MEIS1 is a three–amino acid loop extension class homeodomain-containing homeobox (HOX) cofactor that plays key roles in normal hematopoiesis and leukemogenesis. Expression of Meis1 is rate-limiting in MLL-associated leukemias and potently interacts with Hox and NUP98-HOX genes in leukemic transformation to promote self-renewal and proliferation of hematopoietic progenitors. The oncogenicity of MEIS1 has been linked to its transcriptional activation properties. To further reveal the pathways triggered by Meis1, we assessed the function of a novel engineered fusion form of Meis1, M33-MEIS1, designed to confer transcriptional repression to Meis1 target genes that are otherwise up-regulated in normal and malignant hematopoiesis. Retroviral overexpression of M33-Meis1 resulted in the rapid and complete eradication of M33-Meis1–transduced normal and leukemic cells in vivo. Cell-cycle analysis showed that M33-Meis1 impeded the progression of cells from G1-to-S phase, which correlated with significant reduction of cyclin D3 levels and the inhibition of retinoblastoma (pRb) hyperphosphorylation. We identified cyclin D3 as a direct downstream target of MEIS1 and M33-MEIS1 and showed that the G1-phase accumulation and growth suppression induced by M33-Meis1 was partially relieved by overexpression of cyclin D3. This study provides strong evidence linking the growth-promoting activities of Meis1 to the cyclin D-pRb cell-cycle control pathway.
Introduction
Multiple lines of evidence now point to the key roles of MEIS1, a three–amino acid loop extension class homeodomain-containing homeobox (HOX) cofactor, in both normal hematopoiesis and leukemogenesis. In the context of normal hematopoiesis, Meis1 is preferentially expressed in primitive bone marrow (BM) cells enriched for hematopoietic stem cells (HSCs),1 and Meis1 knockout mice are embryonic lethal presenting with severely impaired hematopoiesis.2,3 Deregulated expression of Meis1 accelerates the onset of disease induced by various Hox and NUP98-HOX fusion genes, including genes without leukemogenic potential on their own (reviewed in Argiropoulos and Humphries4 ). Meis1 expression is also essential and rate limiting for the leukemogenicity of multiple MLL fusions,5,6 and HOX and MEIS1 gene expression is frequently dysregulated in leukemia patient samples (reviewed in Argiropoulos and Humphries4 ).
Despite the well-documented role of Meis1 in the promotion of acute myelogenous leukemia (AML), the regulatory networks controlled by Meis1 are not fully known. The identification of Flt3, Cd34, Erg1,7,8 c-Myb,9 and Trib210 as Meis1 target genes have provided valuable insight into the function of Meis1. A group of these genes can replace Meis1 and serve as collaborating genes in leukemic transformation, albeit, with decreased potencies compared with Meis1.10,–12 However, as in the case with Flt3, the target may be dispensable.13 Thus, the potency of Meis1 to induce leukemia appears to be related to its ability to modulate multiple pathways. Interesting in this regard is the recently reported link between Meis1 and cell-cycle control. In the context of mixed lineage leukemia (MLL)–fusion leukemias, Meis1 overexpression was observed to correlate with increased cell-cycle entry and modest up-regulation of Bmi1.5 Moreover, gene expression profiling of murine Mll-AF9 leukemic BM cells transduced with Meis1 short-hairpin RNA showed reduced expression of genes associated with cell-cycle entry, consistent with the impaired cell growth of these cells.6 The linkage to cell cycle gains currency in light of 2 recent reports that showed a correlation between Meis1 activity, retinal progenitor cell proliferation, and cyclin D1 expression in the developing chick and zebrafish.14,15 Together, these data provide the basis for further investigation into the role of Meis1 in cell-cycle regulation.
Experiments with the Drosophila melanogaster ortholog of Meis1, homothorax (hth), have shown that HTH fused to the potent transcriptional activation domain of VP16 resulted in gain-of-function phenotypes, whereas a trans-repressing form of HTH, fused to the repressive domain of Engrailed, phenocopied loss-of-function hth mutations.16 Correspondingly, in the murine BM transplantation model, retroviral overexpression of the hypermorph VP16-Meis1 produced an autonomous oncoprotein and accelerated Hoxa9-mediated AML compared with wild-type Meis1.8,17 Reciprocally, loss of the C-terminal transactivation domain of MEIS118 abolished oncogenic activity.8,10,17 These data show that transcriptional activation is a primary function of MEIS1 in embryonic development and in AML.
As an alternative approach to short interfering RNA knockdown strategies, we hypothesized that further insights into the genes/pathways regulated by Meis1 could be shown by comparing and contrasting the effects of wild-type Meis1 and a novel engineered form of Meis1 designed to confer transcriptional repression to its target genes, whereby exaggerating Meis1 loss-of-function phenotypes. To achieve this, we generated a novel chimeric protein fusing the repressive domain of M33, a member of the Polycomb group (PcG), to MEIS1.
The PcG genes, first identified in Drosophila melanogaster as negative regulators of homeotic (Hox) gene expression, encode the transcriptional repressors of the cellular memory system.19 PcG-mediated silencing is accomplished by the concerted function of at least 3 distinct multimeric protein complexes that are recruited in a hierarchal manner to specific target genes through Polycomb responsive elements, resulting in transcriptional silencing of these loci in a clonally stable manner (reviewed in Schwartz and Pirrotta20 ). The requirement for a Polycomb responsive element can be circumvented by artificially tethering a PcG protein to a locus by fusing it to a specific DNA-binding domain.21,22 These experiments show that the chimeric DNA-binding domain/PcG fusion protein is capable of regulating gene silencing through recruitment of a functional PcG silencing complex by protein-protein interactions between PcG components. Thus, we hypothesized that fusion of the repressive domain of M33, amino acids 318 to 519,23 a member of Polycomb repressive complex 1 (PRC1), to MEIS1 will facilitate PcG complex formation at all direct MEIS1 target genes and result in transcriptional repression of these genes.
Herein, we show that M33-MEIS1 associates with MEIS1 target genes and silences gene transcription of these loci through the recruitment of PRC1 components and the subsequent loss of active epigenetic marks. Transplanted murine primary BM transduced with M33-Meis1 are rapidly lost from circulation, and enforced overexpression of M33-Meis1 in NUP98-HOXD13, HoxA9, and MN1 AML models resulted in the eradication of the leukemic clones in vivo. Cell-cycle analysis showed that M33-Meis1 induced a partial G1-phase block that correlated with aberrant expression of multiple G1-phase regulators, including significant down-regulation of Ccnd3, encoding cyclin D3, and the inhibition of Retinoblastoma (pRb) hyperphosphorylation. Chromatin immunoprecipitation (ChIP) experiments showed that MEIS1 and M33-MEIS1 associated with cyclin D3 intronic sequences and overexpression of cyclin D3 partially relieved the M33-Meis1–induced G1-phase accumulation and growth suppression. Our study provides the novel insight that Meis1 promotes hematopoietic and leukemic stem cell (LSC) proliferation in part by modulating G1-to-S phase progression through the pRb pathway by direct transcriptional control of cyclin D3.
