox), or a specific ALK inhibitor (PF-2341066), to mice exhibiting florid disease led to a complete regression of ALK-associated lesions. Our results suggest that NPM-ALK and TPM3-ALK oncogenes are sufficient for tumor development and are required for tumor maintenance, therefore placing ALK as an optimal therapeutic target and further support the notion that, in mouse, ALK preferentially malignantly transforms B cells.22,23
Methods
Generation of transgenic constructs and transgenic founder mice
The human NPM-ALK cDNA, from pBluescript2 SK+ plasmid, a generous gift of Dr S. W. Morris (St Jude Children's Research Hospital),1 was subcloned into HindIII- and NotI-restricted pBIL vector (Clontech). The human TPM3-ALK cDNA, cloned in our laboratory,2 from the pcDNA3 plasmid,12 was subcloned into the HindIII-restricted pBIL vector. DNA fragments (7267 base pair [bp] for NPM-ALK construct and 7517 bp for TPM3-ALK construct) were isolated (Kit QIAGEN), diluted at 5 μg/mL in Brinster buffer [Tris (tris(hydroxymethyl)aminomethane) 10mM, pH 7.5; EDTA (ethylenediaminetetraacetic acid) 0.1mM], microinjected into the pronuclei of FVB/N zygotes (R. Janvier Breeding Center), and implanted into pseudopregnant foster mothers (Transgenesis facility, IFR30). Screening of transgenic founder mice were performed by polymerase chain reaction (PCR) genotyping with the following primers: NA forward, 5′-AGAGGCAATGAATTACGAAGGCAGT-3′,and NA reverse, 5′-AGCAGTAGTTGGGGT TGTAGTCGGT-3′, for NPM-ALK (287 bp) and TPM3 forward, 5′-CTGGCAGAGTCCCGTTGCCGAG-3′, and ALK reverse, 5′-GGCTCTGCAGCTCCAT-CTGCATGG-3′, for TPM3-ALK (270 bp). Endogenous myogenin gene (Myo) was used as an internal control: Myo forward, 5′-CCAAGTTGGTGTCAAAAGCC-3′, and Myo reverse, 5′-CTCTCTGCTTTAAGGAGTCAG-3′ (170 bp).
Assessment of tetracycline-regulated NPM-ALK, TPM3-ALK, and luciferase expression in vitro
After collagenase type I isolation (1 mg/mL in phosphate-buffered saline; Sigma-Aldrich), ear fibroblasts isolated from founder mice were transfected with the tTA-coding plasmid (Clontech). Transfections were performed with or without Dox (Sigma-Aldrich) in the culture media (20 ng/mL), and NPM-ALK or TPM3-ALK expression was analyzed by Western blotting and luciferase expression, as previously described.17 Positive founder lines were maintained by crossing with wild-type FVB/N mice.
Generation of double-transgenic mice
The NPM-ALK and TPM3-ALK heterozygous founder transgenic mice were bred with EμSRα-tTA homozygous transgenic mice, provided by Dr D. W. Felsher (Stanford University).26 PCR genotyping was performed with the NPM-ALK or TPM3-ALK primers described in “Generation of transgenic constructs and transgenic founder mice,” and the following tTA gene primers: tTA forward, 5′-AGGCCTGTACGGAAGTGT-3′; tTA reverse, 5′-CTCTGCACCTTGGTGATC-3′. All transgenic mice were housed under pathogen-free conditions at Toulouse, IFR150-IFR BMT (ex IFR30) animal facility, as per animal protocols approved by Inserm.
Doxycycline administration
Dox was added in the drinking water (100 μg/mL) of pregnant females or moribund double-transgenic animals. In addition, moribund animals were also injected once intraperitoneally with 100 μL of Dox solution (100 μg/mL).
ALK inhibitor treatment Racemic
PF-2341066 (3-[1-(2,6-dichloro-3-fluoro-phenyl)-ethoxy]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)-pyridin-2-ylamine) was synthesized according to the method described in the patent international application WO 2006/021881. Moribund double-transgenic mice were administered PF-2341066 in sterile water by oral gavage daily for 10 days at 100 mg/kg/day.
Double-transgenic mouse monitoring
Mice were observed biweekly for early detection of disease symptoms. When mice were moribund with palpable tumor burden, they were humanely killed.
Bone marrow transplantation
Bone marrow cells were harvested from the femurs and tibias of double-transgenic mice (maintained with Dox for 4 weeks after birth) and littermate mice. Unfractionated bone marrow cells were treated with 0.15M NH4Cl, 1mM KHCO3, and 0.1mM Na2EDTA to eliminate erythrocytes before injection into the retro-orbital plexus of lethally γ-irradiated FVB/N mice (8.5 Gy) or sublethally γ-irradiated nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (1 Gy; 137Cs source). Recipient mice irradiated 1 day before injections (5 × 106 bone marrow cells in 200 μL of phosphate-buffered saline) were not treated with Dox and were given antibiotic-containing water for 4 weeks after irradiation. All irradiated mice were monitored closely over their life spans, and, at the first sign of illness, they were killed. Peripheral blood, bone marrow, lymph nodes, spleen, skin, lung, kidney, and liver were collected for cytopathologic and histopathologic analyses, as described in “Histology and immunohistochemistry.”
Western blotting
Western blotting was performed with the use of conventional techniques17 with monoclonal antibodies (mAbs) against ALK (ALKc; a gift from Dr B. Falini, Institute of Hematology, University of Perugia; 1/20027 ), phosphotyrosine (clone 4G10; 1/1000; Upstate Biotechnology), and β-actin (clone AC-15; ascites fluid; 1/20 000; Sigma-Aldrich). NPM-ALK–positive Karpas cell line was used as positive control.
Histology and immunohistochemistry
Sections from formalin-fixed and paraffin-embedded tissues were stained with hematoxylin and eosin. Immunohistochemical analysis was performed with several mAbs or polyclonal antibodies directed against B220/CD45R (rat mAb; clone RA3-6B2; 1 /400), CD3 (rat mAb; clone CD3-12; 1/100), and F4/80 (rat mAb; clone CI:A3-1; 1/100) purchased from AbD Serotec; anti–CD138/Syndecan-1 (rat mAb; clone 281-2; 1/100; BD PharMingen), anti–Pax-5 (goat polyclonal antibody; sc-1974; 1/50; Santa Cruz Biotechnologies), and rabbit anti-ALK mAb (clone SP8; 1/100; Lab Vision Corporation). Antibody binding was detected with the streptavidin-biotin-peroxidase complex method (Vector Laboratories). The heat antigen retrieval procedure was used when necessary.
Confocal immunofluorescence microscopy
Sections were immunostained with a mouse monoclonal anti–human ALK protein (Clone ALK1, 1/100; Dako) and processed as previously described.28 Nuclei were stained with TO-PRO-3 iodide (1/1000; Molecular Probe). Antibody binding was detected using a Leica DMR microscope equipped with the DFC300FX camera and a 40×/0.85 NA objective lens. Image processing was performed using IM50 software from Leica.
Flow cytometry
Cells isolated from tumor tissues were incubated with 5 mg/mL Fc receptors blocking antibody (anti–mouse CD16/32; clone 93; eBioscience) and 3% of heat-inactivated mouse serum diluted in staining buffer. Cells (1 × 106/well) were immunostained for 30 minutes at 4°C with mixtures of cell-surface markers, including directly labeled fluorescein isothiocyanate (FITC) anti-CD3 (clone 145-2C11; eBioscience), allophycocyanin–cyanin 5.5 (APL-Cy5.5) anti-CD19 (clone 6D5; eBioscience), phycoerythrin (PE)–-Cy7 anti-CD23 (clone B3B4; eBioscience), PE-Cy7 anti-CD25 (clone PC61.5; eBioscience), FITC anti-CD43 (clone eBioR2/60; eBioscience), biotin-conjugated anti–pre-B cell receptor (clone SL156; BD PharMingen), and biotin-conjugated anti–immunoglobulin M (clone II,41; eBioscience) for surface cytofluorimetric analysis. Biotin-conjugated antibodies were shown by allophycocyanin streptavidin (BD PharMingen; 554067). Cells were fixed and permeabilized with the use of the BD Cytofix/Cytoperm kit (554714) and stained with PE anti-ALK or isotype control (BD PharMingen; 559257) or FITC anti–μ heavy chain (1021-02; Southern Biotech) or FITC anti–κ light chain (BD PharMingen; 550003). Cell phenotype was determined with the use of an LSRII flow cytometer, followed by BD FACSDiva or WinMDI software analyses.
Apoptosis detection
Apoptosis was assessed by immunohistochemical analysis for caspase 3 (R&D Systems; AF835) and by fluorescence-activated cell sorting (FACS) for annexin V–PE (BD Biosciences) and 7-amino-actinomycin D (7-AAD; BD Biosciences) stainings, according to the manufacturer's instructions, and analyzed by LSRII flow cytometer and by BD FACSDiva software.
Statistical analysis
Statistical significance of differences was determined with the Mann-Whitney test. The minimal level of significance was a P value less than .05. Overall survival was assessed with Kaplan-Meier curves. Statistical comparison of Kaplan-Meier curves is based on the log-rank test. All statistical analyses were performed with GraphPad Prism software (GraphPad Software Inc).
Results
Generation of a conditional transgenic system for expression of the NPM-ALK or TPM3-ALK oncogenes
To generate transgenic mice conditionally expressing the ALK oncogenes (NPM-ALK or TPM3-ALK), we used the tetracycline regulatory system (Tet system) in which the tTA protein mediates the transcription of a transgene placed under the control of the tetracycline-responsive promoter (Tet-O).29 In the Tet off version of this system, the addition of tetracycline or Dox, an analog of tetracycline, inhibits tTA action, promoting silencing of transgene expression.
Our conditional transgenic mice were generated in 2 steps. First, the human NPM-ALK and TPM3-ALK cDNAs were inserted downstream of a Tet-regulated cytomegalovirus minimal promoter into the pBIL vector,30 allowing the concomitant expressions of ALK and firefly luciferase.17 This construct (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) was introduced into the pronucleus of freshly fertilized FVB/N mouse oocytes. The resulting NPM-ALK or TPM3-ALK transgenic mouse lines were designated pBIL-NA and pBIL-TA, respectively. Three transgenic founder mice were obtained for pBIL-NA (pBIL-NA-1; pBIL-NA-2, and pBIL-NA-3) and 2 for pBIL-TA (pBIL-TA-1 and pBIL-TA-2) (supplemental Figure 1B). All transgenic lines developed into phenotypically normal and fertile mice. To assess ALK oncogene and luciferase expression, fibroblast primary cultures were generated from ear biopsies from each founder line. Then, these cells were transfected with a Tet-Off plasmid allowing tTA protein expression, and, after 2 days in culture with or without Dox (20 ng/mL), we observed that ALK oncogene and luciferase expression were induced in pBIL-NA-1–, pBIL-NA-2–, and pBIL-TA-2–derived cells (supplemental Figure 1C-D). NPM-ALK and TPM3-ALK were not detected in tTA-untransfected fibroblasts (S.G. and F.M., unpublished data, June 2006), showing a highly regulated expression in our system. Next, the conditional and lymphoid lineage-restricted activation of ALK transgene expression was achieved by breeding pBIL-NA-1, pBIL-NA-2, and pBIL-TA-2 founder mice with “driver” mice expressing tTA protein under the control of the EμSRα promotor/enhancer cassette to restrict the expression of the ALK transgenes to B or T lymphocytes26,31,32 (Figure 1A).
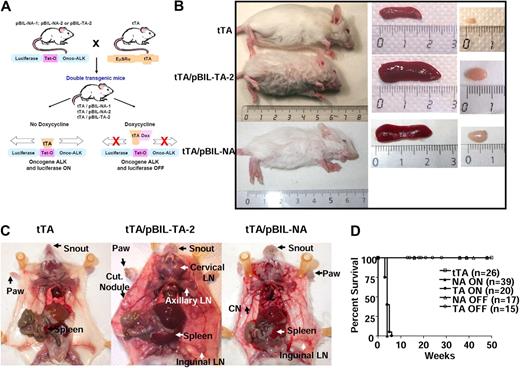
Macroscopic analysis of ALK-expressing double-transgenic mice. (A) Schematic representation of the generation of double-transgenic mice conditional for the expression of the ALK oncogene in the lymphoid lineage. Three heterozygous transgenic founder mice (pBIL-NA-1, pBIL-NA-2, and pBIL-TA-2) were crossed with homozygous EμSRα tTA mice. Double-transgenic mice were designated as tTA/pBIL-NA-1, tTA/pBIL-NA-2, and tTA/pBIL-TA-2. According to the absence (ON) or presence (OFF) of Dox, the tTA transactivator allows or not the expression of both ALK oncogene and luciferase genes. (B) The size of representative 4-week-old tTA/pBIL-TA-2 (TPM3-ALK) and tTA/pBIL-NA (NPM-ALK) double-transgenic mice are shown in comparison with a control age- and sex-matched tTA littermate animal. Spleen and lymph node enlargements in tTA/pBIL-TA-2 and tTA/pBIL-NA double-transgenic versus control tTA littermate mice are also shown. (C) Necropsy findings in tTA/pBIL-TA-2 and tTA/pBIL-NA double-transgenic mice show (arrows) disseminated lymphadenopathies (LN indicates lymph node) and spleen enlargement associated with multiple skin lesions and hypertrophy of the snout and paws (Cut Nodule indicates cutaneous nodules) in comparison with a control age- and sex-matched tTA littermate animal. (D) Survival curves showing the reduced life time of tTA/pBIL-TA-2 (TA ON) and tTA/pBIL-NA (NA ON) double-transgenic mice compared with control tTA littermate mice and Dox-treated tTA/pBIL-TA-2 (TA OFF) and tTA/pBIL-NA (NA OFF) double-transgenic mice.