Methods
Retroviral vectors and cDNA
The murine stem cell virus–based vectors carrying expression cassettes consisting of Flag-NUP98-HOXD13 internal ribosomal entry site–enhanced green fluorescent protein (GFP) (ND13-GFP) and hemagglutinin (HA) tagged-Meis1 internal ribosomal entry site–yellow fluorescent protein (YFP) (Meis1-YFP) have been previously described.12,24 The remaining vectors are illustrated and described in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Retroviral infection and culture of primary BM cells
Primary mouse BM cells were transduced as previously described.24 Selection of puromycin- or neomycin-resistant cells was performed with 1.6 μg/mL of puromycin and 1.4 mg/mL of G418 for 5 to 10 days after transduction.
Cell lines
BM transplantation and animal analysis
Mice were bred and maintained at the British Columbia Cancer Research Center Animal Facility. All experimental protocols were approved by the University of British Columbia Animal Care Committee. For BM transplantation experiments, donors of primary BM cells were Ly5.1+ (C57BL/6Ly-Pep3b × C3H/HeJ) F1, and recipients were Ly5.2+ (C57BL/6J × C3H/HeJ) F1. All recipients were lethally irradiated (750 cGy) and transplanted by tail vein injection along with a life-sparing dose of 250 000 normal BM cells.
Donor-derived engraftment and reconstitution were monitored by flow cytometry of GFP+ and/or YFP+ expression in the peripheral blood of the animals that received transplants. Immunophenotypic fluorescence-activated cell sorting (FACS) analysis was performed as previously described.10
Chromatin immunoprecipitation assay
ChIP assays were performed with the use of a ChIP assay kit (Upstate Biotechnology) with the modifications previously described.10 For each immunoprecipitation reaction, 2 μg of the following antibodies were used: purified mouse immunoglobulin G1 (IgG1) isotype control antibody (BD PharMingen), monoclonal anti-HA (Sigma-Aldrich), monoclonal anti-BMI1 (Abcam), rabbit polyclonal anti-RING1 (Abcam), rabbit polyclonal anti–acetyl H3 (Upstate Biotechnology), rabbit polyclonal anti–acetyl H4 (Upstate Biotechnology), rabbit polyclonal anti–trimethylated H3K27 (Upstate Biotechnology). The fold enrichments were measured by quantitative real-time polymerase chain reaction (qRT-PCR) and calculated as previously described.27 PCR primer sequences are shown in supplemental Table 1.
Quantitative reverse transcription–PCR
RNA extraction and quantitative real-time reverse transcription–PCR was performed as previously described.10 Reverse transcription–PCR primers for all genes tested were designed by the Universal Probe Library Assay Design Center (Roche Applied Science). Gene expression levels relative to Abl levels28 were calculated as described in ABI User Bulletin no. 2.
Affymetrix microarray analysis
The microarray analysis was performed as previously described.10
Cell-cycle analysis
Cell-cycle status was assessed by multiparameter flow cytometry after incubation with Hoechst 33342 (Sigma-Aldrich) and pyronin Y (Sigma-Aldrich).
Western blot analyses and stimulation experiments
Western blot analyses were performed as previously described.12 For the stimulation experiments, U937 cells were serum deprived for 16 hours and stimulated with human stem cell factor (SCF; StemCell Technologies) for 10 minutes. Antibodies used were the monoclonal Rb1 (1F8) nuclear marker (Abcam) and monoclonal anti–glyceraldehyde phosphate dehydrogenase (RDI). The relative phosphorylation of pRb was quantified by densitometry analysis with the use of Image Quant 5.2 software (Molecular Dynamics).
Intracellular phospho-pRb staining
BM cells were fixed and permeabilized with BD Phosflow Fix Buffer I (BD Biosciences) and BD Phosflow Perm Buffer III (BD Biosciences). The cells were stained with phycoerythrin-conjugated anti–phospho-pRb (pSer780) monoclonal antibody (BD Biosciences). The orthologous phosphorylation site in mouse is serine 773. Flow cytometry was performed on a FACSVantage SE (BD Biosciences), and the data were analyzed with the use of FlowJo version 8.8.2 (TreeStar Inc.)
Electrophoretic mobility shift assay and coimmunoprecipitation
Electrophoretic mobility shift assay (EMSA) and coimmunoprecipitation procedures are described in detail in supplemental Methods.
Results
M33-Meis1 potently inhibits growth of transformed and normal hematopoietic cells
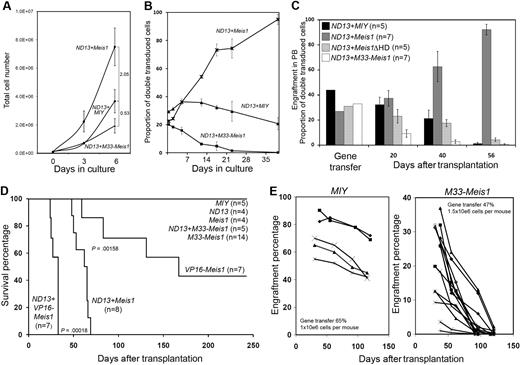
Given that the oncogenicity of MEIS1 has been linked to its transactivation function,8,17 we hypothesized that its conversion to a transcriptional repressor would have potent growth inhibitory effects and may serve as a way to exaggerate and identify novel Meis1 loss-of-function phenotypes. We tested this in the context of a cloned myeloid BM progenitor cell line generated by overexpression of the NUP98-HOXD13 fusion gene (hereafter ND13-GFP).25 In vitro growth properties of ND13-GFP BM cells transduced with empty vector (MIY), Meis1-YFP, or M33-Meis1-YFP (supplemental Figure 1) were assessed with FACS-purified (Figure 1A) and bulk (Figure 1B) populations in standard growth conditions. The quantitative growth measures showed that Meis1 enhanced the in vitro proliferation of ND13-GFP BM cells (2.05-fold change in 6 days), whereas M33-Meis1 conferred a growth disadvantage (0.53-fold change; Figure 1A). Similarly, cell competition experiments showed that the ND13-GFP+Meis1-YFP BM cells out-competed the ND13-GFP BM cells as shown by the increasing proportion of double-transduced BM cells (GFP positive and YFP negative) over time (Figure 1B). Conversely, the ND13-GFP+M33-Meis-YFP BM cells were out-competed by ND13-GFP and were lost from in vitro culture.
M33-Meis1 confers growth inhibition on a myeloid progenitor cell line and to normal hematopoietic cells. (A) In vitro growth kinetics of indicated FACS-sorted cells plated in standard growth conditions. The fold changes compared with ND13+MIY are indicated. (B) In vitro cell expansion of unsorted transduced cells expressed as double-positive GFP+ and YFP+ cells over time. (A-B) The data are expressed as the mean ± SD of 3 independent transductions. (C) Peripheral blood chimerism as measured by FACS analysis. Data are plotted as the percentage (mean ± SD) of GFP+ and YFP+ cells. Gene transfer percentage refers to the proportion of transduced BM cells in the transplanted inoculum. (D) Kaplan-Meier survival curves for mice that received a transplant with BM transduced with the indicated gene(s). Statistical significance was determined by the log-rank test. (E) Peripheral blood chimerism of mice that received a transplant with MIY and M33-Meis1–transduced primary BM as measured by FACS analysis. Data are plotted as the percentage of circulating YFP+ cells for each mouse.
M33-Meis1 confers growth inhibition on a myeloid progenitor cell line and to normal hematopoietic cells. (A) In vitro growth kinetics of indicated FACS-sorted cells plated in standard growth conditions. The fold changes compared with ND13+MIY are indicated. (B) In vitro cell expansion of unsorted transduced cells expressed as double-positive GFP+ and YFP+ cells over time. (A-B) The data are expressed as the mean ± SD of 3 independent transductions. (C) Peripheral blood chimerism as measured by FACS analysis. Data are plotted as the percentage (mean ± SD) of GFP+ and YFP+ cells. Gene transfer percentage refers to the proportion of transduced BM cells in the transplanted inoculum. (D) Kaplan-Meier survival curves for mice that received a transplant with BM transduced with the indicated gene(s). Statistical significance was determined by the log-rank test. (E) Peripheral blood chimerism of mice that received a transplant with MIY and M33-Meis1–transduced primary BM as measured by FACS analysis. Data are plotted as the percentage of circulating YFP+ cells for each mouse.
We next investigated whether the growth inhibitory effect of M33-Meis1 was active in vivo. As previously shown,10,25 the short-term repopulating ND13-GFP+MIY BM cells failed to reconstitute recipient mice beyond 40 days after transplantation (Figure 1C). The ND13-GFP+Meis1-YFP cotransduced BM cells showed rapid engraftment, reaching 86% to 97% in 56 days after transplantation (n = 7) (Figure 1C). The ND13-GFP+M33-Meis1-YFP–cotransduced BM cells did not induce AML, were not detected in peripheral blood beyond 40 days after transplantation (n = 7), and exhibited a more rapid rate of eradication than the MIY control and a dominant-negative form of Meis1, Meis1ΔHD,10 indicating a more robust suppressive effect of M33-Meis1 over a dominant-negative form of Meis1 (Figure 1C-D).
Consistent with its role as a transcriptional activator, we confirmed that transplantation of VP16-Meis1–transduced primary BM, but not wild-type Meis1, is sufficient to cause AML8,17 (Figure 1D; data not shown). Furthermore, co-overexpression of ND13 with VP16-Meis1 significantly reduced the latency period of AML compared with ND13+Meis1 (compare median latency of 34 with 61 days, respectively; Figure 1D; data not shown). Conversely, transplantation of M33-Meis1–transduced primary BM into lethally irradiated recipients failed to induce AML (Figure 1D). Analysis of engraftment in peripheral blood showed a rapid loss of circulating M33-Meis1–transduced BM cells compared with MIY (Figure 1E), wild-type Meis1, and Meis1ΔHD-transduced BM (data not shown).
To further investigate the growth inhibitory effect of M33-Meis1 on normal hematopoiesis, lineage-depleted, Sca1+, c-kit+ (LSK) BM cells were transduced with MIY, Meis1, or M33-Meis1, and the growth properties of the transduced cells were evaluated in a methylcellulose serial replating assay. Although the MIY-transduced population did not exhibit a significant growth advantage over the input, the Meis1-transduced population showed an initial proliferative advantage followed by a decrease below input levels by the third replating (supplemental Figure 2). In contrast, the M33-Meis1–transduced LSK cells failed to grow in methylcellulose beyond the first plating, showing that M33-Meis1 confers growth inhibitory effects on primitive clonogenic hematopoietic progenitors.
Collectively, these results indicate that M33-Meis1 confers a strong in vitro and in vivo growth inhibitory effect on normal primitive BM and transformed myeloid progenitor cells.
M33-MEIS1 heterodimerizes with PBX1 on or off DNA and nucleates the formation of a PcG complex on Meis1 target genes
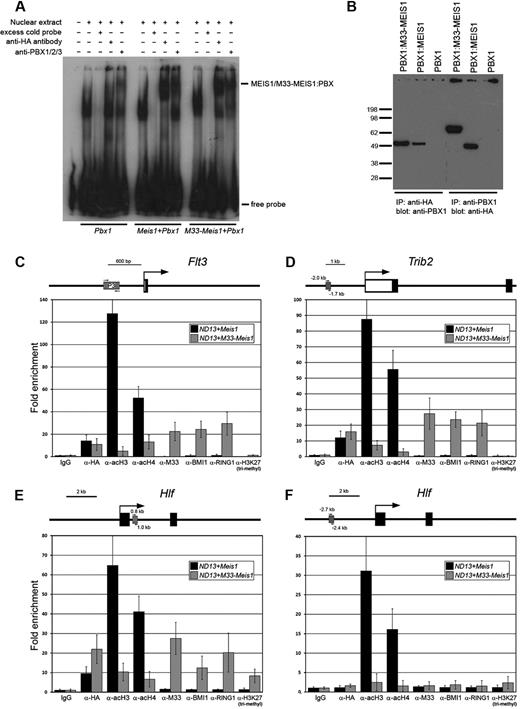
The oncogenicity of Meis1 overexpression may be attributed to the up-regulation of Hox/Meis1/Pbx1,29,30 Meis1/Hox, Meis1/Pbx1, or Meis1 target genes. In addition, overexpression of MEIS1 may titrate PBX1 and thus deregulate Hox/Pbx1 and Pbx1 targets, although this latter mechanism is presumably not relevant to the observed effects of M33-MEIS1 if it interacts with PBX1 in the same manner as MEIS1. Thus, to show that the normal biochemical properties of MEIS1 are retained in the M33-MEIS1 chimera, we assessed the ability of M33-MEIS1 to heterodimerize with PBX1 on or off DNA in cell lines overexpressing Pbx1, Pbx1+Meis1, and Pbx1+M33-Meis1. EMSA showed that both MEIS1 and M33-MEIS1 form nucleoprotein complexes with PBX1 on DNA containing a MEIS1-PBX1 consensus binding sequence7 (Figure 2A). Furthermore, coimmunoprecipitation experiments showed that both MEIS1 and M33-MEIS1 heterodimerize with PBX1 in the absence of DNA (Figure 2B). These data show that the M33 moiety does not interfere with the ability of MEIS1 to heterodimerize with PBX1 on or off DNA.