Macroscopic analysis of ALK-expressing double-transgenic mice. (A) Schematic representation of the generation of double-transgenic mice conditional for the expression of the ALK oncogene in the lymphoid lineage. Three heterozygous transgenic founder mice (pBIL-NA-1, pBIL-NA-2, and pBIL-TA-2) were crossed with homozygous EμSRα tTA mice. Double-transgenic mice were designated as tTA/pBIL-NA-1, tTA/pBIL-NA-2, and tTA/pBIL-TA-2. According to the absence (ON) or presence (OFF) of Dox, the tTA transactivator allows or not the expression of both ALK oncogene and luciferase genes. (B) The size of representative 4-week-old tTA/pBIL-TA-2 (TPM3-ALK) and tTA/pBIL-NA (NPM-ALK) double-transgenic mice are shown in comparison with a control age- and sex-matched tTA littermate animal. Spleen and lymph node enlargements in tTA/pBIL-TA-2 and tTA/pBIL-NA double-transgenic versus control tTA littermate mice are also shown. (C) Necropsy findings in tTA/pBIL-TA-2 and tTA/pBIL-NA double-transgenic mice show (arrows) disseminated lymphadenopathies (LN indicates lymph node) and spleen enlargement associated with multiple skin lesions and hypertrophy of the snout and paws (Cut Nodule indicates cutaneous nodules) in comparison with a control age- and sex-matched tTA littermate animal. (D) Survival curves showing the reduced life time of tTA/pBIL-TA-2 (TA ON) and tTA/pBIL-NA (NA ON) double-transgenic mice compared with control tTA littermate mice and Dox-treated tTA/pBIL-TA-2 (TA OFF) and tTA/pBIL-NA (NA OFF) double-transgenic mice.
Embryonic expression of the ALK oncogene is lethal
When conditional transgenic mice gestations occurred in the absence of Dox (No Dox), progeny number was consistently reduced (4 ± 1 newborns for the tTA/pBIL-NA-1 line, 6 ± 1 newborns for the tTA/pBIL-NA-2 line, and 4.3 ± 2.5 newborns for the tTA/pBIL-TA-2 line in comparison with 10 ± 2 newborns for normal wild-type FVB/N; supplemental Table 1; S.G. and F.M., unpublished data, August 2006). In addition, we failed to identify progeny that expressed both transgenes (supplemental Table 1). Among animals born alive, no NPM-ALK– or TPM3-ALK–positive animals were detected with the use of PCR genotyping (supplemental Table 1). These results strongly suggested that embryonic expression of the ALK oncogene is lethal. Preliminary results suggest that ALK fusion protein is expressed very early during embryogenesis (data not shown). To circumvent embryonic lethality, pregnant females were given Dox in their drinking water to block tTA protein and transgenes expression during gestation. Under these conditions, normal progeny number was observed, and 50% of double-transgenic mice (tTA/pBIL-NA or tTA/pBIL-TA) expressing ALK protein were obtained (supplemental Table 1). Such a frequency was expected because the tTA parents were homozygous and pBIL-NA and pBIL-TA were heterozygous.
Expression of NPM-ALK or TPM3-ALK in newborn mice resulted in aggressive lymphoma/leukemia
Expression of the ALK oncogene was induced in newborn mice by removing Dox from their drinking water. We observed that double-transgenic animals were easily identified because they developed an unexpected skin disease affecting the snout and paws, first detectable at 3 weeks of age (Figure 1B-C). These mice also exhibited general growth retardation (Figure 1B; supplemental Figure 2A-B) and died within a mean latency of 4 weeks (Figure 1D; P < .001). Note, we did not observe a significant difference in overall survival of NPM-ALK or TPM3-ALK double-transgenic mice (Figure 1D; P = .113).
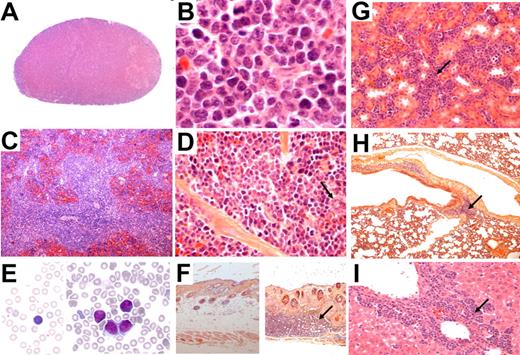
At necropsy, we observed that mice exhibited multiple findings consistent with lymphoid malignancy, including marked splenomegaly (supplemental Figure 2C-D) and generalized lymphadenopathy (Figure 1B-C). Histopathologic studies showed that the architecture of lymph nodes (Figure 2A-B; supplemental Figure 3A-B) and spleen (Figure 2C-D; supplemental Figure 3C-D) was obliterated by malignant lymphoid cells. The lymphoid neoplasia consisted of medium to large-sized cells showing slightly irregular nuclei, with fine chromatin and small nucleoli, and scanty cytoplasms (Figure 2B-D; supplemental Figure 3B-D). Cytologic examination of blood smear showed an increase in circulating lymphocytes (supplemental Table 2), including large cells displaying a clumped nuclear chromatin (Figure 2E; supplemental Figure 3E), suggesting a leukemic dissemination of the disease. Large cells also invaded the skin (Figure 2F; supplemental Figure 3F), kidney (Figure 2G; supplemental Figure 3G), lung (Figure 2H; supplemental Figure 3H), and liver (Figure 2I; supplemental Figure 3I). We conclude that TPM3-ALK (Figure 2) and NPM-ALK (supplemental Figure 3) induce the same aggressive lymphoma/leukemia. Of note, no significant involvement was found in the thymus.
Histologic and cytologic analysis of TPM3-ALK–positive lymphoma and leukemic cells in blood from tTA/pBIL-TA double-transgenic mice. Hematoxylin and eosin staining on tissue sections from lymph node, spleen, kidney, lung, liver, and skin. The lymph node architecture is obliterated by abnormal lymphoid cells showing slightly irregular nuclei and scanty cytoplasms (A, original magnification ×50; B, original magnification ×1000). In the spleen, lymphoma cells are predominantly localized in the white pulp and show comparable morphologic features as seen in the lymph nodes. Note scattered megakaryocytes (arrow) (C, original magnification ×50; D, original magnification ×640). Wright-Giemsa–stained smear of peripheral blood showing presence of circulating abnormal lymphoid cells (E right, original magnification ×1000) compared with normal circulating lymphoid cells from control age-matched tTA littermate mice (E left, original magnification ×1000). All animals presented with skin nodules consisting of a hyperplasia of the epidermis, suggesting a keratoacanthoma-like lesion (F right, original magnification ×50) in comparison with normal skin from control age-matched tTA littermate mice (F left, original magnification ×50). (F right) As shown, a lymphomatous infiltration (arrow) was sometimes observed in the dermis below the keratoacanthoma-like lesion. Infiltration of various intensity was also found in kidney (G, original magnification ×400; arrow), lung (H, original magnification ×100; arrow), and liver (I, original magnification ×400; arrow).
Histologic and cytologic analysis of TPM3-ALK–positive lymphoma and leukemic cells in blood from tTA/pBIL-TA double-transgenic mice. Hematoxylin and eosin staining on tissue sections from lymph node, spleen, kidney, lung, liver, and skin. The lymph node architecture is obliterated by abnormal lymphoid cells showing slightly irregular nuclei and scanty cytoplasms (A, original magnification ×50; B, original magnification ×1000). In the spleen, lymphoma cells are predominantly localized in the white pulp and show comparable morphologic features as seen in the lymph nodes. Note scattered megakaryocytes (arrow) (C, original magnification ×50; D, original magnification ×640). Wright-Giemsa–stained smear of peripheral blood showing presence of circulating abnormal lymphoid cells (E right, original magnification ×1000) compared with normal circulating lymphoid cells from control age-matched tTA littermate mice (E left, original magnification ×1000). All animals presented with skin nodules consisting of a hyperplasia of the epidermis, suggesting a keratoacanthoma-like lesion (F right, original magnification ×50) in comparison with normal skin from control age-matched tTA littermate mice (F left, original magnification ×50). (F right) As shown, a lymphomatous infiltration (arrow) was sometimes observed in the dermis below the keratoacanthoma-like lesion. Infiltration of various intensity was also found in kidney (G, original magnification ×400; arrow), lung (H, original magnification ×100; arrow), and liver (I, original magnification ×400; arrow).
All transgenic mice also presented with skin nodules consisting of a hyperplasia of the epidermis, suggesting a keratoacanthoma-like lesion (Figure 2F; supplemental Figure 3F). A lymphomatous infiltration was sometimes observed in the dermis below the keratoacanthoma-like lesion (Figure 2F; supplemental Figure 3F). Histopathologic examination showed that snout and paw abnormalities were also because of keratoacanthoma-like lesions. Because all founder lines of double-transgenic mice (tTA/pBIL-NA-1, tTA/pBIL-NA-2, and tTA/pBIL-TA-2) developed the same hematopoietic and cutaneous diseases, we decided to focus our investigations only on one transgenic line for each oncogene. Those lines are now referred to as tTA/pBIL-NA and tTA/pBIL-TA.
NPM-ALK and TPM3-ALK are expressed in tumors
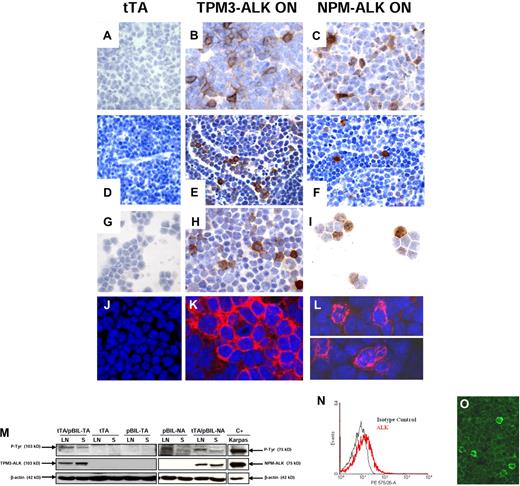
Immunohistochemistry with anti-ALK antibody performed on spleen (Figure 3B-C) and lymph node (Figure 3E-F) tissue sections from 10 diseased double-transgenic mice showed a heterogeneous staining of lymphoma cells. Some cells from tTA/pBIL-NA transgenic mice exhibited a strong cytoplasmic, nuclear, and nucleolar staining. A significant proportion of the remaining cells displayed a weak positivity (Figure 3C,F,I). Confocal microscopy highlighted weakly positive cells, and the subcellular distribution of NPM-ALK protein (Figure 3L,O). As observed in TPM3-ALK–positive human tumors, lymphoma cells from tTA/pBIL-TA showed a restricted cytoplasmic staining (Figure 3B,K). In lymph nodes, scattered lymphoma cells were also observed in lymphatic sinuses (Figure 3E-F). As expected, abnormal lymphoid cells in peripheral blood were positive for ALK (Figure 3H,I). The heterogeneity of lymphoma cells regarding the level of ALK expression was further confirmed by FACS analysis. Indeed, an overlay representation of ALK and isotype control intracellular staining showed that lymphoma cells isolated from lymph nodes expressed the ALK oncogene (right shift), in a continuum from weak to strong expression (Figure 3N), a feature also seen on confocal microscopy (Figure 3O). Immunoblotting of protein extracts from pathologic lymph nodes and spleen confirmed the expression of the 75-kDa NPM-ALK and 103-kDa TPM3-ALK fusion proteins as well as their tyrosine phosphorylation (Figure 3M). As expected, ALK oncogene was not expressed in age-matched littermate controls (tTA) and pBIL-NA and pBIL-TA single-transgenic mice (Figure 3M). Of note, the keratoacanthoma-like lesions of the skin were also strongly positive for ALK. In the thymus, only epithelial cells of the Hassall corpuscles were found to be positive for ALK protein. We conclude that conditional NPM-ALK and TPM3-ALK transgenic mice developed hematopoietic and cutaneous diseases exhibiting robust transgene expression.
ALK oncogene expression in spleen, lymph node, and blood of TPM3-ALK and NPM-ALK double-transgenic mice. Immunohistochemical analysis of spleen (A-C, original magnification ×1000), lymph node (D, original magnification ×640; E-F, original magnification ×1000), blood lymphoid cells (G-I, original magnification ×1000) from control age-matched tTA (A,D,G), TPM3-ALK ON (tTA/pBIL-TA) (B,E,H), and NPM-ALK ON (tTA/pBIL-NA) (C,F,I) double-transgenic mice. Sections were stained with the rabbit anti-ALK antibody, and nuclei were counterstained with hematoxylin. NPM-ALK–positive tumor cells show a cytoplasmic and nuclear and nucleolar staining (C,F,I) in comparison with TPM3-ALK–positive tumor cells, which harbored a cytoplasmic and membrane staining (B,E,H). The staining intensity varies from cell to cell. Scattered ALK-positive lymphoma cells are observed in lymphatic sinuses (E-F). Confocal microscopy analysis shows the restricted cytoplasmic staining in TPM3-ALK (K) and the cytoplasmic and nucleolar staining in NPM-ALK (L) double-transgenic mice. Sections were stained with the mouse anti-ALK antibody, and nuclei were counterstained with TO-PRO-3 iodide. (M) Western blotting analysis of TPM3-ALK and NPM-ALK expressions. Protein lysates (100 μg) extracted from lymph nodes (LN) and spleens (S) of tTA/pBIL-TA and tTA/pBIL-NA double-transgenic mice; tTA, pBIL-TA, and pBIL-NA control transgenic mice; and positive control Karpas cell line overexpressing NPM-ALK (lane C+) were subjected to Western blotting analysis with anti-ALKc, antiphosphotyrosine, and anti–β-actin antibodies. (N) Flow cytometric analysis of ALK expression in lymph node cells from NPM-ALK double-transgenic mice. The histograms show ALK expression (red line) and isotype control (black line). Confocal microscopy (O) of lymphoma cells shows the heterogeneity of ALK expression as seen with flow cytometric analysis.