M33-MEIS1 heterodimerizes with PBX1 on or off DNA and nucleates the formation of a Polycomb group complex on MEIS1 target genes. (A) EMSA was performed with nuclear extracts from E86-Pbx1, E86-Pbx1+Meis1, and E86-Pbx1+M33-Meis1 viral producers (indicated at bottom) and incubated with a radiolabeled oligonucleotide containing a Meis1/Pbx1 consensus binding sequence.7 Each nuclear extract was incubated with probe and a combination of probe with molar excess of unlabeled (cold) probe, anti-HA antibody, or anti-PBX1 antibody. The MEIS1/M33-MEIS1:PBX1 nucleoprotein complexes are indicated. (B) Coimmunoprecipitation experiments were performed with nuclear extracts from E86-Pbx1, E86-Pbx1+Meis1, and E86-Pbx1+M33-Meis1 viral producers (indicated at top). PBX1 is specifically immunoprecipitated by an anti-HA antibody in Pbx1+Meis1 and Pbx1+M33-Meis1–overexpressing cells and recognized by an anti-PBX1 antibody. In the reciprocal experiment, MEIS1 and M33-MEIS1 are specifically immunoprecipitated by an anti-PBX1 antibody and recognized by an anti-HA antibody. (C-F) Schematic representation of Flt3 (C), Trib2 (D), and Hlf (E-F) showing the putative transcription start sites (arrows), the exons (black boxes), the 5′ untranslated region (empty box), and the position of the amplicons assayed in the ChIP analysis (striped box). The ND13+Meis1 and ND13+M33-Meis1–transduced BM cells were coimmunoprecipitated with the antibody indicated on the x-axis. The data represent the mean of fold enrichment relative to the immunoglobulin G (IgG) control samples ± SD of 3 independent experiments. AcH3 indicates acetylated histone H3; AcH4, acetylated H4; H3K27 tri-methyl, trimethylated Lys27 on H3.
M33-MEIS1 heterodimerizes with PBX1 on or off DNA and nucleates the formation of a Polycomb group complex on MEIS1 target genes. (A) EMSA was performed with nuclear extracts from E86-Pbx1, E86-Pbx1+Meis1, and E86-Pbx1+M33-Meis1 viral producers (indicated at bottom) and incubated with a radiolabeled oligonucleotide containing a Meis1/Pbx1 consensus binding sequence.7 Each nuclear extract was incubated with probe and a combination of probe with molar excess of unlabeled (cold) probe, anti-HA antibody, or anti-PBX1 antibody. The MEIS1/M33-MEIS1:PBX1 nucleoprotein complexes are indicated. (B) Coimmunoprecipitation experiments were performed with nuclear extracts from E86-Pbx1, E86-Pbx1+Meis1, and E86-Pbx1+M33-Meis1 viral producers (indicated at top). PBX1 is specifically immunoprecipitated by an anti-HA antibody in Pbx1+Meis1 and Pbx1+M33-Meis1–overexpressing cells and recognized by an anti-PBX1 antibody. In the reciprocal experiment, MEIS1 and M33-MEIS1 are specifically immunoprecipitated by an anti-PBX1 antibody and recognized by an anti-HA antibody. (C-F) Schematic representation of Flt3 (C), Trib2 (D), and Hlf (E-F) showing the putative transcription start sites (arrows), the exons (black boxes), the 5′ untranslated region (empty box), and the position of the amplicons assayed in the ChIP analysis (striped box). The ND13+Meis1 and ND13+M33-Meis1–transduced BM cells were coimmunoprecipitated with the antibody indicated on the x-axis. The data represent the mean of fold enrichment relative to the immunoglobulin G (IgG) control samples ± SD of 3 independent experiments. AcH3 indicates acetylated histone H3; AcH4, acetylated H4; H3K27 tri-methyl, trimethylated Lys27 on H3.
To provide evidence that the growth inhibitory effects of M33-Meis1 are a direct result of M33-MEIS1 binding to and facilitating the formation of a PcG complex on MEIS1 target genes, we used ChIP to examine the co-occupancy of PRC1 components on known MEIS1 target genes. ChIP performed on ND13+Meis1 and ND13+M33-Meis1–transduced BM showed that there was no discrepancy in association between wild-type MEIS1 and M33-MEIS1 at 3 Meis1 target genes, Flt38 (Figure 2C), Trib2,10 (Figure 2D) and Hlf (Figure 2E), indicating that M33-MEIS1 is recruited to Meis1 targets and that M33 moiety does not interfere with MEIS1 binding. Examination of whether M33-MEIS1 recruits PRC1 components to Meis1 target genes showed that the regions bound by M33-MEIS1 were co-occupied by M33, BMI1, and RING1 (Figure 2C-E). Antibodies to M33, BMI1, and RING1 did not coimmunoprecipitate DNA fragments bound by MEIS1. Furthermore, random genomic regions that served as negative controls for MEIS1 binding were also negative for M33-MEIS1, BMI1, RING1, and M33 (Figure 2F; data not shown). Taken together, these data show that MEIS1 and M33-MEIS1 associate with identical target sequences and that recruitment of PRC1 components to Meis1 target genes depend on the M33 moiety of the chimeric protein.
To assess whether artificial recruitment of PRC1 components alters the chromatin structure of Meis1 target genes, we examined epigenetic modifications associated with transcriptionally active and PcG-silenced chromatin. Consistent with transcriptional activation, acetylation of histones H3 and H4 was enriched at all MEIS1-bound regulatory regions tested, and this enrichment was significantly reduced by M33-MEIS1 (Figure 2C-E). Enrichment of the epigenetic modification associated with the initiation of PcG silencing, trimethylation of Lys27 of H3 (H3K27),31 was not significantly different between MEIS1 and M33-MEIS1. We hypothesize that the requirement for trimethylation of H3K27, which is mediated by the PRC2 members EZH2, a histone methyltransferase,32 and SUZ12 and is necessary for PRC1 recruitment and transcriptional silencing, is bypassed by M33-MEIS1.
M33-Meis1 represses transcription of genes normally up-regulated by Meis1
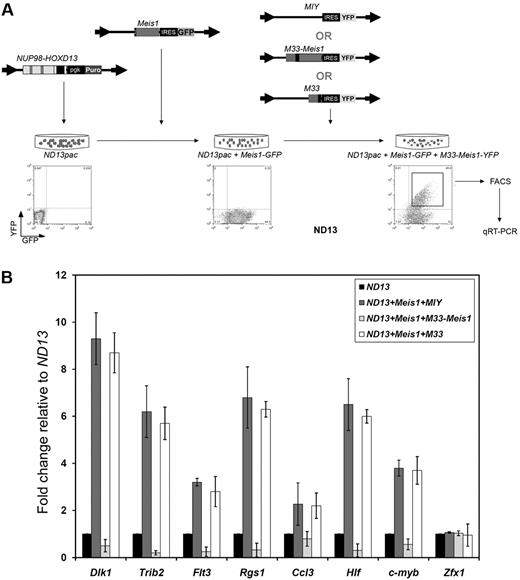
To evaluate the transcriptional effect of M33-Meis1 on Meis1 target genes, qRT-PCR was performed in a ND13 pgk-puromycin BM cell line (ND13pac) on a panel of genes up-regulated by Meis1 and, as a negative control, Zfx1.7,9,10 We have previously demonstrated that overexpression of ND13 does not influence endogenous Meis1 levels33 ; thus, the magnitude of gene expression increase detected in this assay exclusively depends on the enforced overexpression of Meis1. The experimental design is illustrated in Figure 3A. As expected, ND13pac BM cells transduced with Meis1-GFP and MIY showed elevated expression of Dlk1, Trib2, Flt3, Rgs1, Ccl3, Hlf, and c-myb, known Meis1 target genes, but not of Zfx1, relative to the ND13pac BM cells (Figure 3B). In contrast, ND13pac+Meis1-GFP BM cells transduced with M33-Meis1-YFP resulted in significant repression of all genes tested, excluding Zfx1. To control for possible nonspecific effects, the repressive domain of M33 was overexpressed in ND13pac+Meis1-GFP BM cells and found not to affect the expression of the tested genes (Figure 3B). Similarly, the expression levels of genes adjacent to Flt3, Trib2, Ccl3, and Hlf were not affected by M33-Meis1 (data not shown), indicating that M33-Meis1 activity is specific for Meis1 targets. These data show that M33-Meis1 represses transcription of Meis1 target genes in a dominant manner.
M33-Meis1 represses transcription of Meis1 target genes. (A) General experimental design. (B) Quantitative RT-PCR analysis showing the mean mRNA expression level of the indicated genes (x-axis) in each cell population relative to ND13 cells. The error bars represent the mean ± SD from 3 independent experiments.
M33-Meis1 represses transcription of Meis1 target genes. (A) General experimental design. (B) Quantitative RT-PCR analysis showing the mean mRNA expression level of the indicated genes (x-axis) in each cell population relative to ND13 cells. The error bars represent the mean ± SD from 3 independent experiments.
M33-Meis1 suppresses Hox-, NUP98-HOX–, and MN1-induced AML
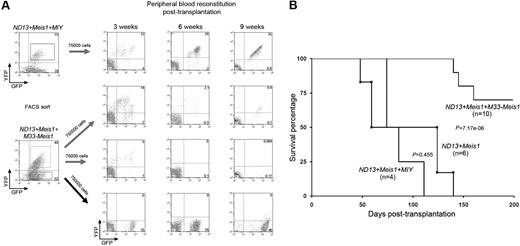
The growth inhibitory effect of M33-Meis1 on myeloid progenitors and normal BM cells prompted us to examine whether M33-Meis1 can suppress LSC function. To ascertain this, leukemic ND13pac+Meis1-GFP BM cells were transduced with M33-Meis1-YFP or MIY, underwent FACS, and transplanted over a 2-log dose range (75 000 and 750 000 cells) into lethally irradiated mice (Figures 3A and 4A). Peripheral blood chimerism measured at 3, 6, and 9 weeks after transplantation showed a rapid, progressive loss of circulating ND13+Meis1+M33-Meis1 triple transduced BM (Figure 4A). Although all ND13+Meis1+MIY BM recipients (n = 4) succumbed to AML with similar kinetics to ND13+Meis1 BM recipients (n = 6) (P = .455), mice receiving ND13+Meis1+M33-Meis1 cotransduced BM did not develop AML (n = 10), although 3 of 10 animals died with no hematologic malignancy detected on necropsy (Figure 4B). Similarly, Hoxa9+Meis1 BM cells transduced with M33-Meis1-YFP or MIY, underwent FACS, and transplanted over a 2-log dose range (20 000 and 200 000 cells) into lethally irradiated mice showed a rapid loss of Hoxa9+Meis1+M33-Meis1 cotransduced BM cells in vivo (supplemental Figure 3). Thus, expression of M33-Meis1 in ND13+Meis1 or HoxA9+Meis1 leukemic BM cells resulted in eradication of the leukemic clones as tested at 2 transplant doses, consistent with a multilog reduction in LSC number.
M33-Meis1 suppresses ND13+Meis1-induced AML. (A) General experimental design and representative FACS profiles showing engraftment of mice that received a transplant with indicated cells. Numbers denote the percentage of cells within the quadrant. (B) Kaplan-Meier survival curves for mice that received a transplant with BM cotransduced with the indicated combination of genes.
M33-Meis1 suppresses ND13+Meis1-induced AML. (A) General experimental design and representative FACS profiles showing engraftment of mice that received a transplant with indicated cells. Numbers denote the percentage of cells within the quadrant. (B) Kaplan-Meier survival curves for mice that received a transplant with BM cotransduced with the indicated combination of genes.
To extend our findings beyond Hox- and NUP98-HOX–induced AML models, we examined the effects of M33-Meis1 on MN1-transduced BM. We have previously demonstrated that the potent oncogene MN1 effectively transforms murine BM cells and induces rapid AML in the murine BM transplantation model.26 MN1-GFP BM cells were transduced with MIY or M33-Meis1-YFP, and 750 000 bulk cells were transplanted into lethally irradiated recipients. Although all MN1+MIY and MN1+M33-Meis1 animals succumbed to AML with similar kinetics as previously reported (median survival of 38 days), FACS analysis of BM and peripheral blood from diseased mice showed a significant loss of MN1+M33-Meis1–transduced cells compared with MN1+MIY–transduced cells (supplemental Figure 4). These data provide further support that M33-Meis1 suppresses LSC function.
Finally, we tested the ability of M33-Meis1 to inhibit the proliferation of human leukemia cell lines. Our analyses showed that overexpression of M33-Meis1 in the human leukemia cell lines U937 and HL60, expressing high and low levels of endogenous MEIS1, respectively (data not shown), resulted in markedly increased rates of eradication of both cell types in vitro compared with the MIY control (supplemental Figure 5).