ALK oncogene expression in spleen, lymph node, and blood of TPM3-ALK and NPM-ALK double-transgenic mice. Immunohistochemical analysis of spleen (A-C, original magnification ×1000), lymph node (D, original magnification ×640; E-F, original magnification ×1000), blood lymphoid cells (G-I, original magnification ×1000) from control age-matched tTA (A,D,G), TPM3-ALK ON (tTA/pBIL-TA) (B,E,H), and NPM-ALK ON (tTA/pBIL-NA) (C,F,I) double-transgenic mice. Sections were stained with the rabbit anti-ALK antibody, and nuclei were counterstained with hematoxylin. NPM-ALK–positive tumor cells show a cytoplasmic and nuclear and nucleolar staining (C,F,I) in comparison with TPM3-ALK–positive tumor cells, which harbored a cytoplasmic and membrane staining (B,E,H). The staining intensity varies from cell to cell. Scattered ALK-positive lymphoma cells are observed in lymphatic sinuses (E-F). Confocal microscopy analysis shows the restricted cytoplasmic staining in TPM3-ALK (K) and the cytoplasmic and nucleolar staining in NPM-ALK (L) double-transgenic mice. Sections were stained with the mouse anti-ALK antibody, and nuclei were counterstained with TO-PRO-3 iodide. (M) Western blotting analysis of TPM3-ALK and NPM-ALK expressions. Protein lysates (100 μg) extracted from lymph nodes (LN) and spleens (S) of tTA/pBIL-TA and tTA/pBIL-NA double-transgenic mice; tTA, pBIL-TA, and pBIL-NA control transgenic mice; and positive control Karpas cell line overexpressing NPM-ALK (lane C+) were subjected to Western blotting analysis with anti-ALKc, antiphosphotyrosine, and anti–β-actin antibodies. (N) Flow cytometric analysis of ALK expression in lymph node cells from NPM-ALK double-transgenic mice. The histograms show ALK expression (red line) and isotype control (black line). Confocal microscopy (O) of lymphoma cells shows the heterogeneity of ALK expression as seen with flow cytometric analysis.
NPM-ALK– and TPM3-ALK–induced lymphoma/leukemia are derived from B cells
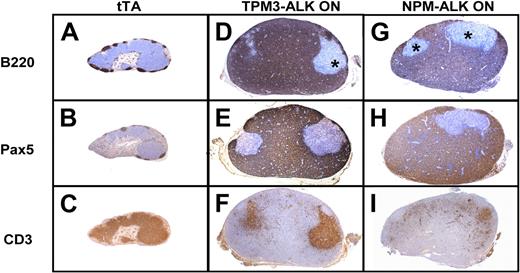
We further characterized the phenotype of the lymphoma/leukemia. We found that the lymphomas induced by TMP3-ALK or NPM-ALK showed a massive expansion of CD45R/B220 and Pax5-positive large cells (B-cell markers) associated with residual normal CD3+ T cells (Figure 4D-I). Lymphoma cells were negative for the plasma cell–associated marker CD138 and the macrophage marker F4/80 (S.G. and F.M., unpublished data, June 2007). Flow cytometric analysis with either CD19 or CD3 antibodies showed that the lymphoid population of pathologic lymph nodes consisted of 60% B cells and 28% T cells, whereas tTA normal lymph nodes displayed 75% T cells and 12% B cells (Figure 5A). To evaluate if the tumors were derived from B cells, tumor cells were stained for CD19, CD3, and ALK.33 We found a population of CD19+ lymphoma cells positive for ALK (16% for TPM3-ALK and 15% for NPM-ALK cells, respectively; red plotted; Figure 5B-C), in agreement with results of ALK immunohistochemical staining (Figure 3). Taken together, these data showed that the NPM-ALK and TPM3-ALK double-transgenic mice developed the same ALK-positive B-cell lymphomas.
ALK oncogene double-transgenic mice develop B-cell lymphoma. Comparison of immunohistochemical stainings of lymph nodes from tTA control littermate (A-C), TPM3-ALK (ON; D-F), and NPM-ALK (ON; G-I) double transgenic mice. Serial sections show a massive involvement by lymphoma cells of B phenotype positive for B220 (D,G) and Pax5 (E,H). Negative areas (*) correspond to residual T cells positive for CD3 (F,I; original magnification ×40).
ALK oncogene double-transgenic mice develop B-cell lymphoma. Comparison of immunohistochemical stainings of lymph nodes from tTA control littermate (A-C), TPM3-ALK (ON; D-F), and NPM-ALK (ON; G-I) double transgenic mice. Serial sections show a massive involvement by lymphoma cells of B phenotype positive for B220 (D,G) and Pax5 (E,H). Negative areas (*) correspond to residual T cells positive for CD3 (F,I; original magnification ×40).
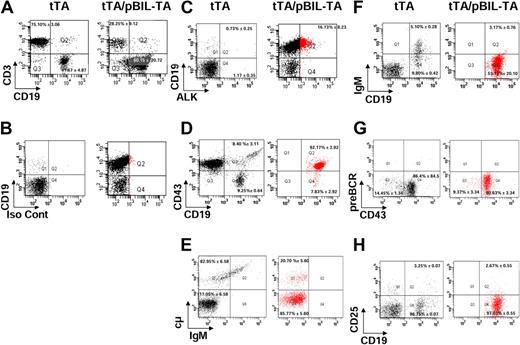
Flow cytometric analysis of the B-cell differentiation stage in TPM3-ALK double-transgenic mice. Representative dot plots of flow cytometric analysis of lymph node cells prepared from 4-week-old control tTA littermate and TPM3-ALK (tTA/pBIL-TA) double-transgenic mice. CD19 versus CD3 profiles of live gated lymphocytes are shown (A). ALK or isotype control (Iso Cont) versus membrane CD19 FACS profiles are shown (B-C). Lymph node cells were labeled with anti-CD19 and with anti-ALK antibodies and with anti-CD43 (D), anti-μ heavy chain (E), anti-immunoglobulin M (IgM; F), anti–pre-B cell receptor (BCR; G), and CD25 (H) antibodies. ALK-positive cells are plotted in red (C-H). Vertical and horizontal cursors were set so that > 99% of events in control unstained samples were in the bottom left quadrants. The numbers indicate the median percentage of cells with SD in a quadrant, relative to the plots of tTA and tTA/pBIL-TA transgenic mice.