Overexpression of M33-Meis1 induces a partial G1-phase block in AML models
Our demonstration of the growth inhibitory effect of M33-Meis1 led us to test whether M33-Meis1–transduced BM cells are undergoing apoptosis, terminally differentiating, and/or undergoing cell-cycle perturbations. To test these possibilities, annexin V staining, FACS immunophenotyping, and cell-cycle analyses were performed, respectively.
To investigate whether M33-Meis1 induced apoptosis, LSK BM cells were transduced with MIY, Meis1, or M33-Meis1, stained with annexin V, and quantified by flow cytometry. This analysis showed that M33-Meis1 did not induce but rather modestly inhibited apoptosis compared with MIY and Meis1 (supplemental Figure 6A).
Immunophenotypic analysis of Hoxa9neo BM cells transduced with MIY, Meis1, and M33-Meis1 showed that, although Meis1 stimulated high c-Kit and low Mac1 expression, which is consistent with more primitive, less differentiated cell populations, M33-Meis1 did not significantly alter c-Kit and Mac1 expression compared with the MIY control (supplemental Figure 6B). Examination of B220, ter119, CD19, CD4, CD8, immunoglobulin E, and Gr1 cell surface markers did not show significant differences between M33-Meis1 and MIY-transduced Hoxa9neo BM cells (supplemental Figure 6B; data not shown). Similar results were obtained in the ND13 BM model (data not shown). These results indicate that M33-Meis1 did not induce an aberrant terminal differentiation program.
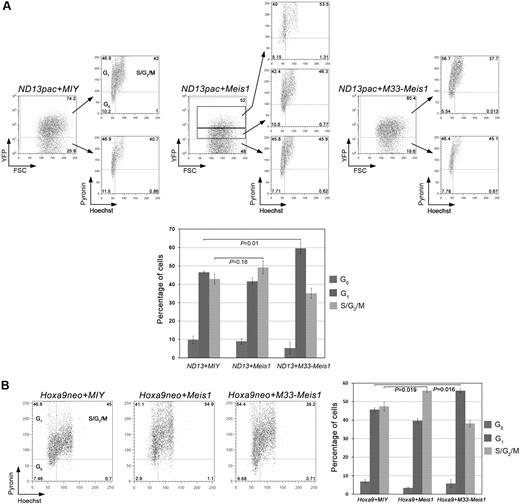
Analysis of cell-cycle distribution showed dramatic differences in the cell-cycle profiles of primary BM cells cotransduced with ND13pac, HoxA9neo, or MN1-GFP and M33-Meis1 compared with MIY or Meis1. In all cellular contexts tested, overexpression of M33-Meis1, but not of MIY or Meis1, resulted in a significant increase in the percentage of cells in the G1 phase of the cell cycle as measured by Hoechst-Pyronin staining (Figure 5) and, independently, by 5-bromodeoxyuridine incorporation (supplemental Figure 7). Moreover, overexpression of Meis1-YFP in all leukemia models tested correlated with increased cell-cycle entry compared with the MIY control. Indeed, cell-cycle analysis of ND13pac+Meis1 BM cells gated for high versus low Meis1 expression, as gauged by high versus low YFP expression, respectively, showed a strong correlation between increased Meis1 expression and cell-cycle entry (Figure 5A).
M33-Meis1 imposes a partial G1-phase cell-cycle block on ND13- and Hoxa9-transduced BM cells. (A) Primary BM cotransduced with ND13pac and MIY, Meis1, or M33-Meis1 were incubated with Hoechst and Pyronin and analyzed by FACS analysis. Cell-cycle profiles of untransduced (YFP−) and transduced (YFP+) are shown for each experimental condition. Numbers denote the percentage of cells within the quadrant. (B) Cell-cycle profiles of primary BM cotransduced with Hoxa9neo and MIY, Meis1, or M33-Meis1. Data in the bar graphs are expressed as the mean ± SD of 3 independent transductions. Numbers denote the percentage of cells within the quadrant.
M33-Meis1 imposes a partial G1-phase cell-cycle block on ND13- and Hoxa9-transduced BM cells. (A) Primary BM cotransduced with ND13pac and MIY, Meis1, or M33-Meis1 were incubated with Hoechst and Pyronin and analyzed by FACS analysis. Cell-cycle profiles of untransduced (YFP−) and transduced (YFP+) are shown for each experimental condition. Numbers denote the percentage of cells within the quadrant. (B) Cell-cycle profiles of primary BM cotransduced with Hoxa9neo and MIY, Meis1, or M33-Meis1. Data in the bar graphs are expressed as the mean ± SD of 3 independent transductions. Numbers denote the percentage of cells within the quadrant.
Meis1 induces a gene expression signature consistent with cell-cycle entry
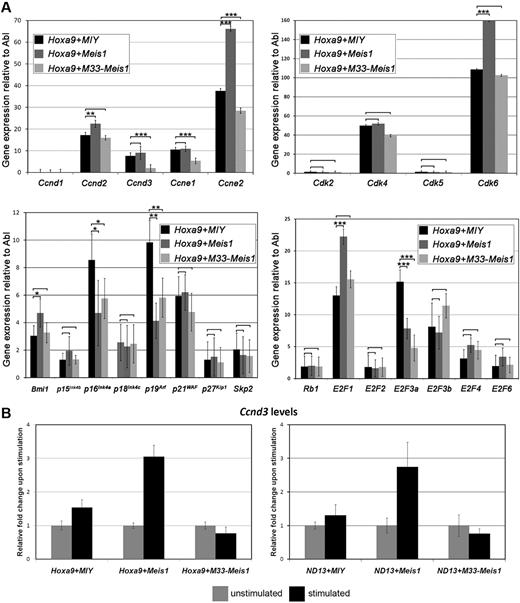
The foregoing results and previous studies showing perturbations of cell-cycle regulators in MLL-AF9-Meis1−/− BM,5,6 suggested a pivotal role for Meis1 in G1-to-S phase progression in hematopoietic cells. Thus, to explore the role of Meis1 and M33-Meis1 in the transcriptional control of cell-cycle regulators, we assayed the expression level of 24 G1-phase regulators in the Hoxa9 AML model. Primary Hoxa9-transduced BM cells were infected in triplicate with MIY, Meis1-YFP, or M33-Meis1-YFP, and doubly transduced cells were isolated by FACS 2 days after transduction for assessment of gene expression by qRT-PCR. Consistent with the promotion of cell-cycle entry, Hoxa9+Meis1 BM cells showed elevated levels of the positive cell-cycle regulators cyclin D2, cyclin E2, Cdk6, Bmi1, and E2F1 compared with Hoxa9+MIY BM cells (Figure 6A). Conversely, Hoxa9+M33-Meis1 BM cells showed significant reduction of the core-positive cell-cycle regulators cyclin D3 and cyclin E2, an effect dependent on the novel activity of the engineered fusion.
Meis1 induces a gene expression signature consistent with cell-cycle entry. (A) Quantitative RT-PCR analysis on 24 G1-phase regulators. The data are plotted relative to the endogenous control Abl and are expressed as the mean ± SD of 3 independent transductions. P > .05 are unlabeled, *P < .05, **P < .01, and ***P < .001. (B) Cytokine stimulation of Hoxa9 and ND13 BM cells transduced with Meis1 show a significant induction of Ccnd3 levels compared with MIY and M33-Meis1 stimulation levels.
Meis1 induces a gene expression signature consistent with cell-cycle entry. (A) Quantitative RT-PCR analysis on 24 G1-phase regulators. The data are plotted relative to the endogenous control Abl and are expressed as the mean ± SD of 3 independent transductions. P > .05 are unlabeled, *P < .05, **P < .01, and ***P < .001. (B) Cytokine stimulation of Hoxa9 and ND13 BM cells transduced with Meis1 show a significant induction of Ccnd3 levels compared with MIY and M33-Meis1 stimulation levels.
To confirm the role of Meis1 as a cell-cycle regulator, we performed Affymetrix gene expression profiling in the context of the ND13 BM cell model and assessed the gene ontology annotations of the ND13+MIY, ND13+Meis1,10 and ND13+M33-Meis1 transcriptomes (GEO accession no. GSE15541). Ingenuity Pathway Analysis (Ingenuity Systems) showed a significant enrichment in several gene ontology annotations in the comparison of the ND13+Meis1 and ND13+MIY datasets, including cell cycle (supplemental Figure 8). Comparison of the ND13+M33-Meis1 and ND13+MIY datasets highlighted a significant enrichment of cell-cycle regulators, specifically, G1-to-S phase regulators. Of particular interest was the significant down-regulation of cyclin D3 induced by M33-Meis1 compared with MIY (3.44-fold decrease; P < .001).
Although M33-Meis1 significantly repressed cyclin D3 levels in all leukemia models tested, overexpression of wild-type Meis1 did not significantly up-regulate cyclin D3 under standard growth conditions. These data strongly suggested that Meis1 may be permissive or may modulate cyclin D3 transcription under the appropriate mitogenic conditions. SCF and its receptor c-kit are important for the survival and proliferation of HSCs and progenitor cells34 and cooperate with Meis1 to induce proliferation of Hoxa9-transduced BM cells.35 Interestingly, SCF/c-kit up-regulates cyclin D3 to promote cell-cycle progression.36 Indeed, Hoxa9+Meis1 and ND13+Meis1 BM cells deprived of growth factors and subsequently stimulated with a high concentration of SCF showed a greater than 2.5-fold induction of cyclin D3 levels compared with an approximately 1.5-fold induction observed in Hoxa9+MIY and ND13+MIY BM cells (Figure 6B). High SCF concentrations could not abrogate the M33-Meis1–induced repression of cyclin D3 (Figure 6B) or the G1-phase accumulation (data not shown). These findings suggest that the proliferative synergy between SCF and Meis1 in Hoxa9 BM cells35 depends on cyclin D3 and supports a model in which Meis1 intensifies SCF signaling by modulating cyclin D3 transcription.
Down-regulation of cyclin D3 by M33-Meis1 correlates with loss of pRb hyperphosphorylation
Cyclin D activity is rate limiting and essential for cell-cycle progression through the G1 phase of the cell cycle.37 This is achieved through its interaction with the cyclin-dependent kinases (Cdk4 or Cdk6) and the subsequent hyperphosphorylation of pRb, which leads to the dissociation of pRb from promoter-bound E2Fs, allowing transcription of E2F-regulated genes and progression into the S phase.38 For this reason, we chose to further investigate the role of cyclin D3 in the M33-Meis1–induced G1-phase accumulation.
To test whether the phosphorylation status of pRb is tempered by M33-Meis1, U937 cells were transduced with Meis1 or M33-Meis1, serum deprived, and subsequently stimulated with SCF. Western blot analyses showed that, although increasing amounts of SCF induced higher levels of pRb hyperphosphorylation in the Meis1-transduced cells, M33-Meis1 abrogated the SCF response and inhibited pRb phosphorylation (Figure 7A). This is consistent with the observed accumulation of U937+M33-Meis1 cells in G1 phase and the concomitant reduction of Cyclin D3 levels (supplemental Figure 9A and B, respectively). These findings were independently confirmed by the statistically significant reduction of intracellular phospho-pRb (pSer773) staining in Hoxa9+M33-Meis1 and ND13+M33-Meis1 BM cells compared with Hoxa9+MIY and ND13+Meis1, respectively (Figure 7B). These data provide strong correlative evidence linking the M33-Meis1–induced G1-phase accumulation to the inhibition of pRb hyperphosphorylation.
Cyclin D3 is functionally downstream of Meis1. (A top) Western blot analysis of U937+Meis1 and U937+M33-Meis1 cells stimulated with SCF and immunoblotted for hypophosphorylated (pRb) and hyperphosphorylated (ppRb) forms of Rb. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as the loading control. (Bottom) densitometry analysis quantifying ppRb. (B) Intracellular phospho-pRb staining of Hoxa9 and ND13 BM cells transduced with Meis1 or M33-Meis1. The cells were immunostained (shaded) or untreated (unshaded) with anti–phospho-pRb and analyzed by flow cytometry. The Kolmogorov-Smirnov chi-square value and the super enhanced D-max (SED %Positive) statistics are shown. (C top) Schematic representation of cyclin D3 showing the putative start site (arrow), exons (black boxes), 5′ and 3′ untranslated regions (unshaded boxes), and introns. The PCR amplicons are labeled C1, C2, and C3. (Bottom) Data represent the mean of fold enrichment relative to immunoglobulin G ± SD of 3 independent experiments. (D) ND13pac BM cells were cotransduced with M33-Meis1-YFP and cyclin D3-GFP, stained with Hoechst-pyronin, and analyzed by flow cytometry. (E) In vitro growth kinetics of indicated cells plated in standard growth conditions. The data are expressed as the mean ± SD of 2 independent transductions.