Flow cytometric analysis of the B-cell differentiation stage in TPM3-ALK double-transgenic mice. Representative dot plots of flow cytometric analysis of lymph node cells prepared from 4-week-old control tTA littermate and TPM3-ALK (tTA/pBIL-TA) double-transgenic mice. CD19 versus CD3 profiles of live gated lymphocytes are shown (A). ALK or isotype control (Iso Cont) versus membrane CD19 FACS profiles are shown (B-C). Lymph node cells were labeled with anti-CD19 and with anti-ALK antibodies and with anti-CD43 (D), anti-μ heavy chain (E), anti-immunoglobulin M (IgM; F), anti–pre-B cell receptor (BCR; G), and CD25 (H) antibodies. ALK-positive cells are plotted in red (C-H). Vertical and horizontal cursors were set so that > 99% of events in control unstained samples were in the bottom left quadrants. The numbers indicate the median percentage of cells with SD in a quadrant, relative to the plots of tTA and tTA/pBIL-TA transgenic mice.
NPM-ALK– and TPM3-ALK–positive B-cell lymphoma development is independent of skin disease and can be induced by adoptive transfer into normal mice
Because conditional NPM-ALK and TPM3-ALK transgenic mice developed hematopoietic and cutaneous diseases exhibiting robust transgene expression, we investigated whether lymphoma development was autonomous to the hematopoietic system and not dependent on the skin disease. Bone marrow transplantation was performed with cells from (1) wild-type donor mice (n = 3) and (2) double-transgenic donor mice, maintained with Dox for 4 weeks after birth, into irradiated pure-bred recipient FVB/N (n = 10) or NOD/SCID (n = 3) mice. By 17 to 20 weeks after transplantation, 100% of the recipient FVB/N and NOD/SCID mice exhibited ALK-positive large cells in spleen and lymph nodes without skin lesions (supplemental Figures 5-6). Lymphoma cells positive for ALK were also observed in blood (data not shown) and bone marrow smears (supplemental Figure 6G-H). In addition, macroscopic examination of one NOD/SCID mouse showed a large mesenteric tumor mass (supplemental Figure 6D-F). Histopathologic examination of this mass showed that the architecture of lymph nodes was obliterated by sheets of ALK-positive B cells, confirming that the ALK-positive lymphoma cells were transplantable and able to grow independently of skin lesions.
NPM-ALK– and TPM3-ALK–induced B-cell lymphoma/leukemia are blocked at an early B-cell stage
Several markers were used to precisely identify the B-cell differentiation stage of the ALK/CD19-positive lymphoma cells. According to the Melchers-Rolinks classification,34 ALK/CD19+ cells (Figure 5C plotted in red) corresponded to pro-B/pre-B-1 cells because they were positive for CD19 and CD43 (92.17% ± 2.92%; Figure 5D) and a small proportion (20.70% ± 5.60%) also were positive for cytoplasmic μ heavy chain (Figure 5E). These cells were negative for serum immunoglobulin M, κ light chain, pre-B cell receptor, CD23, and CD25 (Figure 5F-H). Altogether, these results suggested that the ALK oncogene began to be expressed in pro-B cells but block the differentiation at the early pre-B cell stage. Similar results were obtained with ALK/CD19+ lymphoma cells from tTA/pBIL-NA transgenic mice: CD43 (93.57% ± 2.29%) and μ heavy chain (17.33% ± 5.27%; data not shown).
ALK oncogene inactivation is sufficient to induce tumor regression
To determine whether the suppression of ALK transgene expression is sufficient to cause regression of lymphadenopathies, splenomegaly, and skin lesions, transgenic mice that were moribund with tumors were treated with Dox for 12 days to suppress ALK expression. Within a few days of treatment, the mice became more physically active, gained weight, and exhibited a major clearing of skin lesions and the regrowth of hair within 3 weeks (Figure 6A-B; supplemental Figure 4A-B). In addition, Dox administration resulted in the reduction in abdominal girth, and lymph nodes and spleen returned to their normal sizes (Figure 6G,H; supplemental Figure 4G,H). Twelve days after the initiation of Dox treatment, 10 mice were killed (10 regressed tTA/pBIL-NA and tTA/pBIL-TA; ie ALK-OFF), and tumor regression and disappearance of lymphoma cells were confirmed by histopathologic and immunophenotypic analyses of the tumor tissues (mesenteric and axillary lymph nodes, spleen, liver, kidney, lung, skin, and blood) after treatment. All these organs now were found to have normal architecture, and no ALK-positive cells were detected (Figures 6C-F,I,J and 7; supplemental Figures 4C-F,I,J and 7). In the skin, we observed a regression of keratoacanthoma-like lesions associated with an exfoliation (Figure 6C-D; supplemental Figure 4C-D) of apoptotic ALK-positive cells (Figure 6F). We then checked that tumor regression occurred as a result of Dox-induced ALK oncogene mRNA (data not shown) and protein expression (Figure 6K; supplemental Figure 4K) down-regulation. Thus, ALK inactivation for 12 days appeared sufficient to lead to tumor cell eradication from lymph node, spleen, blood, skin, and other organs. We conclude that in conditional NPM-ALK and TPM3-ALK transgenic mice the inactivation of ALK is sufficient to reverse tumorigenesis.
Lymphoma and cutaneous disease regression on ALK oncogene inactivation in TPM3-ALK mice. Clinical examination and autopsy findings show that all animals with fully developed disease (TPM3-ALK ON; n=10) presented with disseminated lymphoma with lymph node and spleen enlargement and skin nodules mostly related to keratoacanthoma-like lesions involving the skin, snout, and paws (TPM3-ALK ON: A,C,E,G,I). Ten additional moribund mice were treated with Dox in drinking water (100 μg/mL) and 1 intraperitoneal injection of 100 μL of Dox solution (100 μg/mL; TPM3-ALK OFF; B,D,F,H,J). Compared with the ON mice, the OFF mice exhibited, after 12 days of Dox treatment, a major clearing of the skin lesions (A,C vs B,D) associated with the regrowth of hair within 3 weeks, a regression of spleen and lymph node enlargement (G vs H), and an ALK oncogene expression down-regulation as assessed by anti-ALK immunostaining (E,I vs F,J) and Western blotting analysis (K lane ON vs OFF). Note, in panel K, a vertical line has been inserted to indicate a repositioned gel lane. In panel F, apoptotic ALK-positive cells admixed with exfoliated material (arrow). Original magnifications ×50 (C-F) and ×640 (I-J).
Lymphoma and cutaneous disease regression on ALK oncogene inactivation in TPM3-ALK mice. Clinical examination and autopsy findings show that all animals with fully developed disease (TPM3-ALK ON; n=10) presented with disseminated lymphoma with lymph node and spleen enlargement and skin nodules mostly related to keratoacanthoma-like lesions involving the skin, snout, and paws (TPM3-ALK ON: A,C,E,G,I). Ten additional moribund mice were treated with Dox in drinking water (100 μg/mL) and 1 intraperitoneal injection of 100 μL of Dox solution (100 μg/mL; TPM3-ALK OFF; B,D,F,H,J). Compared with the ON mice, the OFF mice exhibited, after 12 days of Dox treatment, a major clearing of the skin lesions (A,C vs B,D) associated with the regrowth of hair within 3 weeks, a regression of spleen and lymph node enlargement (G vs H), and an ALK oncogene expression down-regulation as assessed by anti-ALK immunostaining (E,I vs F,J) and Western blotting analysis (K lane ON vs OFF). Note, in panel K, a vertical line has been inserted to indicate a repositioned gel lane. In panel F, apoptotic ALK-positive cells admixed with exfoliated material (arrow). Original magnifications ×50 (C-F) and ×640 (I-J).
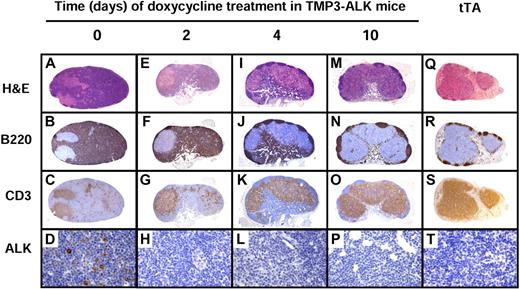
Histopathologic changes of lymph nodes after ALK inactivation by Dox treatment in lymph nodes of TMP3-ALK mice. The architecture of lymph node from mice with fully developed disease (day 0; A-D) is obliterated by lymphoma cells strongly positive for B220 (B). Negative area corresponds to residual T cells as seen in panel C. Lymphoma cells are positive for ALK (D). After Dox treatment (days 2-10: E-P), the lymph node progressively recovers its normal immunoarchitecture together with the decrease of ALK-positive lymphoma cells (H,L,P) and increase in normal B and T cells (M-O). Lymph node from tTA transgenic mice was used as a normal control (Q-T). Original magnification ×50 (A-C), ×40 (E-G,I-K,M-O,Q-S), and ×640 (D,H,L,P,T).
Histopathologic changes of lymph nodes after ALK inactivation by Dox treatment in lymph nodes of TMP3-ALK mice. The architecture of lymph node from mice with fully developed disease (day 0; A-D) is obliterated by lymphoma cells strongly positive for B220 (B). Negative area corresponds to residual T cells as seen in panel C. Lymphoma cells are positive for ALK (D). After Dox treatment (days 2-10: E-P), the lymph node progressively recovers its normal immunoarchitecture together with the decrease of ALK-positive lymphoma cells (H,L,P) and increase in normal B and T cells (M-O). Lymph node from tTA transgenic mice was used as a normal control (Q-T). Original magnification ×50 (A-C), ×40 (E-G,I-K,M-O,Q-S), and ×640 (D,H,L,P,T).
To determine whether ALK suppression exerted any effect on the viability of lymphoma cells, TPM3-ALK (supplemental Figure 8) and NPM-ALK (data not shown) double-transgenic mice were treated for 3 and 15 hours with Dox. After this time, B- and T-cell populations from lymph nodes were analyzed by immunohistochemistry with caspase 3 and FACS annexin V/7-AAD staining (supplemental Figure 8). We found an increase in the percentage of dead CD19+ B cells (ie, lymphoma cells) during Dox treatment (from 14% in the first 3 hours to 22% at 15 hours; supplemental Figure 8). We also found a correlation between the percentage of caspase 3 and annexin V/7-AAD–positive cells, indicating that apoptosis was involved in dying cells. Beyond 15 hours of Dox treatment, the percentage of apoptotic cells decreased close to the value observed in untreated animals.
To further confirm that the tumor regression was related to the loss of ALK oncogene functional activity, we examined the affects of the treatment of NPM-ALK transgenic mice with a novel ALK phosphorylation inhibitor PF-2341066.35 Seven moribund transgenic mice were treated for 10 days with this compound. We observed a progressive clearing of skin lesions together with an improvement of the general health status of treated mice compared with untreated controls. Necropsy findings showed tumor regression, confirmed by histopathologic examination, as after Dox treatment (supplemental Figures 7,9). Of note, the lymphoma regression after PF-2341066 treatment was not due to the inhibition of c-met phosphorylation (another target of this inhibitor)35 because lymphoma cells in our model are negative for this receptor (date not shown). Hence, we conclude that this inhibitor of ALK is sufficient to induce reversal of tumorigenesis in our ALK conditional transgenic tumor model.
Discussion
Our results suggest that ALK-induced tumorigenesis can be reversed through the inactivation of ALK. They have important implications for the understanding of the role of ALK in the initiation and maintenance of tumorigenesis. We have developed a mouse model that will be useful for dissecting the contribution of pathologic activation of ALK to the pathogenesis of lymphoma/leukemia. Pathologic activation of ALK is well known to be associated with 2 major types of non-Hodgkin lymphomas, namely systemic anaplastic large cell lymphoma, T/Null and rare cases of diffuse large B-cell lymphomas with immunoblastic/plasmablastic features.1,5,6 Most systemic T/Null anaplastic large cell lymphomas expressed NPM-ALK or TPM3-ALK, whereas ALK-positive diffuse large B-cell lymphomas are mostly associated with Clathrin-ALK and more rarely with NPM-ALK fusion protein.8,9,11 However ALK tyrosine kinase is also involved in some nonhematopoietic neoplasia such as inflammatory myofibroblastic tumors (TPM3-ALK) as well as in some (< 5%) non–small cell lung tumors in which ALK protein is fused to EML4, the echinoderm microtubule-associated protein-like 4.6,36,37 Our conditional transgenic model appears to recapitulate only some of the features of human ALK-positive lymphoma because lymphoma cells are of pro-B/pre-B phenotype (negative for CD138) and not of terminal B-cell stage of differentiation as seen in human ALK-positive B-cell lymphomas that are positive for CD138 antigen.8,9,11
Our results are consistent with previous reports that ALK activation can result in tumorigenesis in transgenic mouse models. The first description of a murine model to investigate the oncogenic events mediated by NPM-ALK was reported by Kuefer et al,18 who used a retrovirus-mediated gene transfer strategy. After this report, several transgenic lines were generated in which the full-length NPM-ALK cDNA was placed under the control of various promoters such as CD4,20 vav,38 Lck,21 or CD2.39 Whatever the promoter used, transgenic mice developed mostly B-cell tumors, some with plasma cell differentiation,19,20,38 and only a few T-cell lymphomas (mostly lymphoblastic lymphoma) have been reported.20,21 As suggested by Chiarle et al,20 the high incidence of B-cell tumors in NPM-ALK transgenic mice could be due to the forced expression of ALK oncogene. Our results are in agreement with those previously reported because NPM-ALK and TPM3-ALK transgenic mice developed lymphoma/leukemia of B-cell phenotype, further stressing the fact that in mouse, the ALK oncogene preferentially transforms the B-cell lineage. However, our results suggest that ALK oncogene overexpression could block B-cell differentiation at early B stage, even if the Eμ enhancer was previously described to be active throughout the entire B-lymphoid compartment, as reported in Eμ-myc and Eμ-BrD2 transgenic mice.40,–42
An important unique feature of our model system is that we have generated a transgenic mouse model in which ALK is conditionally expressed with the Tet-off system. We demonstrated that transgenic mice conditionally expressing the NPM-ALK or TPM3-ALK oncoprotein with tyrosine kinase activity developed a lethal lymphoproliferative disorder of early B-cell origin associated with skin lesions resembling keratoacanthoma. Of note, comparable skin lesions were previously described by a group using the same EμSRα enhancer/promoter to drive Tax transactivator protein overexpression. This is probably due to the high level of tTA expression in skin as reported in tTA/tax mice.43 The results obtained in bone marrow transplantation experiments in FVB/N and NOD/SCID mice clearly showed that the lymphoma development was autonomous to the hematopoietic system and not dependent on the skin disease. The development of a lymphoid proliferation could be anticipated because, using our strategy, the hematopoietic lineage expression is controlled by the EμSRα enhancer/promoter sequence,25,26,40,42 that drives the expression of the tTA protein. By crossing EμSRα-tTA homozygous transgenic mice with Tet-O-NPM-ALK or Tet-O-TPM3-ALK transgenic mice, we consistently obtained double-transgenic mice developing the same early B-cell tumor phenotype. Our results show for the first time, in an animal model, that activation of either NPM-ALK or TPM3-ALK oncogenic tyrosine kinases exhibit equal tumorigenic potential. In addition, our findings are consistent with previous reports showing that ALK fusion proteins can form homodimers (or oligomers) with dimerization sites at the N-terminus of ALK fusion protein, a mechanism which mimics ligand binding and is responsible for the activation of the catalytic domain through the autophosphorylation of the tyrosine kinase domain of ALK.6,13 Our observation of the very short tumor latency suggests that ALK activation alone is sufficient to result in the transformation of early B cells. Moreover, our observation that the inactivation of a conditional ALK transgene by Dox administration leads to the rapid, complete, and stable tumor regression suggests that ALK alone is required for tumor maintenance.