Cyclin D3 is functionally downstream of Meis1. (A top) Western blot analysis of U937+Meis1 and U937+M33-Meis1 cells stimulated with SCF and immunoblotted for hypophosphorylated (pRb) and hyperphosphorylated (ppRb) forms of Rb. Glyceraldehyde phosphate dehydrogenase (GAPDH) served as the loading control. (Bottom) densitometry analysis quantifying ppRb. (B) Intracellular phospho-pRb staining of Hoxa9 and ND13 BM cells transduced with Meis1 or M33-Meis1. The cells were immunostained (shaded) or untreated (unshaded) with anti–phospho-pRb and analyzed by flow cytometry. The Kolmogorov-Smirnov chi-square value and the super enhanced D-max (SED %Positive) statistics are shown. (C top) Schematic representation of cyclin D3 showing the putative start site (arrow), exons (black boxes), 5′ and 3′ untranslated regions (unshaded boxes), and introns. The PCR amplicons are labeled C1, C2, and C3. (Bottom) Data represent the mean of fold enrichment relative to immunoglobulin G ± SD of 3 independent experiments. (D) ND13pac BM cells were cotransduced with M33-Meis1-YFP and cyclin D3-GFP, stained with Hoechst-pyronin, and analyzed by flow cytometry. (E) In vitro growth kinetics of indicated cells plated in standard growth conditions. The data are expressed as the mean ± SD of 2 independent transductions.
Cyclin D3 is a direct transcriptional target of MEIS1 and partially restores the M33-Meis1–induced G1-phase accumulation
The above data raised the possibility that cyclin D3 may be directly controlled by M33-Meis1 and, by extension, Meis1. To address this hypothesis we tested putative MEIS1-associated regions within the Ccnd3 locus, as determined by ChIP-Solexa sequencing (E.Y., B.A., R.K.H., manuscript in preparation), for MEIS1 co-occupancy. ChIP–qRT-PCR assays verified that MEIS1 and M33-MEIS1 associate with the same intronic region of cyclin D3 (Figure 7C). The various fold enrichments of the C3 region by MEIS1 and M33-MEIS1 does not reflect differential binding affinities of these proteins to this region because this assay cannot distinguish relative binding affinities across experimental arms. EMSA was used to independently confirm the specificity of MEIS1 binding to the cyclin D3 C3 region (supplemental Figure 10).
We next sought to determine whether the G1-phase accumulation induced by M33-Meis1 was specifically due to the reduction of cyclin D3 levels. ND13pac+M33-Meis1-YFP BM cells engineered to overexpress cyclin D3-GFP showed a marked increase in the proportion of cells in the S phase (Figure 7D) and were not out-competed in prolonged in vitro culture compared with the ND13pac+M33-Meis1 BM cells (Figure 7E). Although the ND13+Ccnd3 BM cells exhibited a modest increase in proliferation in vitro (Figure 7E), overexpression of cyclin D3 was insufficient to cooperate with ND13 to promote AML in mice (data not shown), reinforcing our hypothesis that the leukemogenicity of Meis1 depends on its ability to deregulate multiple pathways.
Collectively, these results show that down-regulation of cyclin D3 by M33-Meis1 is direct and that the growth suppression and G1-phase accumulation induced by M33-Meis1 is partially relieved by overexpression of cyclin D3.
Discussion
The ability of a repressive form of MEIS1 to potently inhibit normal hematopoietic and leukemic cell proliferation by impeding cell-cycle progression shows Meis1 as a positive cell-cycle regulator. Our results confirm and extend accumulating evidence linking Meis1 to cell-cycle regulation and provide the first demonstration that Meis1 directly controls the expression of a key G1 phase cell-cycle regulator, cyclin D3, and indirectly, the phosphorylation of pRb.
Cyclin D3–null mice are viable and displayed no apparent HSC or progenitor defect.39 However, these mice displayed neutropenia and resistance to stimulation by granulocyte colony-stimulating factor40 and exhibited defects in the expansion of immature T lymphocytes39 and in the development of pre-B cells.41 Consistent with our data, inhibition of cyclin D3 blocked G1-to-S phase progression in T-lymphoma cells,42 endometrial carcinoma cells,43 and U937 cells.44
Overexpression of cyclin D3 has been observed in many human cancers.45,–47 In leukemias, Notch and FLT3/ITD signaling promote cell-cycle entry by stimulating cyclin D3 transcription.48,49 Moreover, overexpression of cyclin D3 in the murine myeloid 32Dcl3 cell line suppressed granulocyte colony-stimulating factor–induced granulocyte differentiation,50 a function also described for Meis1.35 Although we were unable to document a positive correlation between MEIS1 and cyclin D3 expression in AML patient samples (data not shown), it does not preclude the model that MEIS1 modulates cyclin D3 expression.
The Meis1 genetic signature includes genes implicated in stem cell self-renewal and differentiation.7,9,10 In light of the current and recent studies, it is becoming apparent that several key cell-cycle regulators belong to this signature. Our study advances the Meis1 genetic signature to include the G1-cyclins D2, D3, and E2, and the G1-regulators Cdk6 and E2F1. We also confirm the observation made by Wong et al5 that expression of Bmi1 is modulated by Meis1. The discrepant down-regulation of the BMI1 target genes p16 and p19 by both Meis1 and M33-Meis1 shows the complexity of cell-cycle gene regulation and can be interpreted as indirect effects. In this regard, although we provide evidence for cyclin D3, it remains to be determined if the other responsive genes are directly controlled by Meis1/M33-Meis1. Furthermore, we propose that studies combining the gene expression analyses with examination of posttranslational modifications induced by Meis1 will be tremendously insightful.
Although the use of transgenic models, in particular short-hairpin RNA expressing and knockout animals, are the methods of choice to study gene function in vivo, we present a novel method of gene suppression that uses a well-characterized, trackable trans-acting factor that exaggerates Meis1 loss-of-function phenotypes and is more amenable to biofunctional assays. The identification of Meis1 as a positive G1-phase regulator has important implications for the treatment of AML. The inhibition of cyclin D-Cdk4/6 kinases in combination with drugs that target other Meis1-regulated pathways, such as Flt3 inhibitors, may have merit. However, because our results highlight the ability of Meis1 to deregulate multiple oncogenic pathways, it may be more pertinent to design therapies that reduce aberrant MEIS1 transcription and/or abrogate MEIS1 activity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Terry Fox Foundation, Genome Canada/BC, the Cancer Research Society, the Networks of Centres of Excellence of Canada, Stem Cell Network, and the Leukemia Research Fund of Canada.
Authorship
Contribution: B.A. designed and performed the research, analyzed the data, and wrote the manuscript; E.Y., P.X., C.Y.L., F.K., L.P., C.R., M.H., S.S., P.R., A.M., S.-L.G., and M.F. performed research, analyzed the data, and edited the manuscript; and R.K.H. designed the research, analyzed the data, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Keith Humphries, Terry Fox Laboratory, British Columbia Cancer Agency, 675 West 10th Ave, Vancouver, BC, Canada, V5Z 1L3; e-mail: khumphri@bccrc.ca.