Our results are the first demonstration that ALK-induced tumorigenesis is reversible, even at a very advanced stage of the disease. The sustained regression on ALK oncogene inactivation suggests that malignant ALK-positive cells depend on the continued activation of ALK oncogene, a finding in agreement with the concept of “oncogene addiction” that was proposed.44,–46 Indeed, it is unlikely that the ALK oncogene promotes the emergence of additional genetic lesions that could compensate for ALK inactivation in the maintenance of the tumor phenotype. Our findings are in agreement to what has been reported in human anaplastic large cell lymphoma because, except for rare studies,47,48 neither NPM-ALK nor other ALK fusion oncogenes have been described to induce genomic instability both in human cancers and in animal models. The striking loss of lymph node and spleen enlargement under Dox treatment raises the question of the underlying mechanism(s) responsible for the disappearance of ALK-positive lymphoma cells.
The relatively low percentage of apoptotic lymphoma cells in lymph nodes of animals treated for 15 hours together with the rapid recovery of the normal lymph node immunoarchitecture lead us to speculate that after the switch-off of ALK oncogene, a significant proportion of lymphoma cells can take a normal B-cell differentiation process again. Such a hypothesis is in agreement with what has been previously described by Felsher and Bishop26 in mice overexpressing the myc oncogene. Similarly, the associated resolution of the ALK-induced keratoacanthoma-like lesions on ALK inactivation appears to be secondary to the exfoliation of apoptotic ALK-positive cells and regrowth of hair. Interestingly, preliminary results that we observed with the specific ALK inhibitor (PF-2341066)35 are comparable with those obtained with Dox treatment. Thus, our results generally support the notion that oncogene inactivation can result in sustained tumor regression.24 They also strongly suggest that NPM-ALK or TPM3-ALK tyrosine kinases are therapeutic targets comparable with Bcr-Abl in chronic myeloid leukemia and that ALK-specific inhibitors16,35,49 would give therapeutic effects comparable with imatinib mesylate (STI571) that targets the Bcr-Abl oncogene in chronic myeloid leukemia.50 It is now important to determine whether the targeted inactivation of the ALK tyrosine kinase oncogene would be sufficient to induce tumor regression in human neoplasia.
In conclusion, we have developed a conditional transgenic mouse model that mimics some of the features of human ALK-positive non-Hodgkin lymphomas. Our model offers a new tool to investigate in vivo the molecular mechanisms involved in the initiation and maintenance of ALK-associated lymphoproliferative disorders. Moreover, we have shown that our model can be used to preclinically test protein tyrosine kinase inhibitors, potentially useful for patients with ALK-associated tumors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank members of the Pr G. Delsol laboratory, Dr S. Mancini, Dr B. Payrastre, and Dr K. Rabin for helpful discussions and critical reading. We thank Dr M. Rousseau (Centre de Distribution, Typage et Archivage animal, CNRS, Orléans) and Dr F. Cormier (Institut Curie, Paris) for their help to transfer the EμSRα-tTA mice from Paris to Toulouse. We thank, for their excellent technical assistance, S. Pillipenko, A. Huchenq, M. Calise, A. Tridon, and M. A. Daussion (Services de transgenèse et de zootechnie, IFR150-IFR BMT [ex IFR30], CHU Purpan, Toulouse); F. Capilla and D. Lestrade (Plateau technique d'histopathologie expérimentale, IFR150-IFR BMT, CHU Purpan, Toulouse); F. Ezzahra L'Faqihi-Olive and V. Duplan-Eche (Plateau technique de cytométrie, IFR150-IFR BMT, CHU Purpan, Toulouse); D. Roda and D. Lhuillier (Laboratoire d'Anatomie et Cytologie pathologiques, CHU Purpan, Toulouse); and J. Boyes (Inserm U563, CHU Purpan, Toulouse).
This work was supported by grants from Inserm, the “Cancéropôle Grand Sud-Ouest,” the “Institut National du Cancer” (INCa), the Région Midi-Pyrénées, and the “Pôle de Compétitivité Cancer-Bio Santé/Fonds uniques interministériels des pôles de compétitivité” (G.D.), “la Fondation de France” (F.M.) and Comités 31 et 09 de la Ligue contre le cancer (F.M.); by postdoctoral fellowships from the “Association pour la Recherche sur le Cancer,” “La Fondation de France,” and “Pôle de Compétitivité Cancer-Bio Santé/Fonds uniques interministériels des pôles de compétitivité” (S.G.); by “Pôle de Compétitivité Cancer-Bio Santé/Fonds uniques interministériels des pôles de compétitivité” and “La Fondation de France” (M.F.); and by a PhD fellowship from the “Association pour la Recherche sur le Cancer” (E.D.).
This work is dedicated to the memory of Mr David Mason.
Authorship
Contribution: S.G., G.D., and F.M. designed research; S.G., M.F., E.D., T.A.S., C.D., A.R., and F.M. performed research; A.K., C.S. and D.W.F. helped with discussions and critical reading; S.G., T.A.S., C.D., C.S., G.D., and F.M. analyzed data; and S.G., D.W.F., G.D., and F.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georges Delsol, Inserm, U563, Centre de Physiopathologie de Toulouse Purpan, Toulouse, F-31300 France; e-mail: georges.delsol@inserm.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal